Abstract
Carbon tetrachloride (CCl4) is widely used as an animal model of hepatotoxicity and the mechanisms have been arduously studied, however, the contribution of the sympathetic nervous system (SNS) in CCl4-induced acute hepatotoxicity remains controversial. It is also known that either CCl4 or SNS can affect systemic inflammatory responses. The aim of this study was to establish the effect of chemical sympathectomy with 6-hydroxydopamine (6-OHDA) in a mouse model of CCl4-induced acute hepatotoxicity and systemic inflammatory response. Mice exposed to CCl4 or vehicle were pretreated with 6-OHDA or saline. The serum levels of aminotransferases and alkaline phosphatase in the CCl4-poisoning mice with sympathetic denervation were significantly lower than those without sympathetic denervation. With sympathetic denervation, hepatocellular necrosis and fat infiltration induced by CCl4 were greatly decreased. Sympathetic denervation significantly attenuated CCl4-induced lipid peroxidation in liver and serum. Acute CCl4 intoxication showed increased expression of inflammatory cytokines/chemokines [eotaxin-2/CCL24, Fas ligand, interleukin (IL)-1α, IL-6, IL-12p40p70, monocyte chemoattractant protein-1 (MCP-1/CCL2), and tumor necrosis factor-α (TNF-α)], as well as decreased expression of granulocyte colony-stimulating factor and keratinocyte-derived chemokine. The overexpressed levels of IL-1α, IL-6, IL-12p40p70, MCP-1/CCL2, and TNF-α were attenuated by sympathetic denervation. Pretreatment with dexamethasone significantly reduced CCl4-induced hepatic injury. Collectively, this study demonstrates that the SNS plays an important role in CCl4-induced acute hepatotoxicity and systemic inflammation and the effect may be connected with chemical- or drug-induced hepatotoxicity and circulating immune response.
Introduction
Carbon tetrachloride (CCl4) is a chlorinated hydrocarbon that has widespread use in various industries as a solvent and in medicine as a vermifuge. It is found in low levels in ambient air and water [1,2]. Acute exposure to high levels of CCl4 vapor results in central nervous system depression and even coma. Lower levels of exposure leads to renal and especially hepatic toxicity [3,4]. Indeed CCl4 intoxication in the mouse is a widely used experimental model for the study of hepatotoxicity [1,5–7].
The mechanisms of CCl4 toxicity are recognized. CCl4 is mainly activated by cytochrome P450 2E1 to form the trichloromethyl peroxy radical, which initiates lipid peroxidation by pulling out a hydrogen atom in the vicinity of a polyunsaturated fatty acid double bond [1,2,8]. After propagation of the peroxidation process, lipids are finally degraded in small molecules such as malondialdehyde (MDA), a highly reactive aldehyde that can damage the plasma membranes [9]. The end result inactivates calcium pump activity with calcium influx. All these alterations eventually lead to liver cell death accompanied by the release into the blood of intrahepatic enzymes [1,3]. However, the mechanisms of CCl4-induced steatosis remain speculative [10].
A close relationship between the hepatic sympathetic nerve supply and acute and chronic liver injury has previously been suggested [11]. Some studies indicated that CCl4-induced acute hepatotoxicity was promoted by the systemic sympathetic tone or adrenoceptor stimulation [12–16], whereas other investigators found inconsistent effects of the sympathetic nervous system (SNS) on the acute hepatotoxicity of CCl4 [17–19]. Thus the role of SNS action in CCl4 hepatotoxicity remains controversial and detailed mechanisms of SNS effect do not appear to have been sufficiently elucidated.
Moreover, CCl4 is a well-known compound for the inducing immune responses and inflammation [20–22]. Immune cells express various adrenergic and purinergic receptors that are sensitive to transmitters of the SNS. The production of cytokines/chemokines is modulated by activation of these receptors [23]. We therefore hypothesized that the sympathetic activity has an effect on secretion of cytokines/chemokines in an animal with CCl4 intoxication. Here, we applied chemical sympathectomy to investigate the role of the SNS on (1) acute toxic liver necrosis and steatosis induced by CCl4, and (2) systemic pattern of pro- and anti-inflammatory proteins in CCl4-poisoned mice.
Materials and Methods
Animals
Wild-type C57Bl/6JNarl male mice (8 weeks old) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and maintained under specific-pathogen-free conditions in the Laboratory Animal Center of National Defense Medical Center (Taipei, Taiwan). The animals were housed in a constant temperature room (22 ± 1°C) and subjected to a 12:12 hr light/dark cycle (lights on at 07:00 AM) with water and food ad libitum. To decrease the stress status and be relaxed and calm during the time of investigation, animals were regularly handled for a week prior to commencing an experiment. The protocols for all experiments involving mice were approved by the Committee on Institutional Animal Care and Use (IACUC-14–123, IACUC-14–311, and IACUC-15–032), National Defense Medical Center.
Chemical sympathectomy
The peripheral sympathetic nerve terminals of mice were ablated by intraperitoneal injection of a neurotoxin, 100 mg/kg of 6-hydroxydopamine (6-OHDA) (Sigma, St. Louis, MO, USA), for 5 consecutive days. 6-OHDA was freshly prepared in saline containing 0.1% (wt/vol) ascorbic acid (Sigma). Control animals received solvent only. As 6-OHDA does not cross the blood-brain barrier in adult animals, it selectively ablates peripheral sympathetic nerves [24–26]. The regimen of 6-OHDA treatment has been proven to deplete peripheral catecholamine in rodent tissues [17]. Sympathectomy was confirmed by staining fresh frozen sections of liver with an anti-tyrosine hydroxylase (TH) antibody [27].
Mice model of acute hepatotoxicity
One day after sympathetic denervation, CCl4 (Sigma) was administered intraperitoneally using the 100 μL Hamilton syringe at a single dose of 2 mL/kg, 12.5% diluted in olive oil (Sigma) for acute hepatotoxicity model. This dose which represents approximately one-fifth of the lethal dose in C57BL/6 mouse was chosen to ensure hepatocellular necrosis with low or negligible mortality [28,29]. Control mice were given olive oil at the same dose.
Experimental design
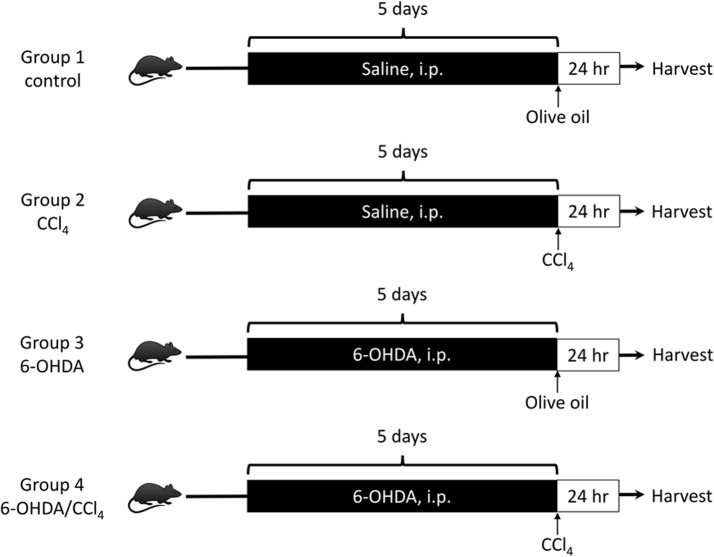
The experimental protocol is described in Fig. 1. Mice were divided into 4 groups (6 mice/group): Group 1 (control), Group 2 (CCl4), Group 3 (6-OHDA), and Group 4 (6-OHDA/CCl4). After a single dose of CCl4 or its vehicle, animals were sacrificed and their livers and blood were harvested for analysis at 24 hours. Livers were divided with lobes then fixed in 10% neutral buffered formalin for histology, preserved in optimum cutting temperature (OCT) (Tissue-Tek, Sakura, CA, USA) compound for frozen sections, and snap-frozen in liquid nitrogen for further protein analysis.
Fig 1. Schematic diagram of the study protocol.
Mice were randomly assigned in a blinded manner to one of four groups: group 1, control (n = 6), group 2, CCl4 (n = 6), group 3, OHDA (n = 6), and group 4, 6-OHDA/CCl4 (n = 6). The animals underwent 5 days of intra-peritoneal (i.p.) administration of 6-OHDA or saline. After ablation of the sympathetic nervous system, the animals were given CCl4 or vehicle on day 6, and sacrificed 24 hours after CCl4 poisoning. 6-OHDA = 6-hydroxydopamine, CCl4 = carbon tetrachloride.
A further set of male C57Bl/6JNarl 8-week old mice was included for additional experiments to establish dexamethasone-mediated effects on the inflammatory response. Dexamethasone (Sigma) was administered by intraperitoneal injection (10 mg/kg), at 15 hours and 2 hours prior to CCl4 administration. Two groups of mice (n = 4 for each group) were analyzed in this study: mice pretreated with phosphate-buffered saline (PBS) and subsequently injected with CCl4 and mice pretreated with dexamethasone and subsequently injected with CCl4. Twenty-four hours later, a blood sample was obtained to confirm liver damage through hepatic enzymes concentrations and a cytokine antibody array.
Histological and immunofluorescent assay
For histological analysis, formalin-fixed tissues were paraffin-embedded, sectioned (5 μm) and stained with hematoxylin and eosin. Cryostat sections of fresh frozen tissue were stained with Oil-Red-O (Sigma) for lipid detection or fixed in a mixture of methanol and acetone (1:1) at −20°C for 5 min for immunofluorescence. Following blocking of nonspecific activity by incubation with 2% bovine serum albumin (BSA) (Sigma) in TBST (12.5 mM Tris/HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20) for 20 min, sections were incubated for 1 hr at room temperature with an antibody against TH (Millipore, Billerica, MA, USA), then washed with TBST. Next, they were incubated with donkey anti-rabbit Alexa Fluor 488 (Invitrogen Corporation, Carlsbad, CA, USA) (1:5000 diluted in 2% BSA) for 1 hr at room temperature. Nuclei were counterstained with 4',6-diamidino-2-phenylindole (DAPI) (Sigma). Images were captured using an Olympus microscope BX51 and digital camera DP71 (Olympus, Tokyo, Japan). As described previously [30], the relative areas of centrilobular necrosis (the areas full of vacuolated and necrotic hepatocytes) and steatosis with Oil-Red-O staining were quantitated by ImageJ software (US National Institutes of Health) in six random different fields.
Measurement of hepatic enzymes and lipid profile
Measurement of serum lactate dehydrogenase (LDH, IU/L), alanine aminotransferase (ALT, IU/L), aspartate aminotransferase (AST, IU/L), alkaline phosphatase (ALP, IU/L), cholesterol (mg/dL), triglycerides (mg/dL), and high-density lipoprotein (HDL, mg/dL), was performed using standard enzymatic procedures by Union Clinical Laboratory (Taipei, Taiwan). Low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) were estimated by using Freidewald's equation [31].
Determination of the atherogenic index (AI) and the cardiac risk factor (CRF)
The AI was calculated as (cholesterol-HDL)/HDL. The CRF was calculated as (cholesterol/HDL) [32].
Liver homogenate preparation and lipid peroxidation assay
Liver samples were lysed using ice-cold standard RIPA buffer (Sigma), 100 μM Na3VO4 (Sigma), and protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany). Liver lysates were collected after centrifugation at 13,300 rpm for 15 min. The steady-state level of MDA was analyzed by measuring the formation of thiobarbituric acid reactive substances (TBARS) using a commercial assay kit (Cayman Chemical, Ann Arbor, MI, USA) in accordance with the manufacturer’s protocol. TBARS concentration was calculated from a MDA standard curve and normalized for protein content.
Measurement of inflammatory cytokines/chemokines in serum
Serum samples were analyzed with a cytokine antibody array, specifically the RayBio Mouse Inflammation Antibody Array 1 (RayBiotech, Inc., Norcross, GA, USA). Briefly, arrays were blocked with blocking buffer, equal amounts of serum were added, and the arrays were incubated at room temperature for 30 minutes. Arrays were processed according to the manufacturer’s instructions. The membranes were scanned by a UVP BioSpectrum AC image system (VisionWorks LS, UVP, Upland, CA, USA). Densitometry was performed by ImageJ software to determine the relative protein expression levels between groups.
Statistical analysis
Data were expressed as mean ± SD. Differences were analyzed by Student’s t-test for group comparison using the GraphPad Prism software program (GraphPad Software, La Jolla, CA). A p value less than 0.05 was considered significant.
Results
Identification of the intrahepatic sympathetic nerve fibers
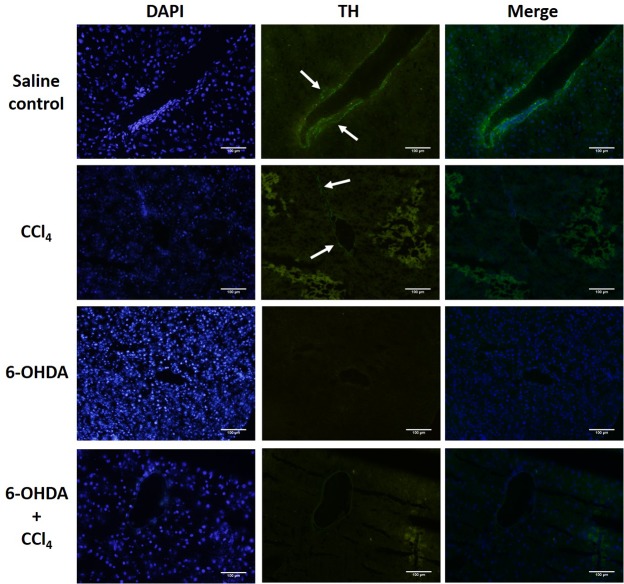
The sympathetic nerve marker tyrosine hydroxylase (TH) was used to identify the presence of intrahepatic sympathetic nerve fibers [33]. Immunofluorescences showed that the TH-positive fibers in the liver without chemical sympathectomy were mainly distributed in the portal tract. Chemical denervation by 6-OHDA effectively depleted the TH-positive fibers in the liver (Fig. 2).
Fig 2. Immunofluorescent analysis of liver tyrosine hydroxylase (TH) reactive nerve fibers.
TH positive nerve fibers (arrows) were seen around the portal area in saline treated mice but not in chemical sympathectomised animals. Magnification × 200. The results are representative of four sets of experiments. Scale bar = 100 μm.
Effect of chemical sympathetic denervation on the CCl4-induced acute hepatotoxicity
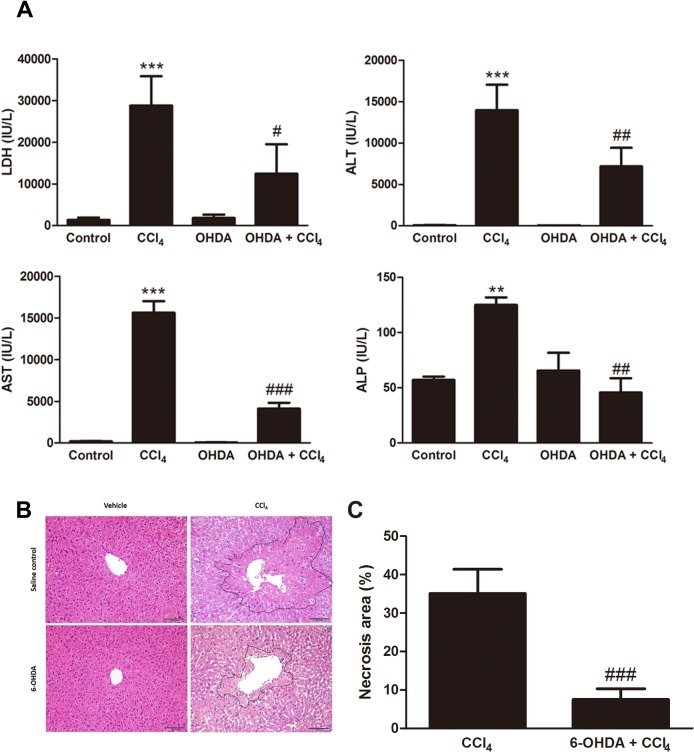
The biochemical effects of chemical sympathectomy on CCl4-induced hepatotoxicity were assayed by assessing serum levels of LDH, ALT, AST, and ALP (Fig. 3A). There was no statistically significant difference between control and 6-OHDA-treated mice. Twenty-four hours after administration of CCl4, LDH level was elevated from 1,351 ± 564 to 28,831 ± 7,027 IU/L (p = 0.0005); ALT level was elevated from 61 ± 33 to 13,970 ± 3,069 IU/L (p = 0.0003); AST level was elevated from 189 ± 70 to 15,620 ± 3,421 IU/L (p = 0.0001); and ALP level was elevated from 57 ± 4 to 125 ± 12 IU/L (p = 0.0049). Intraperitoneal injection of 6-OHDA inhibited the CCl4-induced elevation of serum LDH (12,460 ± 4,072 IU/L, p = 0.0465), ALT (7,180 ± 2,240 IU/L, p = 0.0040), AST (4,115 ± 1,755 IU/L, p < 0.0001), and ALP (46 ± 26 IU/L, p = 0.0047) levels (Fig. 1). Histology demonstrated that acute liver necrosis around pericentral areas in the CCl4-treated mice (35.19 ± 6.3%) was significantly greater than in the 6-OHDA/CCl4-cotreated mice (7.5 ± 2.8%, p = 0.0002). 6-OHDA-treated mice showed no morphological necrosis (Fig. 3B and 3C).
Fig 3. Absence of the sympathetic nervous system attenuated CCl4-induced hepatotoxicity.
(A) Serum isolated from whole blood was used to determine lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels. Bars are means ± SD, n = 6 mice per group. (***p < 0.001 vs. control, # p < 0.05 vs. CCl4, and ## p < 0.01 vs. CCl4) (B) Liver sections after CCl4 administration were stained with hematoxylin and eosin, 200× magnification. Scale bar = 100 μm. (C) The semi-quantitative data of hepatocellular necrosis area showed sympathetic denervation resulted in lower necrosis area (### p < 0.001 vs. CCl4). Images are representative of n = 6 mice per group.
Effect of chemical sympathetic denervation on CCl4-induced hepatic lipid accumulation
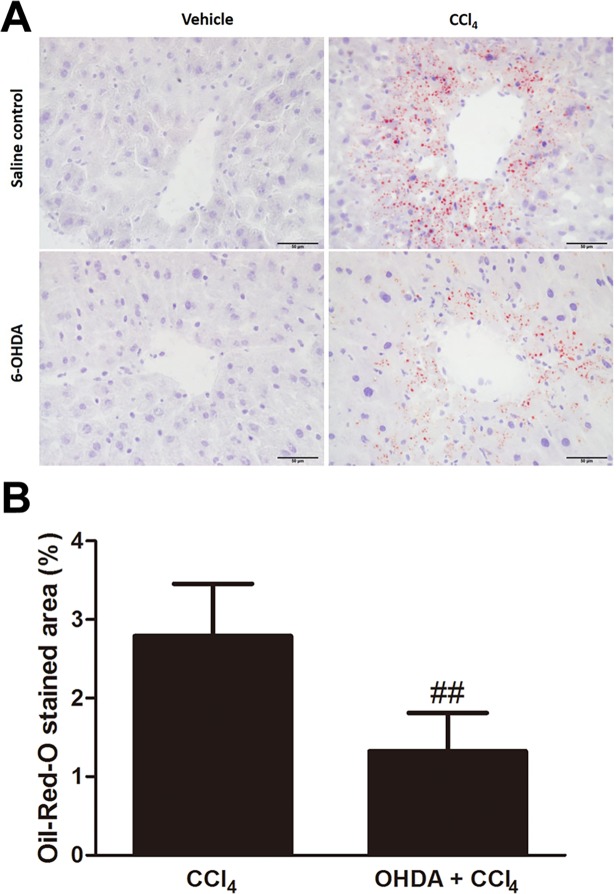
Steatosis was evaluated by assessing the histological area of fat droplets within hepatocytes in Oil-Red-O stained sections (Fig. 4A). There was negligible Oil-Red-O staining in the control mice. In contrast, in CCl4-poisoned mice Oil-Red-O lipid droplets were readily identifiable and amounted to 2.8 ± 0.7% of the total area of the liver sections. In the 6-OHDA/CCl4-cotreated mice, the lipid-staining area was significantly less (1.3 ± 0.5%, p = 0.0014) than that observed in the CCl4-treated group (Fig. 4B).
Fig 4. Absence of the sympathetic nervous system attenuated CCl4-induced steatosis.
(A) Steatosis was evaluated using fat droplets area per 400× field. Scale bar = 50 μm. (B) Semi-quantitatively sympathetic denervation reduced fat droplets deposition prominently in the injured hepatocytes around the central veins compared to control at 24 h. Bars are means ± SD, n = 6 mice per group. (## p < 0.01 vs. CCl4).
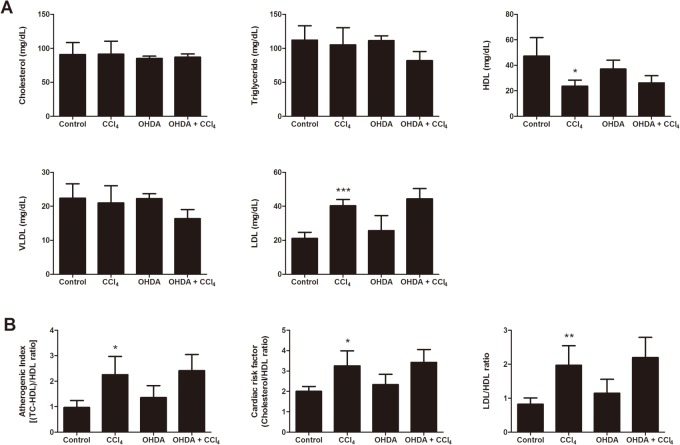
Effect of chemical sympathetic denervation on CCl4-induced lipid profile in serum
Blood lipid levels are shown in Fig. 5. The levels of cholesterol, triglyceride and VLDL showed no significant difference in the different groups. The levels of HDL (24 ± 5 mg/dL) significantly decreased in CCl4-treated mice in comparison to the control mice (47 ± 14 mg/dL, p = 0.0448). In contrast, levels of LDL significantly increased in CCl4-treated mice (40 ± 4 mg/dL) compared with control mice (21 ± 4 mg/dL, p = 0.0003). 6-OHDA/CCl4 co-treatment had no effect on these CCl4 induced changes in the levels of HDL and LDL. Similarly 6-OHDA/CCl4 co-treatment had no influence on the elevated AI index, CRF and LDL/HDL ratio that were seen in the CCl4-treated mice.
Fig 5. Effect of ablation of the sympathetic nervous system on serum lipid profile in mice.
(A) Serum total cholesterol, triglyceride, high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL) in mice administered sympathetic denervation for 5 d following CCl4 intoxication. (B) Serum atherogenic index, cardiac risk factor, and LDL/HDL ratio in mice with sympathetic denervation following CCl4 intoxication. Bars are means ± SD, n = 6 mice per group. In all figures, significant differences are noted as *p < 0.05 vs. control, **p < 0.01 vs. control, and ***p < 0.001 vs. control.
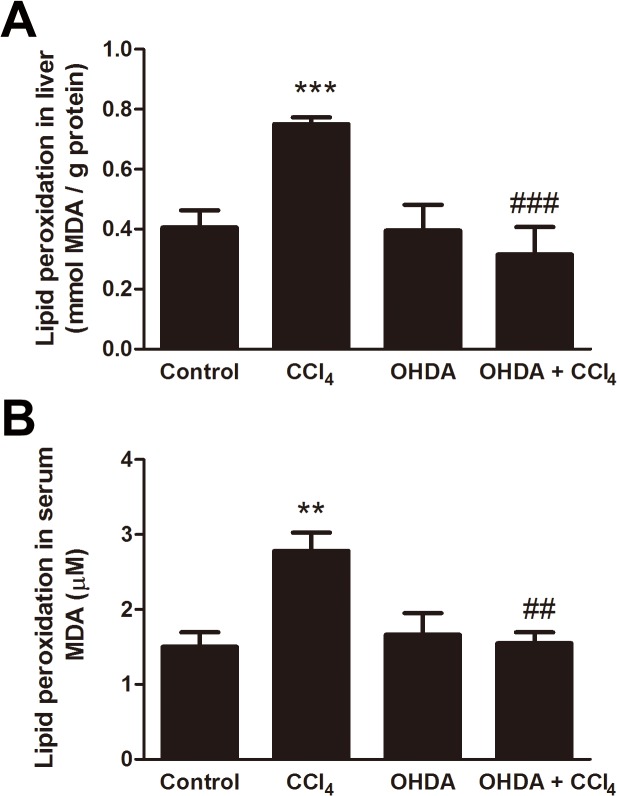
Effects of chemical sympathetic denervation on lipid peroxidation levels in liver and serum of CCl4-induced liver damage
The lipid peroxidation status in the liver of control and treated mice is shown in Fig. 6A. The level of MDA in liver was increased from 0.41 ± 0.06 mmol/g protein in control mice to 0.75 ± 0.02 mmol/g protein in CCl4-treated mice (p < 0.0001). 6-OHDA on its own had no significant effect on MDA level (0.40 ± 0.09 mmol/g protein), but the elevation of MDA caused by CCl4 was blocked in the mice with pretreated with 6-OHDA (0.32 ± 0.09 mmol/g protein, p = 0.0005). Moreover, the level of MDA in serum of CCl4-treated mice (2.78 ± 0.24 μM) was significantly higher than that of control mice (1.50 ± 0.19 μM, p = 0.0068), 6-OHDA-treated mice (1.66 ± 0.29 μM, p = 0.0051), and 6-OHDA/CCl4-cotreated mice (1.55 ± 0.15 μM, p = 0.0013) (Fig. 6B).
Fig 6. Prevention by sympathetic denervation of CCl4-induced lipid peroxidation.
Mice were treated or not treated for 24 h with CCl4, with or without pretreatment with 6-OHDA. Lipid peroxidation was assessed by malondialdehyde (MDA) measurement in liver homogenates (A) and serum (B). Results are expressed as mmol/g protein in liver homogenates and μM in serum. Data are mean ± SD for 6 mice per group. In all figures, significant differences are noted as **p < 0.01 vs. control, ## p < 0.01 vs. CCl4, ***p < 0.001 vs. control, and ### p < 0.001 vs. CCl4.
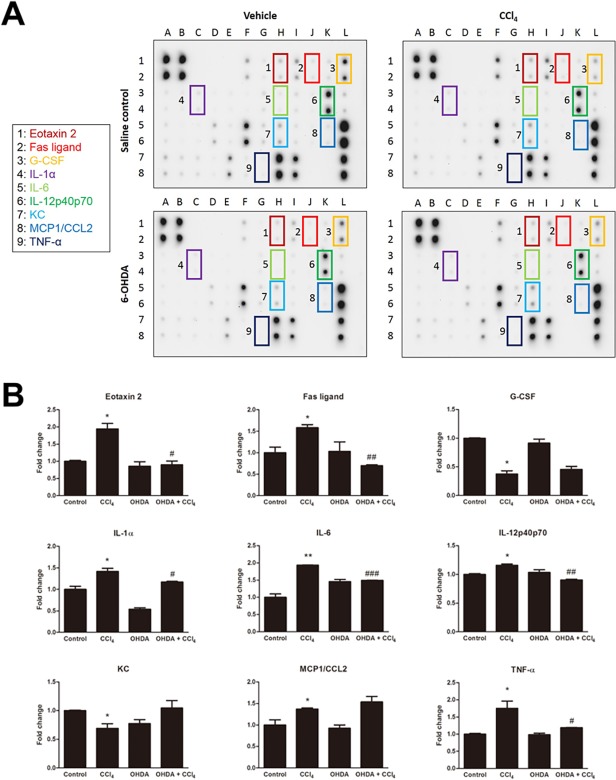
Effects of chemical sympathetic denervation on inflammatory cytokines/chemokines expression in serum of mice after CCl4 exposure
Serum from mice in each treatment group was exposed to the inflammatory cytokines/chemokines antibody arrays (Fig. 7A). The levels of eotaxin-2/CCL24, Fas ligand (FasL), interleukin (IL)-1α, IL-6, IL-12p40p70, monocyte chemoattractant protein-1 (MCP-1/CCL2), and tumor necrosis factor-α (TNF-α) each increased in CCl4-treated mice group and SNS ablation significantly prevented these cytokines and chemokines except MCP-1. Serum samples obtained from CCl4-treated mice expressed decreased levels of the granulocyte colony-stimulating factor (G-CSF) and keratinocyte-derived chemokine (KC) as compared to control mice. Both showed no significant change in 6-OHDA/CCl4-cotreated mice (Fig. 7B).
Fig 7. The effect of sympathetic denervation on CCl4-induced systemic inflammatory markers as measured by antibody array.
(A) Inflammation array in serum from control, CCl4-, 6-OHDA-, and 6-OHDA/CCl4-treated mice. Altered cytokines and chemokines are indicated by boxes. (B) Quantification of the expression of eotaxin-2/CCL24, Fas ligand, granulocyte colony-stimulating factor (G-CSF), interleukin (IL)-1α, IL-6, IL-12p40p70, keratinocyte-derived chemokine (KC), monocyte chemoattractant protein-1 (MCP-1/CCL2), and tumor necrosis factor-α (TNF-α) in each group. Data are presented as the fold of expression. Bars are mean ± SD for two replicated spots on the membrane. In all figures, significant differences are noted as *p < 0.05 vs. control, # p < 0.05 vs. CCl4, **p < 0.01 vs. control, ## p < 0.01 vs. CCl4, and ### p < 0.001 vs. CCl4.
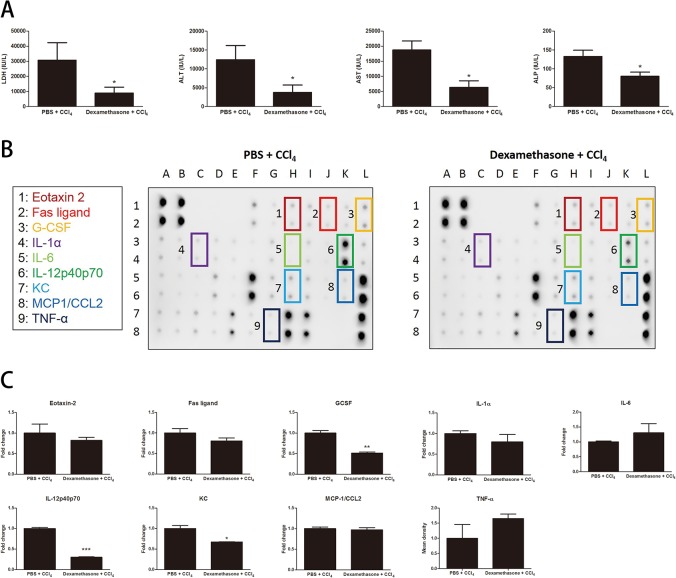
Effects of pretreatment with dexamethasone on the systemic inflammatory response of mice following CCl4 exposure
The biochemical impacts of dexamethasone pretreatment on CCl4-induced hepatotoxicity were assayed by assessing serum levels of LDH, ALT, AST, and ALP (Fig. 8A). Dexamethasone significantly decreased CCl4 induced hepatotoxicity. The levels of liver enzymes in the peripheral blood in dexamethasone/CCl4 vs. PBS/CCl4 treated mice were LDH 8,931 ± 3,957 vs. 30,700 ± 11,610 IU/L (p = 0.0371), ALT 3,739 ± 1,963 vs. 12,390 ± 3,795 IU/L (p = 0.0247), AST 6,370 ± 2,192 vs. 18,700 ± 3,062 IU/L (p = 0.0436), and ALP 80 ± 11 vs. 133 ± 17 IU/L (p = 0.0315). Furthermore, serum from mice in PBS/CCl4 group and dexamethasone/CCl4 group was exposed to the inflammatory cytokines/chemokines antibody arrays (Fig. 8B). Serum samples obtained from dexamethasone/CCl4-treated mice expressed decreased levels of the IL-12p40p70 (0.30 ± 0.01 fold; p = 0.0007), G-CSF (0.51 ± 0.02 fold; p = 0.0101) and KC (0.67 ± 0.00 fold; p = 0.0238) as compared to PBS/CCl4 group. Others tested showed no significant change (Fig. 8C).
Fig 8. The effect of dexamethasone pretreatment on CCl4-induced hepatotoxicity and systemic inflammation.
(A) Serum isolated from whole blood was used to determine LDH, ALT, AST, and ALP levels. Bars are means ± SD, n = 4 mice per group. (*p < 0.05 vs. PBS + CCl4) (B) Inflammation array in serum from PBS + CCl4 and Dexamethasone + CCl4-treated mice. (C) Quantification of the expression of eotaxin-2, FasL, G-CSF, IL-1α, IL-6, IL-12p40p70, KC, MCP-1/CCL2, and TNF-α in each group. Data are presented as the fold of expression. Bars are mean ± SD for two replicated spots on the membrane. In all figures, significant differences are noted as *p < 0.05 vs. PBS + CCl4, **p < 0.01 vs. PBS + CCl4, and ***p < 0.001 vs. PBS + CCl4.
Discussion
The role of SNS in CCl4-induced acute hepatotoxicity is still a matter of debate [12–14,17–19]. It has been shown that selective sympathetic blockade confined to liver could alleviate liver injury by CCl4 [12]. Prior chemical sympathectomy with 6-OHDA abolished the CCl4 toxicity, suggesting that it is mediated through adrenergic stimulation of the liver, but the regulatory mechanism of sympathetic innervation remains poorly understood so far [13,14]. However, it has been demonstrated that the inhibitory effect of noradrenaline on acute hepatotoxicity induced by CCl4 [19]. In addition, it has been reported that pretreatment of a ganglionic blocking agent diminished acute hepatotoxicity of CCl4 only in female Wistar rats rather than in males [18]. In contrast, in the pilot experiment conducted by Dubuisson and colleagues, chemical destruction of noradrenergic fibers or antagonism of noradrenergic signaling through α1 receptor did not decrease the acute toxicity of CCl4, as checked by biological tests and necrosis area measurement [17]. The discrepancy between the previous studies can probably be accounted for by the use of different species, varied chemical sympathectomy, and dissimilar harvest time points. In this report, we found that chemical sympathetic denervation strikingly attenuated CCl4-mediated hepatotoxicity and systemic inflammatory cytokines production in mice. These data suggest that SNS enhances hepatotoxicity and immune response in animals primed with a toxin such as CCl4.
Hepatic sympathectomy may also be carried out surgically, overcoming potential systemic effects of treatment with 6-OHDA. Surgical sympathectomy is not without its difficulties. It is impossible to guarantee full hepatic denervation, previous reports indicating that that the extent of denervation varies widely between 30 and 78% in the surgically denervated rodent liver [34,35]. Furthermore, in studies aimed at identifying the pathophysiologic effect of selective hepatic vagotomy on the hepatic sympathetic nerve no significant difference in norepinephrine levels between the sham-operated and vagotomized livers are seen [36]. On the other hand, peritoneal 6-OHDA is rapidly distributed by the circulation into tissues, where sympathetic fibers are destroyed as a consequence of its internalization into recycling synaptic vesicles, where it is subsequently oxidized to generate neurotoxic free radicals. As 6-OHDA does not cross the blood-brain barrier in adult animals, it selectively ablates peripheral sympathetic nerves [24–26,37,38]. Importantly, when a selective hepatic sympathectomy is performed, there is only a disconnection of the sympathetic input from the hypothalamus to the liver, rather than the systemic inhibition of the SNS caused by chemical sympathectomy [38,39]. It was first proposed that the massive discharge of the SNS, occasioned by an action of CCl4 on the central nervous system, had diminished hepatic blood flow with associated centrilobular hypoxia, which would lead to centrilobular necrosis [2]. Our study, the effect of sympathetic denervation to reduce CCl4-induced acute hepatotoxicity, supports this hypothesis. Indeed the effect of adrenergic signaling in the liver and serum with respect to modulation of CCl4-induced lipid peroxidation has not yet been addressed. Our study demonstrated that, during CCl4 intoxication, the level of MDA was increased in the liver with an associated increase in MDA concentrations in circulation. Chemical sympathetic denervation exhibited protective effects by reducing CCl4-mediated lipid peroxidation through decreased production of free radical derivatives, as evidenced by the decreased MDA levels in liver and serum. Thus, these results indicate that a SNS response to CCl4 metabolic intoxication promote the systemic and hepatic lipid peroxidation.
Several studies have reported that hepatosteatosis occurs after acute exposure to CCl4 albeit by uncertain mechanisms [10,40,41]. First, CCl4 might lead to steatosis due to impaired liver mitochondrial β-oxidation which processes hepatic lipid breakdown [42]. In addition, CCl4-induced TNF-α synthesis via stimulation of Kupffer cells might contribute to steatosis [10,43]. Furthermore, CCl4-induced liver steatosis was shown to be related to cytochrome P450 2E1 expression and activity in vitro [8] and also in vivo [43], respectively. It is also believed that the lipid accumulation, which commences very early, is due to failure of the liver to transport triglyceride-rich LDL into the plasma [44], which arises from the CCl4-mediated ER stress with structural disorganization [9]. Moreover, it has been indicated that a decrease in hepatic glutathione concentration in CCl4-injected mice is closely related to triglyceride accumulation in the liver [45]. Additionally, it has been suggested that the loss of microsomal triglyceride transfer protein (MTP) might be involved in the development of CCl4-hepatosteatosis because CCl4 exposure leads to covalent modification of MTP and its degradation by proteasomes [41]. Finally, CCl4 poisoning might affect glycoprotein processing and maturation at the level of liver microsomes and Golgi apparatus. CCl4 poisoning might then influence the synthesis, maturation, and release of hepatic VLDL, and thereafter cause fat accumulation [46]. The mechanisms whereby the SNS acts on the metabolism of hepatic lipids have not been clearly identified. It is important to note that our study revealed an overall anti-steatosis role in blocking the SNS signaling in the CCl4-induced surfeit of fat in the liver. These observations suggest that the SNS acts as a positive regulator of steatosis signaling pathways in CCl4-intoxicated liver. It further suggests that hepatosteatosis following CCl4 poisoning is not the result of one single event, but rather, the end result of a series of episodes. Therefore, a new project in our laboratory is now in progress to further clarify the influence of chronic sympathetic denervation in drug-induced hepatosteatosis and other forms of fatty liver.
In our study, LDL levels in serum were significantly increased and HDL levels in serum were significantly decreased after CCl4 treatment. These results were reanalyzed for determination of the AI, CRF, and LDL/HDL ratio. However, sympathetic denervation was not effective in modifying serum levels of LDL, HDL, AI, CRF, and LDL/HDL ratio after CCl4 treatment. These findings indicate that the SNS may not be involved in the CCl4-induced alteration of the lipid profile in the circulation. Taken together, the presence of the SNS is considered the critical event in the development of CCl4-induced steatosis which results from an imbalance between lipid synthesis and degradation rather than from a consequence of the failure of triglycerides to move as VLDL from liver to the circulation.
In this study, we showed that acute CCl4 intoxication increased circulating levels of inflammatory cytokines/chemokines, including eotaxin 2, FasL, IL-1α, IL-6, IL-12, MCP-1, and TNF-α. The source of the increased levels of these cytokines/chemokines is not clear. It is likely to be, at least in part, from a variety of intrahepatic cells including CD11b+ Kupffer cells, stellate cells/hepatic myofibroblasts, endothelial cells in addition to peritoneal mesothelial and exudate cells following CCl4 administration [21,47–58]. Previous studies in ischemia and reperfusion models have also demonstrated the ability of hepatocytes to produce IL-12 [59]. In steatotic liver, IL-12 is probably derived from Kupffer cells [60] whereas in chronic viral hepatitis, primary biliary cirrhosis, and fulminant hepatitis B, IL-12 is produced primarily by sinusoidal endothelial cells, hepatic stellate cells, bile ducts, and lymphocytes [61]. It has been shown previously that some catecholamines can induce an inflammatory response in hepatocytes, e.g. overproduction of IL-6 [62]. However, in the current study we have not performed in vitro studies on primary hepatocyte cultures challenged with CCl4 in the presence or absence of catecholamines that could more accurately identify the hepatocyte as being a primary source of particular inflammatory mediator production. Nevertheless, as chemical sympathectomy with 6-OHDA treatment leads to potential global alterations in physiology; e.g., decrease of arterial blood pressure, increase of mesenteric blood flow and microcirculatory blood flow, and blood flow to immune organs [37,63,64] with a dramatic alteration of cytokine production [24,37,65–67], it is unclear to what extent these peripheral events are involved in the alterations in chemokine/cytokine levels seen in the current study.
Immune cells express various adrenergic and purinergic receptors that are sensitive to transmitters of the SNS. The production of cytokines and chemokines is modulated by activation of these receptors. Notably, the production of TNF-α, IL-6, and IL-12 have been shown to be altered by activation of these receptors [23]. Therefore, we analyzed the effect of sympathetic denervation on the release of cytokines and chemokines in CCl4-induced systemic inflammation model. Major hepatotoxic mediators induced by CCl4 are IL-6, TNF, and FasL [21,22,29,68]. In our findings, elevated levels of IL-6, TNF-α, and FasL in CCl4 group were markedly reduced by ablation of the SNS. These data suggest that the SNS exacerbated CCl4-induced hepatotoxicity through the increased level of serum IL-6, TNF-α, and FasL. Previous studies have reported that eotaxin-2/CCL24, IL-1α, IL-12, and TNF-α, respectively, are the important mediators in the steatosis model [60,69–71]. In this study, eotaxin-2/CCL24, IL-1α, IL-12, and TNF-α production in mice treated with CCl4 was increased compared with that in control mice, suggesting these cytokines and chemokines play a critical role in CCl4-induced steatosis. Additionally, these cytokines and chemokines were significantly reduced by ablation of the SNS. Therefore, we speculate the SNS contributes to the progress of CCl4-induced hepatosteatosis via eotaxin-2/CCL24, IL-1α, IL-12, and TNF-α signaling. To investigate whether dexamethasone reproduces the effect of liver sympathetic denervation in CCl4 model, in this study, we found that a simple pretreatment regimen with dexamethasone can partially inhibit CCl4-induced hepatic injury and inflammatory immune responses. These experiments were preliminary; further experiments are needed to explore the relationship between an attenuation of the inflammatory response by glucocorticoids and a blunted activation of sympathetic signaling.
In conclusion, the SNS is found to be involved in the process of CCl4-mediated acute hepatotoxicity and systemic inflammatory responses. This novel finding provides us with important insights into the pathophysiological significance of the SNS in promoting the CCl4 poisoning. As the SNS is early and essential in the onset of CCl4-induced lipid peroxidation and steatosis, the findings raise the possibility that the SNS may be involved in the free radical biology and the development of other forms of fatty liver. Additionally, these data reinforce the importance of the SNS in mechanisms of chemical- or drug-induced hepatotoxicity with associated-systemic inflammatory responses.
Acknowledgments
We acknowledge the skilled technical assistance of Yu-Ju Lin, Chih-Shan Chang, Sz-Chi Chen, Chih-Min Kuo, and Ya-Ting Ku. We also thank Dr. Daniel Steve Villarreal for English-language editing and Ms. Yu-Ting Hsu for help in picture editing.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported in part by grants from the National Science Council, Taiwan (NSC100-2320-B-016-006-MY3); Department of Medical Research of Cardinal Tien Hospital, Taiwan (CTH-103-1-2C04); National Defense Medical Center, Tri-Service General Hospital, Taiwan (TSGH-C102-069, TSGH-C103-078, TSGH-C103-080, and TSGH-C104-175); and Ministry of National Defense Medical Affairs Bureau (MAB-103-066 and MAB-104-089). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33: 105–136. [DOI] [PubMed] [Google Scholar]
- 2. Judah JD, Rees KR. Mechanism of action of carbon tetrachloride. Fed Proc. 1959;18: 1013–1020. [PubMed] [Google Scholar]
- 3. Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25: 185–209. [DOI] [PubMed] [Google Scholar]
- 4. Smialowicz RJ, Simmons JE, Luebke RW, Allis JW. Immunotoxicologic assessment of subacute exposure of rats to carbon tetrachloride with comparison to hepatotoxicity and nephrotoxicity. Fundam Appl Toxicol. 1991;17: 186–196. [DOI] [PubMed] [Google Scholar]
- 5. Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24: 200–208. [DOI] [PubMed] [Google Scholar]
- 6. Tseng CK, Lin CK, Chang HW, Wu YH, Yen FL, Chang FR, et al. Aqueous extract of Gracilaria tenuistipitata suppresses LPS-induced NF-kappaB and MAPK activation in RAW 264.7 and rat peritoneal macrophages and exerts hepatoprotective effects on carbon tetrachloride-treated rat. PLoS One. 2014;9: e86557 10.1371/journal.pone.0086557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, Yu CH, Jiang YP, Peng C, He K, Tang JY, et al. Protective effects of polydatin from Polygonum cuspidatum against carbon tetrachloride-induced liver injury in mice. PLoS One. 2012;7: e46574 10.1371/journal.pone.0046574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boll M, Weber LW, Becker E, Stampfl A. Pathogenesis of carbon tetrachloride-induced hepatocyte injury bioactivation of CCl4 by cytochrome P450 and effects on lipid homeostasis. Z Naturforsch C. 2001;56: 111–121. [DOI] [PubMed] [Google Scholar]
- 9. Knockaert L, Berson A, Ribault C, Prost PE, Fautrel A, Pajaud J, et al. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver. Lab Invest. 2012;92: 396–410. 10.1038/labinvest.2011.193 [DOI] [PubMed] [Google Scholar]
- 10. Kaiser JP, Lipscomb JC, Wesselkamper SC. Putative mechanisms of environmental chemical-induced steatosis. Int J Toxicol. 2012;31: 551–563. 10.1177/1091581812466418 [DOI] [PubMed] [Google Scholar]
- 11. Jensen KJ, Alpini G, Glaser S. Hepatic nervous system and neurobiology of the liver. Compr Physiol. 2013;3: 655–665. 10.1002/cphy.c120018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xia F, He Z, Li K, Wang X, Dong J. Evaluation of the role of sympathetic denervation on hepatic function. Hepatol Res. 2006;36: 259–264. [DOI] [PubMed] [Google Scholar]
- 13. Sato Y, Yoneda M, Nakamura K, Makino I, Terano A. Protective effect of central thyrotropin-releasing hormone on carbon tetrachloride-induced acute hepatocellular necrosis in rats. J Hepatol. 2003;39: 47–54. [DOI] [PubMed] [Google Scholar]
- 14. Hamasaki K, Nakashima M, Naito S, Akiyama Y, Ohtsuru A, Hamanaka Y, et al. The sympathetic nervous system promotes carbon tetrachloride-induced liver cirrhosis in rats by suppressing apoptosis and enhancing the growth kinetics of regenerating hepatocytes. J Gastroenterol. 2001;36: 111–120. [DOI] [PubMed] [Google Scholar]
- 15. Larson RE, Plaa GL, Brody MJ. Immunological Sympathectomy and CCl4 Hepatotoxicity. Proc Soc Exp Biol Med. 1964;116: 557–560. [PubMed] [Google Scholar]
- 16. Calvert DN, Brody TM. Role of the sympathetic nervous system in carbon tetrachloride hepatotoxicity. Am J Physiol. 1960;198: 669–676. [DOI] [PubMed] [Google Scholar]
- 17. Dubuisson L, Desmouliere A, Decourt B, Evade L, Bedin C, Boussarie L, et al. Inhibition of rat liver fibrogenesis through noradrenergic antagonism. Hepatology. 2002;35: 325–331. [DOI] [PubMed] [Google Scholar]
- 18. Kulcsar-Gergely J, Kulcsar A. The influence of a ganglion-blocking agent on experimental liver injury. Toxicology. 1973;1: 125–130. [DOI] [PubMed] [Google Scholar]
- 19. Ishii K, Shimizu M, Karube H, Shibuya A, Shibata H, Okudaira M, et al. Inhibitory effect of noradrenaline on acute liver injury induced by carbon tetrachloride in the rat. J Auton Nerv Syst. 1992;39: 13–18. [DOI] [PubMed] [Google Scholar]
- 20. Kaminski NE, Jordan SD, Holsapple MP. Suppression of humoral and cell-mediated immune responses by carbon tetrachloride. Fundam Appl Toxicol. 1989;12: 117–128. [DOI] [PubMed] [Google Scholar]
- 21. Sato A, Nakashima H, Nakashima M, Ikarashi M, Nishiyama K, Kinoshita M, et al. Involvement of the TNF and FasL produced by CD11b Kupffer cells/macrophages in CCl4-induced acute hepatic injury. PLoS One. 2014;9: e92515 10.1371/journal.pone.0092515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vanden Berghe T, Denecker G, Brouckaert G, Vadimovisch Krysko D, D'Herde K, Vandenabeele P. More than one way to die: methods to determine TNF-induced apoptosis and necrosis. Methods Mol Med. 2004;98: 101–126. [DOI] [PubMed] [Google Scholar]
- 23. Hasko G, Szabo C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem Pharmacol. 1998;56: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 24. Callahan TA, Moynihan JA. Contrasting pattern of cytokines in antigen- versus mitogen-stimulated splenocyte cultures from chemically denervated mice. Brain Behav Immun. 2002;16: 764–773. [DOI] [PubMed] [Google Scholar]
- 25. Joers V, Dilley K, Rahman S, Jones C, Shultz J, Simmons H, et al. Cardiac Sympathetic Denervation in 6-OHDA-Treated Nonhuman Primates. PLoS One. 2014;9: e104850 10.1371/journal.pone.0104850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zapater P, Gomez-Hurtado I, Peiro G, Gonzalez-Navajas JM, Garcia I, Giménez P, et al. Beta-adrenergic receptor 1 selective antagonism inhibits norepinephrine-mediated TNF-alpha downregulation in experimental liver cirrhosis. PLoS One. 2012;7: e43371 10.1371/journal.pone.0043371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tiegs G, Bang R, Neuhuber WL. Requirement of peptidergic sensory innervation for disease activity in murine models of immune hepatitis and protection by β-adrenergic stimulation. J Neuroimmunol. 1999;96: 131–143. [DOI] [PubMed] [Google Scholar]
- 28. Zhu R, Zeng G, Chen Y, Zhang Q, Liu B, Liu J, et al. Oroxylin A accelerates liver regeneration in CCl4-induced acute liver injury mice. PLoS One. 2013;8: e71612 10.1371/journal.pone.0071612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pritchard MT, Cohen JI, Roychowdhury S, Pratt BT, Nagy LE. Early growth response-1 attenuates liver injury and promotes hepatoprotection after carbon tetrachloride exposure in mice. J Hepatol. 2010;53: 655–662. 10.1016/j.jhep.2010.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li J, Gao J, Yan D, Yuan Y, Sah S, Satyal U, et al. Neutralization of chemokine CXCL14 (BRAK) expression reduces CCl4 induced liver injury and steatosis in mice. Eur J Pharmacol. 2011;671: 120–127. 10.1016/j.ejphar.2011.09.174 [DOI] [PubMed] [Google Scholar]
- 31. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18: 499–502. [PubMed] [Google Scholar]
- 32. Haglund O, Luostarinen R, Wallin R, Wibell L, Saldeen T. The effects of fish oil on triglycerides, cholesterol, fibrinogen and malondialdehyde in humans supplemented with vitamin E. J Nutr. 1991;121: 165–169. [DOI] [PubMed] [Google Scholar]
- 33. Chida Y, Sudo N, Takaki A, Kubo C. The hepatic sympathetic nerve plays a critical role in preventing Fas induced liver injury in mice. Gut. 2005;54: 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wheatley AM, Shaw SG, Stuart ET, Zhao D, Gassel HJ, Blumgart LH . Selective sympathectomy of the liver: a comparison of orthotopic liver transplantation and intraportal 6-hydroxydopamine injection. J Auton Pharmacol. 1993;13: 15–22. [DOI] [PubMed] [Google Scholar]
- 35. Holmin T, Ekelund M, Kullendorff CM, Lindfeldt J. A microsurgical method for denervation of the liver in the rat. Eur Surg Res. 1984;16: 288–293. [DOI] [PubMed] [Google Scholar]
- 36. Hiramoto T, Chida Y, Sonoda J, Yoshihara K, Sudo N, Kubo C. The hepatic vagus nerve attenuates Fas-induced apoptosis in the mouse liver via α7 nicotinic acetylcholine receptor. Gastroenterology. 2008;134: 2122–2131. 10.1053/j.gastro.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 37. Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc Natl Acad Sci U S A. 2009;106: 5300–5305. 10.1073/pnas.0808851106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang M, Tanida M, Shibamoto T, Kurata Y. Alpha-adrenoceptor antagonists and chemical sympathectomy exacerbate anaphylaxis-induced hypotension, but not portal hypertension, in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2013;305: R900–907. 10.1152/ajpregu.00120.2013 [DOI] [PubMed] [Google Scholar]
- 39. Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, et al. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One. 2009;4: e5650 10.1371/journal.pone.0005650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967;19: 145–208. [PubMed] [Google Scholar]
- 41. Pan X, Hussain FN, Iqbal J, Feuerman MH, Hussain MM. Inhibiting proteasomal degradation of microsomal triglyceride transfer protein prevents CCl4-induced steatosis. J Biol Chem. 2007;282: 17078–17089. [DOI] [PubMed] [Google Scholar]
- 42. Pessayre D, Fromenty B, Berson A, Robin MA, Letteron P, Moreau R, et al. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44: 34–87. 10.3109/03602532.2011.604086 [DOI] [PubMed] [Google Scholar]
- 43. Liu C, Tao Q, Sun M, Wu JZ, Yang W, Jian P, et al. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab Invest. 2010;90: 1805–1816. 10.1038/labinvest.2010.123 [DOI] [PubMed] [Google Scholar]
- 44. Pencil SD, Brattin WJ Jr, Glende EA Jr, Recknagel RO. Carbon tetrachloride-dependent inhibition of lipid secretion by isolated hepatocytes. Characterization and requirement for bioactivation. Biochem Pharmacol. 1984;33: 2419–2423. [DOI] [PubMed] [Google Scholar]
- 45. Nishida K, Ohta Y, Ishiguro I. Preventive effect of γ-glutamylcysteinylethyl ester on carbon tetrachloride-induced hepatic triglyceride accumulation in mice. Toxicol Lett. 1998;95: 141–146. [DOI] [PubMed] [Google Scholar]
- 46. Poli G, Chiarpotto E, Albano E, Cottalasso D, Nanni G, Marinari UM, et al. Carbon tetrachloride-induced inhibition of hepatocyte lipoprotein secretion: functional impairment of Golgi apparatus in the early phases of such injury. Life Sci. 1985;36: 533–539. [DOI] [PubMed] [Google Scholar]
- 47. Teixeira-Clerc F, Belot MP, Manin S, Deveaux V, Cadoudal T, Chobert MN, et al. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology. 2010;52: 1046–1059. 10.1002/hep.23779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamaji K, Ohnishi K, Zuinen R, Ochiai Y, Chikuma T, Hojo H. Interleukin-6 production by peritoneal mesothelial cells and its regulation by inflammatory factors in rats administered carbon tetrachloride intraperitoneally. Toxicol Appl Pharmacol. 2008;226: 38–45. [DOI] [PubMed] [Google Scholar]
- 49. Wen T, Ren F, Liu Y, Wu ZM, Zhao JY. Expression of Fas antigen and Fas ligand in acute liver injury induced by carbon tetrachloride in rat. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18: 417–420. [PubMed] [Google Scholar]
- 50. Zamara E, Galastri S, Aleffi S, Petrai I, Aragno M, Mastrocola R, et al. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46: 230–238. [DOI] [PubMed] [Google Scholar]
- 51. Chan CC, Lee KC, Huang YH, Chou CK, Lin HC, Lee FY. Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury. J Gastroenterol Hepatol. 2014;29: 603–613. 10.1111/jgh.12366 [DOI] [PubMed] [Google Scholar]
- 52. Gewiese-Rabsch J, Drucker C, Malchow S, Scheller J, Rose-John S. Role of IL-6 trans-signaling in CCl4 induced liver damage. Biochim Biophys Acta. 2010;1802: 1054–1061. 10.1016/j.bbadis.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 53. Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171: 3233–3244. [DOI] [PubMed] [Google Scholar]
- 54. Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, et al. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2013;57: 2026–2036. 10.1002/hep.26196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, et al. Hepatocyte necrosis induced by oxidative stress and IL-1α release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14: 156–165. 10.1016/j.ccr.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Armbrust T, Schmitt E, Ramadori G. Viable rat Kupffer cells synthesize but do not secrete interleukin-1: indications for necrosis-induced maturation of interleukin-1α, but not of interleukin-1β. Biochem Biophys Res Commun. 1995;207: 637–645. [DOI] [PubMed] [Google Scholar]
- 57. Xie W, Li M, Xu N, Lv Q, Huang N, He J, et al. miR-181a regulates inflammation responses in monocytes and macrophages. PLoS One. 2013;8: e58639 10.1371/journal.pone.0058639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Libby P, Ordovas JM, Birinyi LK, Auger KR, Dinarello CA. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986;78: 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, et al. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 60. Kremer M, Thomas E, Milton RJ, Perry AW, van Rooijen N, Wheeler MD, et al. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51: 130–141. 10.1002/hep.23292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leifeld L, Cheng S, Ramakers J, Dumoulin FL, Trautwein C, Sauerbruch T, et al. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology. 2002;36: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 62. Aninat C, Seguin P, Descheemaeker PN, Morel F, Malledant Y, Guillouzo A. Catecholamines induce an inflammatory response in human hepatocytes. Crit Care Med. 2008;36: 848–854. 10.1097/CCM.0B013E31816532BE [DOI] [PubMed] [Google Scholar]
- 63. Obuchowicz R, Sendur R, Pawlik M, Biernat J, Koprowska B, Jaworek J, et al. Myoelectric function, metabolism, intestinal circulation and vagal activity after chemical sympathectomy. Folia Med Cracov. 2002;43: 95–109. [PubMed] [Google Scholar]
- 64. Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension. 1992;20: 612–619. [DOI] [PubMed] [Google Scholar]
- 65. Kruszewska B, Felten SY, Moynihan JA. Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. J Immunol. 1995;155: 4613–4620. [PubMed] [Google Scholar]
- 66. Leo NA, Callahan TA, Bonneau RH. Peripheral sympathetic denervation alters both the primary and memory cellular immune responses to herpes simplex virus infection. Neuroimmunomodulation. 1998;5: 22–35. [DOI] [PubMed] [Google Scholar]
- 67. Grebe KM, Takeda K, Hickman HD, Bailey AL, Embry AC, Bennink JR, et al. Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J Immunol. 2010;184: 540–544. 10.4049/jimmunol.0903395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhu RZ, Xiang D, Xie C, Li JJ, Hu JJ, He HL, et al. Protective effect of recombinant human IL-1Ra on CCl4-induced acute liver injury in mice. World J Gastroenterol. 2010;16: 2771–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Poniachik J, Csendes A, Diaz JC, Rojas J, Burdiles P, Maluenda F, et al. Increased production of IL-1α and TNF-α in lipopolysaccharide-stimulated blood from obese patients with non-alcoholic fatty liver disease. Cytokine. 2006;33: 252–257. [DOI] [PubMed] [Google Scholar]
- 70. Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55: 1086–1094. 10.1016/j.jhep.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Duval C, Thissen U, Keshtkar S, Accart B, Stienstra R, Boekschoten MV, et al. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes. 2010;59: 3181–3191. 10.2337/db10-0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.