Abstract
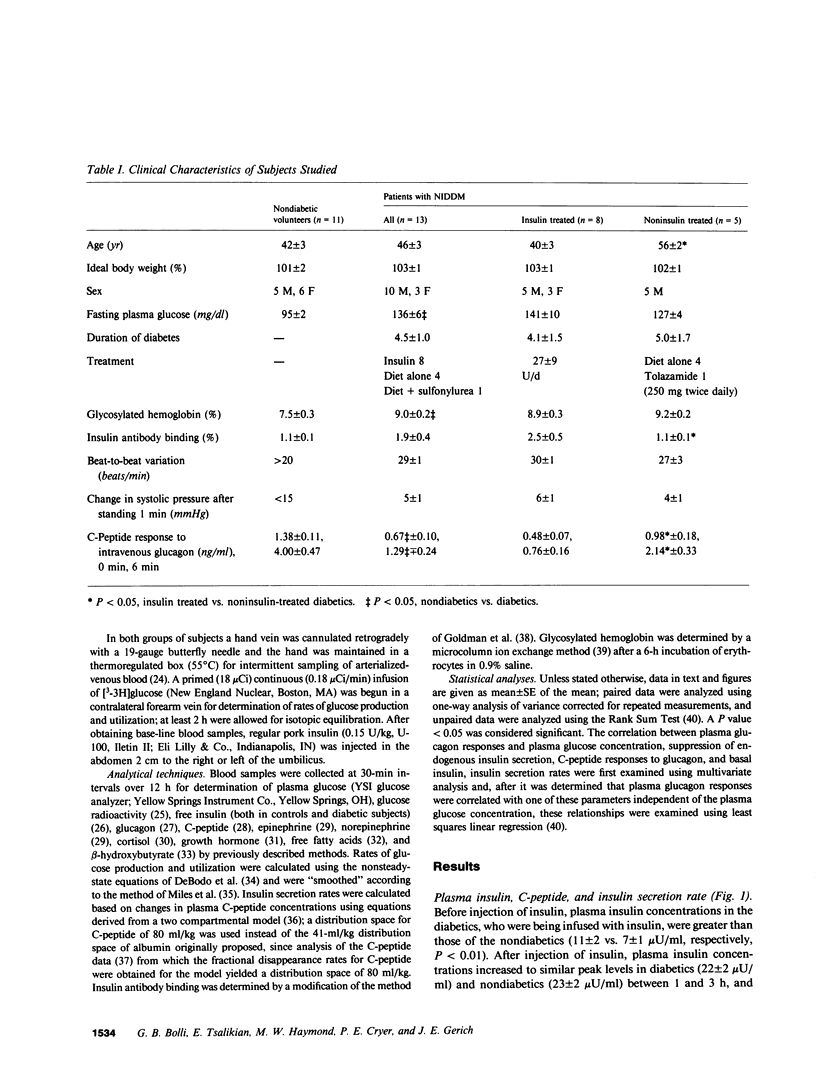
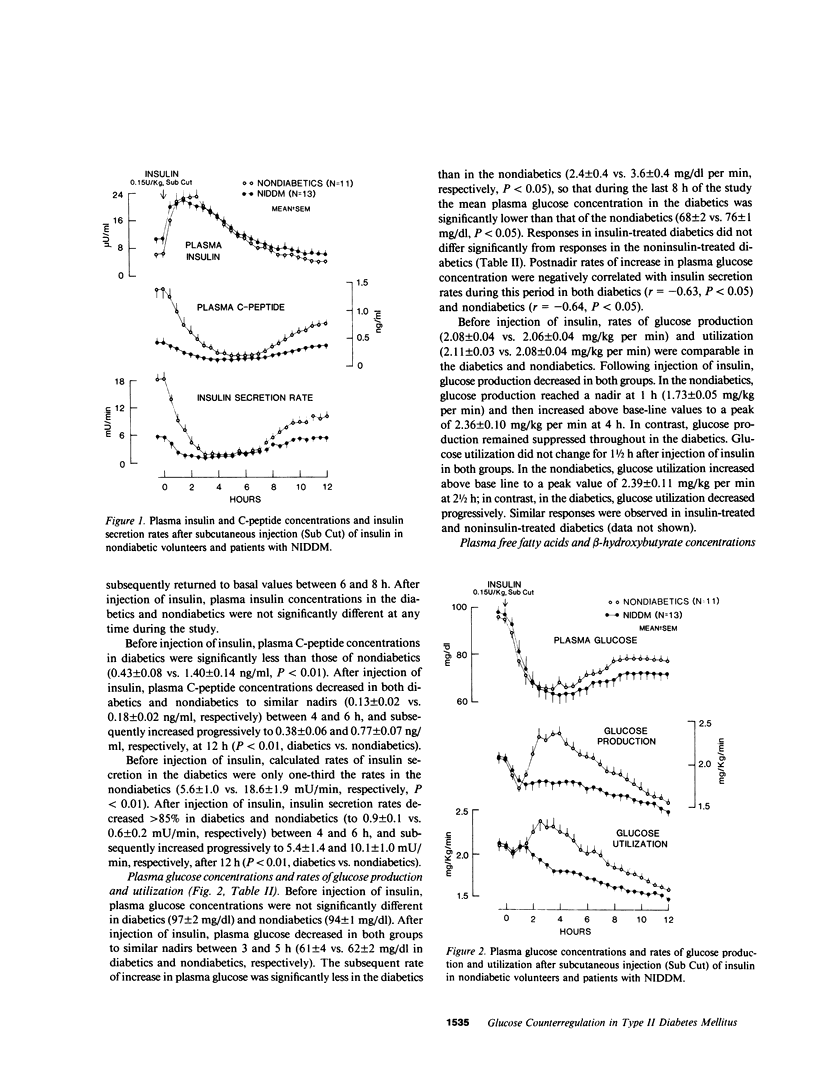
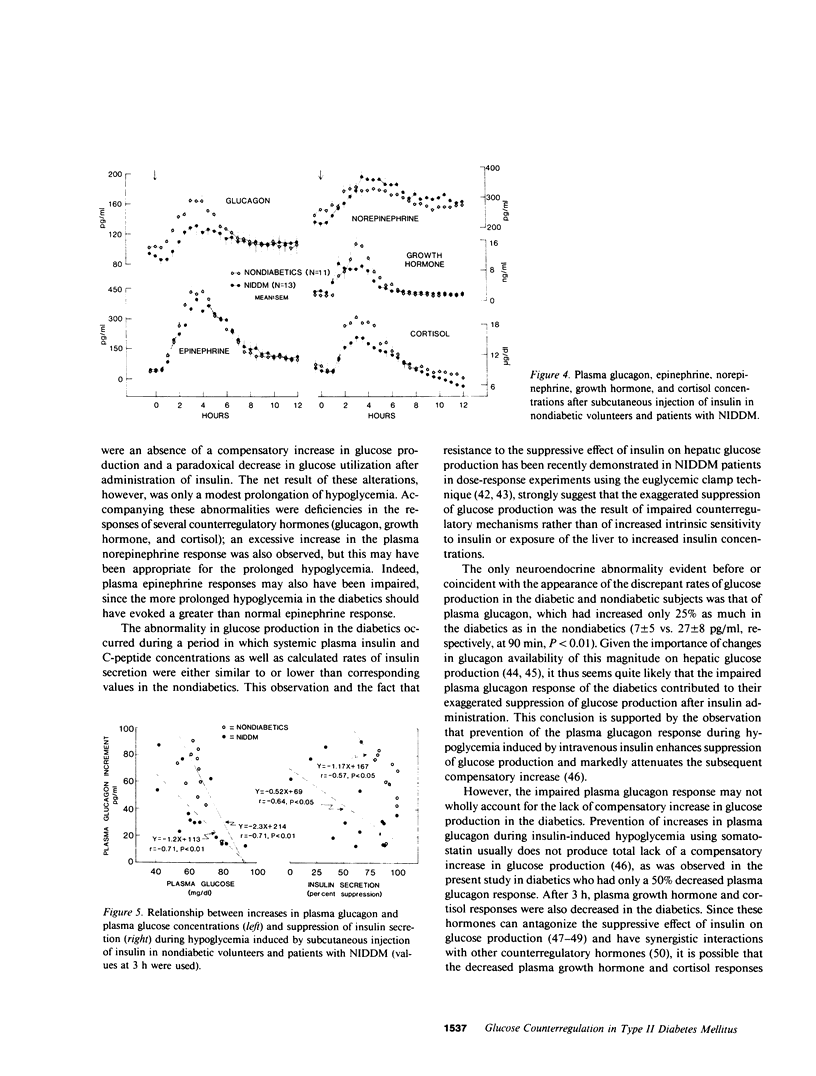
To characterize glucose counterregulatory mechanisms in patients with noninsulin-dependent diabetes mellitus (NIDDM) and to test the hypothesis that the increase in glucagon secretion during hypoglycemia occurs primarily via a paracrine islet A-B cell interaction, we examined the effects of a subcutaneously injected therapeutic dose of insulin (0.15 U/kg) on plasma glucose kinetics, rates of glucose production and utilization, and their relationships to changes in the circulating concentrations of neuroendocrine glucoregulatory factors (glucagon, epinephrine, norepinephrine, growth hormone, and cortisol), as well as to changes in endogenous insulin secretion in 13 nonobese NIDDM patients with no clinical evidence of autonomic neuropathy. Compared with 11 age-weight matched nondiabetic volunteers in whom euglycemia was restored primarily by a compensatory increase in glucose production, in the diabetics there was no compensatory increase in glucose production (basal 2.08 +/- 0.04----1.79 +/- 0.07 mg/kg per min at 21/2 h in diabetics vs. basal 2.06 +/- 0.04----2.32 +/- 0.11 mg/kg per min at 21/2 h in nondiabetics, P less than 0.01) despite the fact that plasma insulin concentrations were similar in both groups (peak values 22 +/- 2 vs. 23 +/- 2 microU/ml in diabetics and nondiabetics, respectively). This abnormality in glucose production was nearly completely compensated for by a paradoxical decrease in glucose utilization after injection of insulin (basal 2.11 +/- 0.03----1.86 +/- 0.06 mg/kg per min at 21/2 h in diabetics vs. basal 2.08 +/- 0.04----2.39 +/- 0.11 mg/kg per min at 21/2 h nondiabetics, P less than 0.01), which could not be accounted for by differences in plasma glucose concentrations; the net result was a modest prolongation of hypoglycemia. Plasma glucagon (area under the curve [AUC] above base line, 12 +/- 3 vs. 23 +/- 3 mg/ml X 12 h in nondiabetics, P less than 0.05), cortisol (AUC 2.2 +/- 0.5 vs. 4.0 +/- 0.7 mg/dl X 12 h in nondiabetics, P less than 0.05), and growth hormone (AUC 1.6 +/- 0.4 vs. 2.9 +/- 0.4 micrograms/ml X 12 h in nondiabetics, P less than 0.05) responses in the diabetics were decreased 50% while their plasma norepinephrine responses (AUC 49 +/- 12 vs. 21 +/- 5 ng/ml X 12 h in nondiabetics, P less than 0.05) were increased twofold (P less than 0.05) and their plasma epinephrine responses were similar to those of the nondiabetics (AUC 106 +/- 17 vs. 112 +/- 10 ng/ml X 12 h in nondiabetics). In both groups of subjects, increases in plasma glucagon were inversely correlated with plasma glucose concentrations (r = -0.80 in both groups, P less than 0.01) and suppression of endogenous insulin secretion (r = -0.57 in nondiabe
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asplin C. M., Paquette T. L., Palmer J. P. In vivo inhibition of glucagon secretion by paracrine beta cell activity in man. J Clin Invest. 1981 Jul;68(1):314–318. doi: 10.1172/JCI110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenstal R. M., Polonsky K. S., Pons G., Jaspan J. B., Rubenstein A. H. Lack of glucagon response to hypoglycemia in type I diabetics after long-term optimal therapy with a continuous subcutaneous insulin infusion pump. Diabetes. 1983 May;32(5):398–402. doi: 10.2337/diab.32.5.398. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Bucolo R. J. Interaction of insulin and glucose in the control of hepatic glucose balance. Am J Physiol. 1974 Dec;227(6):1314–1322. doi: 10.1152/ajplegacy.1974.227.6.1314. [DOI] [PubMed] [Google Scholar]
- Boden G., Reichard G. A., Jr, Hoeldtke R. D., Rezvani I., Owen O. E. Severe insulin-induced hypoglycemia associated with deficiencies in the release of counterregulatory hormones. N Engl J Med. 1981 Nov 12;305(20):1200–1205. doi: 10.1056/NEJM198111123052007. [DOI] [PubMed] [Google Scholar]
- Boden G., Soriano M., Hoeldtke R. D., Owen O. E. Counterregulatory hormone release and glucose recovery after hypoglycemia in non-insulin-dependent diabetic patients. Diabetes. 1983 Nov;32(11):1055–1059. doi: 10.2337/diab.32.11.1055. [DOI] [PubMed] [Google Scholar]
- Bolli G., de Feo P., Compagnucci P., Cartechini M. G., Angeletti G., Santeusanio F., Brunetti P., Gerich J. E. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983 Feb;32(2):134–141. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- Bolli G., de Feo P., Compagnucci P., Cartechini M. G., Angeletti G., Santeusanio F., Brunetti P. Important role of adrenergic mechanisms in acute glucose counterregulation following insulin-induced hypoglycemia in type I diabetes. Evidence for an effect mediated by beta-adrenoreceptors. Diabetes. 1982 Jul;31(7):641–647. doi: 10.2337/diab.31.7.641. [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain P. R., Smith D., DeFronzo R. A. The effect of growth hormone on glucose metabolism and insulin secretion in man. J Clin Endocrinol Metab. 1982 Nov;55(5):973–982. doi: 10.1210/jcem-55-5-973. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. V., Kraegen E. W., Lazarus L. Defective blood glucose counter-regulation in diabetics is a selective form of autonomic neuropathy. Br Med J. 1977 Dec 10;2(6101):1527–1529. doi: 10.1136/bmj.2.6101.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. V., Kraegen E. W., Meler H., Lazarus L. Hormonal responses to insulin infusion in diabetes mellitus. Diabetologia. 1979 Jun;16(6):359–364. doi: 10.1007/BF01223155. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Gerich J. E. Relevance of glucose counterregulatory systems to patients with diabetes: critical roles of glucagon and epinephrine. Diabetes Care. 1983 Jan-Feb;6(1):95–99. doi: 10.2337/diacare.6.1.95. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Day J. L., Anderson J. Abnormalities of glucagon metabolism in diabetes mellitus. Clin Endocrinol (Oxf) 1973 Jul;2(3):211–217. doi: 10.1111/j.1365-2265.1973.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Ewing D. J., Campbell I. W., Burt A. A., Clarke B. F. Vascular reflexes in diabetic autonomic neuropathy. Lancet. 1973 Dec 15;2(7842):1354–1356. doi: 10.1016/s0140-6736(73)93323-0. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C. C-peptide response to glucagon. A test for the residual beta-cell function in diabetes mellitus. Diabetes. 1977 Jul;26(7):605–610. doi: 10.2337/diab.26.7.605. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C., Markussen J., Heding L. G., Naithani V. K., Kuzuya H., Blix P., Horwitz D. L., Rubenstein A. H. Characterization of seven C-peptide antisera. Diabetes. 1978;27 (Suppl 1):170–177. doi: 10.2337/diab.27.1.s170. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Hagen C., Binder C., Markussen J., Naithani V. K., Blix P. M., Kuzuya H., Horwitz D. L., Rubenstein A. H., Rossing N. Kinetics of human connecting peptide in normal and diabetic subjects. J Clin Invest. 1978 Jul;62(1):197–203. doi: 10.1172/JCI109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J. B., Ohta M., Boyle C., Remer A. Potentiation of acetohexamide hypoglycemia by phenylbutazone. N Engl J Med. 1967 Oct 26;277(17):889–894. doi: 10.1056/NEJM196710262771701. [DOI] [PubMed] [Google Scholar]
- Fradkin J., Shamoon H., Felig P., Sherwin R. S. Evidence for an important role of changes in rather than absolute concentrations of glucagon in the regulation of glucose production in humans. J Clin Endocrinol Metab. 1980 Apr;50(4):698–703. doi: 10.1210/jcem-50-4-698. [DOI] [PubMed] [Google Scholar]
- Frey H. M., Rosenlund B. Studies in patients with chlorpropamide-induced hypoglycemia. Diabetes. 1970 Dec;19(12):930–937. doi: 10.2337/diab.19.12.930. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Cryer P. E., Santiago J. V., Haymond M. W., Pagliara A. S., Kipnis D. M. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976 Jul;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Regulation of pancreatic insulin and glucagon secretion. Annu Rev Physiol. 1976;38:353–388. doi: 10.1146/annurev.ph.38.030176.002033. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Gerich J., Cryer P., Rizza R. Hormonal mechanisms in acute glucose counterregulation: the relative roles of glucagon, epinephrine, norepinephrine, growth hormone, and cortisol. Metabolism. 1980 Nov;29(11 Suppl 1):1164–1175. doi: 10.1016/0026-0495(80)90026-8. [DOI] [PubMed] [Google Scholar]
- Goldman J., Baldwin D., Pugh W., Rubenstein A. H. Equilibrium binding assay and kinetic characterization of insulin antibodies. Diabetes. 1978 Jun;27(6):653–660. doi: 10.2337/diab.27.6.653. [DOI] [PubMed] [Google Scholar]
- Hilsted J., Jensen S. B. A simple test for autonomic neuropathy in juvenile diabetics. Acta Med Scand. 1979;205(5):385–387. doi: 10.1111/j.0954-6820.1979.tb06069.x. [DOI] [PubMed] [Google Scholar]
- Hilsted J., Madsbad S., Krarup T., Sestoft L., Christensen N. J., Tronier B., Galbo H. Hormonal, metabolic, and cardiovascular responses to hypoglycemia in diabetic autonomic neuropathy. Diabetes. 1981 Aug;30(8):626–633. doi: 10.2337/diab.30.8.626. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Brodie A. N., Mayson S. M., Jakway J. Microchromatography of hemoglobins. II. A simplified procedure for the determination of hemoglobin A2. J Lab Clin Med. 1975 Oct;86(4):700–702. [PubMed] [Google Scholar]
- Kleinbaum J., Shamoon H. Impaired counterregulation of hypoglycemia in insulin-dependent diabetes mellitus. Diabetes. 1983 Jun;32(6):493–498. doi: 10.2337/diab.32.6.493. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt N. S., Vinik A. I., Sive A. A., Child P., Jackson W. P. Studies on plasma glucagon concentration in maturity-onset diabetics with autonomic neuropathy. Diabetes. 1979 Nov;28(11):1015–1021. doi: 10.2337/diab.28.11.1015. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Haymond M. W., Gerich J. E. Effects of free fatty acids, insulin, glucagon and adrenaline on ketone body production in humans. Ciba Found Symp. 1982;87:192–213. doi: 10.1002/9780470720691.ch11. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Nakagawa S., Nakayama H., Sasaki T., Yoshino K., Yu Y. Y. A simple method for the determination of serum free insulin levels in insulin-treated patients. Diabetes. 1973 Aug;22(8):590–600. doi: 10.2337/diab.22.8.590. [DOI] [PubMed] [Google Scholar]
- Perley M. J., Kipnis D. M. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967 Dec;46(12):1954–1962. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky K., Bergenstal R., Pons G., Schneider M., Jaspan J., Rubenstein A. Relation of counterregulatory responses to hypoglycemia in type I diabetics. N Engl J Med. 1982 Oct 28;307(18):1106–1112. doi: 10.1056/NEJM198210283071802. [DOI] [PubMed] [Google Scholar]
- Popp D. A., Shah S. D., Cryer P. E. Role of epinephrine-mediated beta-adrenergic mechanisms in hypoglycemic glucose counterregulation and posthypoglycemic hyperglycemia in insulin-dependent diabetes mellitus. J Clin Invest. 1982 Feb;69(2):315–326. doi: 10.1172/JCI110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C., Molnar G. D., Horwitz D. L., Rubenstein A. H., Taylor W. F., Jiang N. S. Abnormalities of endogenous glucagon and insulin in unstable diabetes. Diabetes. 1977 Jan;26(1):36–45. doi: 10.2337/diab.26.1.36. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Gerich J. E., Haymond M. W., Westland R. E., Hall L. D., Clemens A. H., Service F. J. Control of blood sugar in insulin-dependent diabetes: comparison of an artificial endocrine pancreas, continuous subcutaneous insulin infusion, and intensified conventional insulin therapy. N Engl J Med. 1980 Dec 4;303(23):1313–1318. doi: 10.1056/NEJM198012043032301. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982 Jan;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes. 1982 Aug;31(8 Pt 1):663–669. doi: 10.2337/diab.31.8.663. [DOI] [PubMed] [Google Scholar]
- Saccà L., Sherwin R., Hendler R., Felig P. Influence of continuous physiologic hyperinsulinemia on glucose kinetics and counterregulatory hormones in normal and diabetic humans. J Clin Invest. 1979 May;63(5):849–857. doi: 10.1172/JCI109384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoon H., Hendler R., Sherwin R. S. Altered responsiveness to cortisol, epinephrine, and glucagon in insulin-infused juvenile-onset diabetics. A mechanism for diabetic instability. Diabetes. 1980 Apr;29(4):284–291. doi: 10.2337/diab.29.4.284. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Hendler R., Sherwin R. S. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab. 1981 Jun;52(6):1235–1241. doi: 10.1210/jcem-52-6-1235. [DOI] [PubMed] [Google Scholar]
- Silverberg A. B., Shah S. D., Haymond M. W., Cryer P. E. Norepinephrine: hormone and neurotransmitter in man. Am J Physiol. 1978 Mar;234(3):E252–E256. doi: 10.1152/ajpendo.1978.234.3.E252. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Raskin P., Srikant C. B., Orci L. Glucagon and the A cells. Recent Prog Horm Res. 1976;33:477–517. doi: 10.1016/b978-0-12-571133-3.50018-0. [DOI] [PubMed] [Google Scholar]
- Unger R. H. The Berson memorial lecture. Insulin-glucagon relationships in the defense against hypoglycemia. Diabetes. 1983 Jun;32(6):575–583. doi: 10.2337/diab.32.6.575. [DOI] [PubMed] [Google Scholar]
- White N. H., Skor D. A., Cryer P. E., Levandoski L. A., Bier D. M., Santiago J. V. Identification of type I diabetic patients at increased risk for hypoglycemia during intensive therapy. N Engl J Med. 1983 Mar 3;308(9):485–491. doi: 10.1056/NEJM198303033080903. [DOI] [PubMed] [Google Scholar]



