Abstract
Background
Maternal gestational smoking, diabetes, alcohol drinking, and pre-pregnancy obesity are thought to increase the risk of cryptorchidism in newborn males, but the evidence is inconsistent.
Method
We conducted a systematic review and meta-analysis of studies on the association between maternal gestational smoking, diabetes, alcohol drinking, and pre-pregnancy obesity and the risk of cryptorchidism. Articles were retrieved by searching PubMed and ScienceDirect, and the meta-analysis was conducted using Stata/SE 12.0 software. Sensitivity analysis was used to evaluate the influence of confounding variables.
Results
We selected 32 articles, including 12 case—control, five nested case—control, and 15 cohort studies. The meta-analysis showed that maternal smoking (OR = 1.17, 95% CI: 1.11–1.23) or diabetes (OR = 1.21, 95%CI: 1.00–1.46) during pregnancy were associated with increased risk of cryptorchidism. Overall, the association between maternal alcohol drinking (OR = 0.97, 95% CI: 0.87–1.07), pre-pregnancy body mass index (OR = 1.02, 95% CI: 0.95–1.09) and risk of cryptorchidism were not statistically significant. Additional analysis showed reduced risk (OR = 0.89, 95% CI: 0.82–0.96) of cryptorchidism with moderate alcohol drinking during pregnancy. No dose—response relationship was observed for increments in body mass index in the risk of cryptorchidism. Sensitivity analysis revealed an unstable result for the association between maternal diabetes, alcohol drinking and cryptorchidism. Moderate heterogeneity was detected in studies of the effect of maternal alcohol drinking and diabetes. No publication bias was detected.
Conclusion
Maternal gestational smoking, but not maternal pre-pregnancy overweight or obesity, was associated with increased cryptorchidism risk in the offspring. Moderate alcohol drinking may reduce the risk of cryptorchidism while gestational diabetes may be a risk factor, but further studies are needed to verify this.
Introduction
Cryptorchidism is a genital malformation of newborn males, in which one or both testes are absent from the scrotum at birth. It has a prevalence of 1.63 to 2.90% [1, 2], and in approximately 70% of affected infants, the testes spontaneously descend during the first 3 to 6 months after their birth [3–5]. Nearly 20% of undescended testes are impalpable [6]. Cryptorchidism can be treated surgically or by hormone therapy, and may have severe long-term complications if untreated. Evidence presented in recent reviews indicates that cryptorchidism carries a high risk of infertility and testicular cancer [7, 8].
The causes of cryptorchidism are not well understood. In addition to genetic alterations [9] and exposure to endocrine-disrupting chemicals [10], maternal smoking [10–13], alcohol drinking [12–14], diabetes [15], and pre-pregnancy obesity [16] are considered potential risk factors for cryptorchidism. However, the mechanisms underlying these relationships remain unclear and the results of epidemiologic studies of associations between maternal exposure to specific risk factors and cryptorchidism are inconsistent. The relationship between maternal gestational smoking and risk of cryptorchidism was assessed in a recent review [17]. But there were some limitations in the methodology; and the studies it included were insufficient. Additional meta-analysis [18] has been well designed, but several studies have published after that. And none of these two meta-analyses have focused on the remaining risk factors mentioned above. The evidence available in recent reviews is thus not comprehensive.
We conducted a systematic review and meta-analysis of current observational studies following the PRISMA statement [19] (S1 PRISMA Checklist). Our primary aim was to quantitatively evaluate the association of maternal gestational smoking, diabetes, alcohol drinking, and pre-pregnancy obesity with the risk of cryptorchidism.
Methods
Eligibility criteria
The studies selected for analysis satisfied the following criteria. (1) The studies included newborn males diagnosed with cryptorchidism in case group. (2) The effects of maternal gestational smoking, diabetes, alcohol drinking or maternal pre-pregnancy body mass index (BMI) were investigated. (3) The control or non-case group comprised boys without cryptorchidism. (4) The study was of a cohort, case—control, or nested case—control design. (5) Complete data were reported; or missing data could be obtained from the study investigators. Response letters or meeting papers were also considered if additional information was provided.
Search strategy
We searched PubMed and ScienceDirect for relevant, English-language studies published to February 10, 2014. The PubMed search terms were: [(gestational diabetes OR gestational glycuresis) OR (gestational smoking OR gestational cigarette exposure) OR (gestational alcohol drinking OR gestational drinking) OR (pre-pregnancy obesity OR pre-pregnancy overweight OR pre-pregnancy body mass index) OR (risk factors)] AND (cryptorchidism OR undescended testes OR cryptorchism) AND (human). We also manually searched the reference lists of retrieved articles and reviews.
Data extraction
Two reviewers (L. Zhang and X.-H. Wang) independently extracted the author names, publication year, country, study type, the number of cryptorchidism cases, controls or non-cases or person years, exposure categories, adjusted or crude relative risk (RR), odds ratio (OR), hazard ratio (HR), or prevalence ratio (PR) with 95% confidence intervals (CIs), and adjusted variables. A standardized data collection form was used. When more than one OR (RR, HR, or PR) was shown, the one from multivariate-adjusted was extracted to account for the influence of potential confounders. If a study included two or more control groups, the one that matched more of the study variables or was population-based was analyzed. A third author (T.Z. Liu) independently reviewed the articles for the extracted information, which were then discussed by all authors to decide on inclusion.
Statistical analysis
In the first part of the analysis, we pooled the ORs and 95% CIs of cryptorchidism for the mother who smoking, drinking, with gestational diabetes or pre-pregnancy obesity. For studies that did not provide valid evaluations, we used the reported tabular data to calculate crude ORs with their 95% confidence intervals (CIs). If the raw data included a zero cell, 0.5 was added to every cell in the 2 x 2 table. Studies with two or more zero cells in 2 x 2 tables were excluded. In the meta-analysis, hazard ratios (HRs) and prevalence ratio (PRs) were considered as RRs; and then relative risk (RRs) and relevant standard error (SE) were converted into ORs using the method described by Zhang et al [20], which requires the incidence of the outcome of interest (P0) in the control group. It should be noted that, this conversion may overestimate the variance of OR [21]. We pooled RR with OR directly if P0 could not be obtained since the incidence of cryptorchidism is low (< 10%) that RR is similar to OR, as described by Zhang et al. [20]. We used I2 statistics to detect statistical heterogeneity, which varied from 0 to 100% and was described as low (0–40%), moderate (30–60%), substantial (50–90%), and considerable (75–100%) [23]. A fixed-effect model was applied when slight heterogeneity (I2 < 30%) was detected; otherwise, a random-effect model was used. Studies that reported results separately by area, sex, or race, the ORs were pooled using a fixed-effects model before combining it to overall meta-analysis. Subgroup analysis (such as geographical region, study design) was used to address heterogeneity. The ORs and 95%CI were log-transformed and the weight was calculated by the inverse variance method.
To determine whether high and low exposure resulted in differences in the risk of cryptorchism (such as alcohol drinking), we pooled the ORs of high or moderate separately, and compared them to ORs of low exposure groups. We also evaluated potential dose-response relationships as previously described by Orsini et al. [24] when a sufficient number of studies reported relevant data for the 4 risk factors (such as BMI). Briefly, a regression model was initially fitted within each study to estimate the RR of cryptorchism for each unit of increase in exposure to a risk factor. Then the RRs in each individual study were pooled in a random-effect model. RR was considered as a general term that included RR, OR, HR, or PR [25]. The assigned doses were estimated as the midpoints of the upper and lower boundaries of each category [25]. For open-ended lower categories, the assigned dose was assumed to be 0.83 (1/1.2) times the cutoff point [26]; for open-ended upper categories, the assigned dose was evaluated as 1.2 times the cutoff point [25].
Egger’s test and Begg’s test were used to detect potential publication bias, and a sensitivity analysis was performed to evaluate the robustness of the results. All the analyses were conducted using Stata/SE 12.0 software (StataCorp LP, College Station, Texas, USA).
Results
Search results and study characteristics
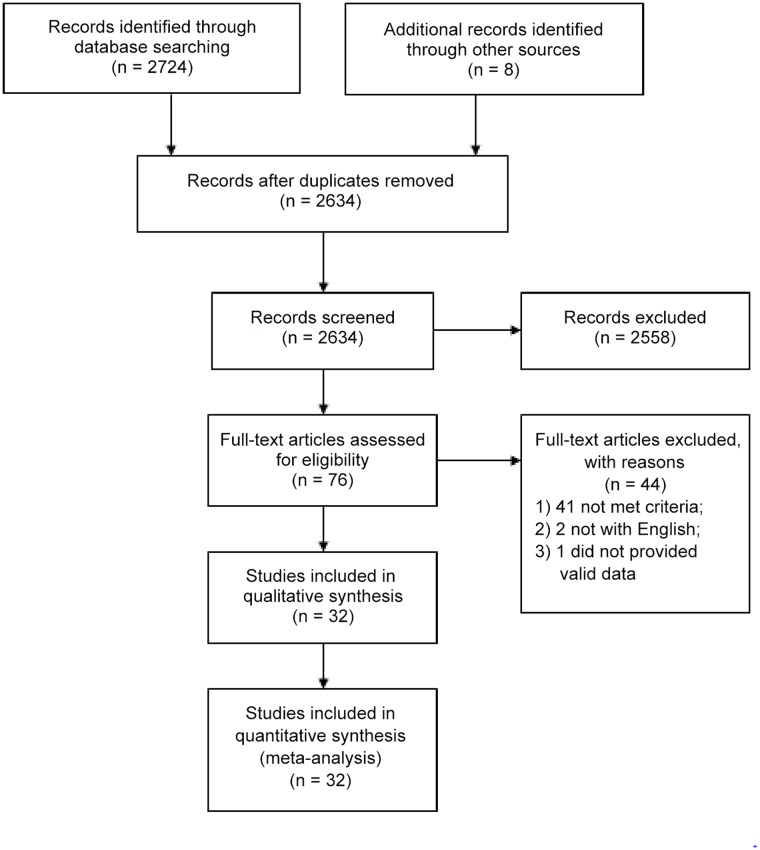
Thirty-two studies were selected [2, 10, 12–16, 22, 27–50]. Among them, there were 12 case—control (all matched with age and sex), five nested case-control, and 15 cohort studies (at least 1 years follow-up), with a total number of 21,774 cryptorchidism cases. Fig. 1 shows the flow diagram. Ten studies were conducted in the United States, seven in Denmark, three in the UK, two in Japan, two in the Netherlands, two in Italy, and one each in Egypt, France, Spain, Sweden, and Lithuania. Two studies, [28, 37] were published by the same author in different years, and four [30, 40] and [34, 41] were studies published by two different authors in different journals. The study [49] was a response letter by Main [48] that reported the information of maternal alcohol consumption and risk of cryptorchism that was not presented in study [48]. Duplicate data were excluded when reported in two or more studies. The study characteristics were shown in Table 1, more complete data were given in S1 Dataset.
Fig 1. Flow diagram of the literature search.
Table 1. Characteristics of included studies in the meta-analysis.
| First Author | Year | Country | Study types and description | Total cases/controls | Risk Factors |
|---|---|---|---|---|---|
| Zakaria | 2013 | Egypt | Nested case-control study with 1 year follow-up. | 29/40 | Maternal smoking, diabetes. |
| Brouwers | 2012 | Netherlands | Hospital based case-control study matched with geographical distribution and sex. | 200/629 | Maternal smoking, alcohol, diabetes, BMI. |
| Jensen1 | 2007 | Denmark | Cohort study with 3 years follow-up. | 270 cases | Maternal smoking. |
| Strandberg | 2009 | Denmark | Cohort study with 6 years follow-up. | 1,598 cases | Maternal alcohol. |
| Kurahashi | 2005 | Japan | Hospital-based case-control study matched with sex. | 96/116 | Maternal smoking, alcohol, BMI. |
| Adams | 2011 | America | Population-based case-control study matched with age and sex. | 2,759/35,268 | Gestational smoking, diabetes, obesity. |
| Berkowitz1 | 1996 | America | Nested case-control study with 3 years follow-up. | 69/219 | Maternal smoking, alcohol, diabetes, BMI. |
| Mori | 1992 | Japan | Hospital-based case-control study matched with age and sex. | 104/104 | Maternal smoking, alcohol. |
| Agopian | 2014 | America | Cohort study with 10 years follow-up. | 4,001 cases | Maternal diabetes. |
| Trabert | 2014 | America | Cohort study with 10 years follow-up. | 3,649 cases | Maternal diabetes. |
| Mongraw-C | 2007 | America | Cohort study with 8 yeas follow-up. | 101 cases | Maternal smoking, alcohol. |
| Damgaard1 | 2008 | Denmark | Cohort study with 2 to 4 years follow-up. | 128 cases | Maternal smoking, alcohol. |
| Fernandez | 2007 | Spain | Nested case-control study with about 2.5 years follow-up. | 47/105 | Maternal smoking, BMI. |
| McGlynn | 2006 | America | Cohort study with7 years follow-up. | 238 cases | Maternal smoking, diabetes. |
| Jones | 1998 | British | Cohort study with 17 years follow-up. | 1,499 cases | Maternal smoking, obesity, diabetes. |
| Giordano | 2008 | Italy | Population-based case-control study matched with age and sex and city. | 48/202 | Maternal smoking, alcohol. |
| Jensen2 | 2007 | Denmark | Cohort study with 3 years follow-up. | 270 cases | Maternal smoking, alcohol. |
| Virtanen | 2006 | Denmark, Finland | Hospital-based case-control study matched with age and sex. | 125 cases | Maternal smoking, gestational diabetes. |
| Pierik | 2004 | Netherlands | Nested case-control study with 2 years follow-up. | 78/313 | Maternal smoking. |
| Biggs | 2002 | America | Hospital-based case-control study matched with age and sex. | 2,395/9,580 | Maternal smoking, alcohol, diabetes. |
| Berkowitz2 | 1995 | America | Nested case-control study with 3 years follow-up | 231 cases | Maternal smoking, BMI. |
| Damgaard2 | 2008 | Denmark | Cohort study with 2 to 4 years follow-up. | 128 cases | Maternal smoking, BMI. |
| Akre | 1999 | Sweden | Hospital-based case-control study matched with age and hospital birth. | 2,782/13,916 | Maternal smoking. |
| Beard | 1984 | America | Case-control study matched with year of delivery, maternal age, gravidity, parity. | 81/159 | Maternal smoking. |
| Preiksa | 2005 | Lithuania | Cohort study with 1 year follow-up. | 69 cases | Maternal smoking. |
| Davies | 1986 | British | Hospital-based case-control study matched with age and hospital birth. | 83/129 | Maternal smoking, alcohol, diabetes. |
| Mcbirde | 1991 | British | Population-based case-control study matched with age and sex. | 244/488 | Maternal smoking, alcohol. |
| Carbone | 2006 | Italy | Population-based case-control study matched with age and city. | 48/203 | Maternal smoking, alcohol. |
| Wagner-Mahler | 2011 | France | Cohort study with 3 years follow-up. | 76/137 | Maternal smoking, alcohol drinking. |
| Shiono | 1986 | America | Cohort study with 3 years follow-up. | 233/424 | Maternal smoking. |
| Main! | 2007, 2008 | Denmark, Finland | Cohort study with 4 years follow-up. | 95/185 | Maternal smoking, alcohol, diabetes. |
Jensen1 and Jensen2, Berkowitz1 and Berkowitz2, Damgaard1 and Damgaard2 are three pairs of studies, and each pair was published by the same author; some of the data are the same, but different risk factors were evaluated.
Maternal gestational smoking and the risk of cryptorchidism
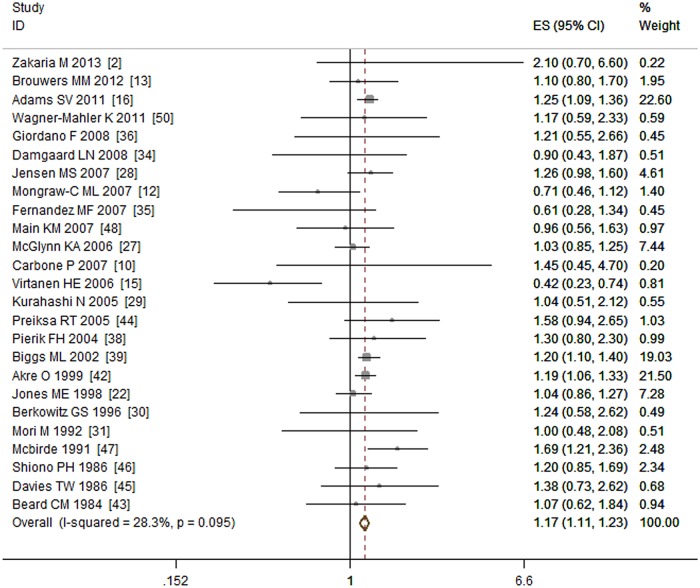
A total of 28 studies [2, 10, 12, 13, 15, 16, 22, 27–30, 31, 34–41, 42–48, 50] that included 11,900 cases investigated the relationship between maternal gestational smoking and the risk of cryptorchidism. Three studies [37, 40, 41] were excluded for duplicate data. Among the remaining 25 studies, nine were cohort studies, twelve were case—control studies and four were nested case—control studies. Our meta-analysis showed that maternal gestational smoking was associated with increased risk of cryptorchidism (OR = 1.17, 95% CI: 1.11–1.23; P < 0.01). That is, mothers who smoked during pregnancy had a 1.17 times higher odds of having a son with cryptorchidism than mothers who did not smoke. There was no obvious heterogeneity (I2 = 28.30%, P = 0.10) among studies. (See Fig. 2)
Fig 2. Forest plot for maternal gestational smoking and risk of cryptorchidism.
Maternal gestational diabetes and risk of cryptorchidism
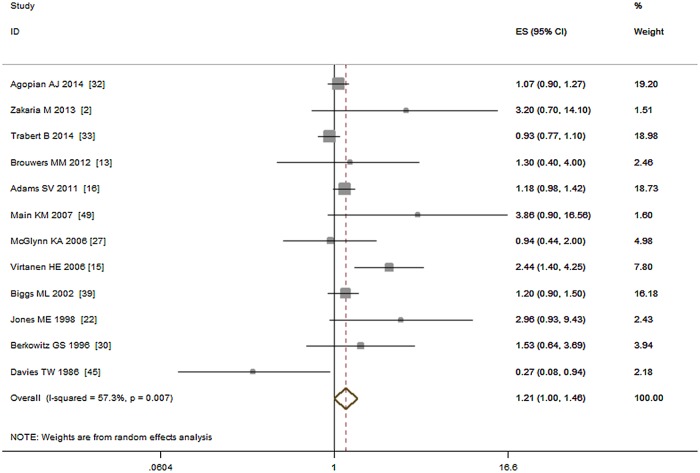
Thirteen studies including 15,373 cases investigated the relationship between maternal gestational diabetes and risk of cryptorchidism [2, 13, 15, 16, 22, 27, 30, 32, 33, 39, 40, 45, 48]. Two studies, [30, 40] were published by same author in different years, we only used the data from [30]. Among the remaining 12 studies, five were cohort studies, five were case—control studies, and two were nested case—control studies. Our meta-analysis showed that gestational diabetes was marginal associated with increased risk of cryptorchidism (OR = 1.21, 95% CI: 1.00–1.46; P = 0.06). Moderate heterogeneity (I2 = 57.30%, P < 0.01) was seen among the studies. A Forest plot for maternal gestational diabetes and risk of cryptorchidism is shown in Fig. 3.
Fig 3. Forest plot for maternal gestational diabetes and risk of cryptorchidism.
Maternal gestational alcohol drinking and the risk of cryptorchidism
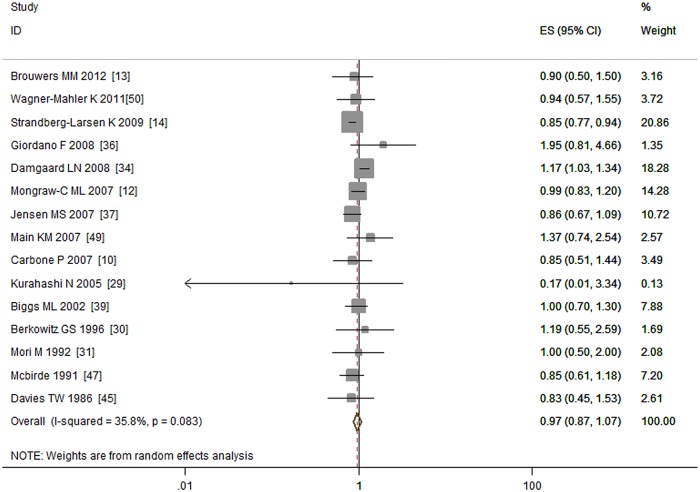
Fifteen studies investigated the association between maternal gestational alcohol drinking and the risk of cryptorchidism [10, 12–14, 29–31, 34, 36, 37, 39, 45, 47, 49, 50] including 5,601 cases in six cohort studies, eight case—control studies, and one nested case—control study. The meta-analysis did not find an association between maternal gestational drinking and the risk of cryptorchidism (OR = 0.97, 95% CI: 0.87–1.07; P = 0.54). Low to moderate heterogeneity (I2 = 35.80%, P = 0.08) was detected (Fig. 4).
Fig 4. Forest plot for maternal gestational alcohol drinking and risk of cryptorchidism.
Maternal pre-pregnancy obesity and the risk of cryptorchidism
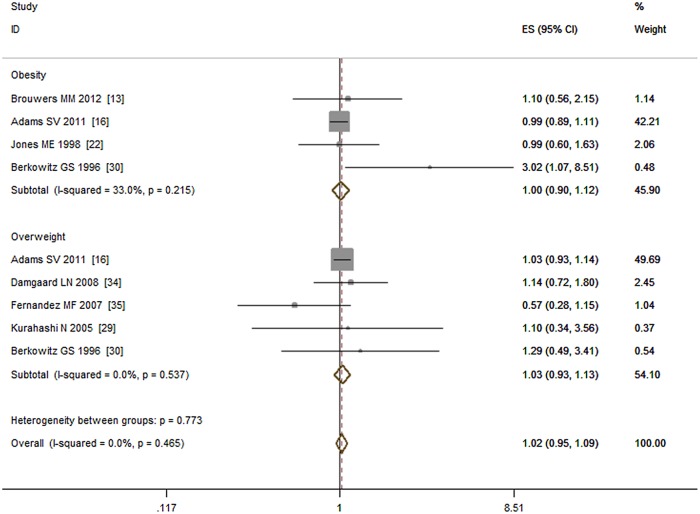
Eight studies investigated the association between maternal pre-pregnancy BMI and the risk of cryptorchidism [13, 16, 22, 29, 30, 35, 40, 41]. A duplicate study [40] was removed from the analysis. The remaining seven reports included 4,397 cases comprising three case—control studies, three cohort studies, and one nested case—control study. Maternal pre-pregnancy BMI > 25 kg /m2 did not associated with increased cryptorchidism risk (OR = 1.02, 95% CI: 0.95–1.09; P = 0.67) compared with those who had a BMI < 25 kg /m2. No obvious heterogeneity (I2 = 0.00%, P = 0.47) was detected. Subgroup analyses showed that neither maternal obesity (BMI ≥ 30 kg /m2) nor pre-pregnancy overweight (25 kg /m2 ≤ BMI < 30 kg/m2) associated with the risk of cryptorchidism (OR = 1.00, 95% CI: 0.90–1.12; P = 0.94; I2 = 33.00% and OR = 1.03, 95% CI: 0.93–1.13; P = 0.61; I2 = 0.00%, respectively), see Fig. 5.
Fig 5. Forest plot for maternal pre-pregnancy BMI and risk of cryptorchidism.
Subgroup analysis was conducted by dividing study participants into an obesity group (BMI >30 kg /m2) and an overweight group (25 kg /m2≤ BMI < 30 kg /m2).
Additional meta-analysis
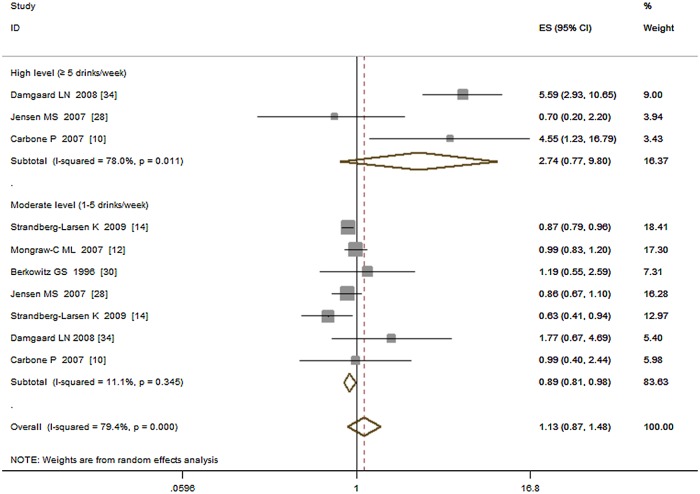
Several studies [12, 10, 14, 30, 34, 37] have investigated the risk of drinking alcohol and reported that high levels of alcohol consumption may increase the risk of cryptorchidism. We then pooled the ORs of high (≥ 5 drinks/week), moderate (1 to 5 drinks /week) vs. low (< 1 or no drinks /week) exposure levels separately and estimated the corresponding risk of cryptorchidism. The results showed that, high-level exposure was associated with increased risk of cryptorchidism (OR = 2.74, 95%CI: 0.77–9.80; P = 0.12; I2 = 78.00%) while moderate-level exposure associated decreased risk (OR = 0.89, 95%CI: 0.82–0.96; P < 0.01; I2 = 0.00%), see Fig. 6.
Fig 6. Forest plot for high and moderate vs. low exposure level of alcohol.
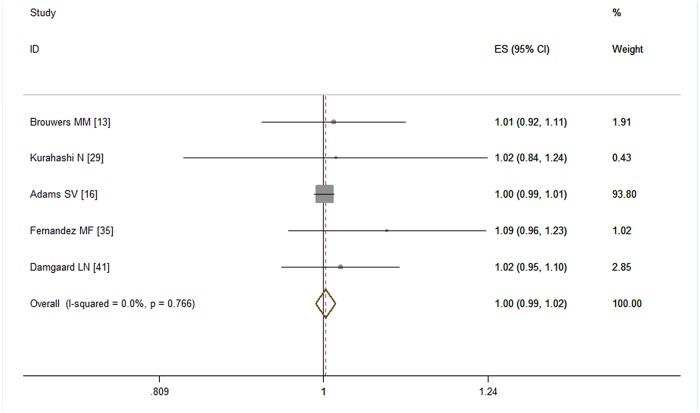
Five studies [13, 16, 29, 40, 41] provided valid dose-response data for pre-pregnancy obesity. Our dose-response meta-analysis (Fig. 7) showed that every 5 kg /m2 increment of BMI was not associated with the risk of cryptorchidism (RR = 1.00, 95% CI: 0.99–1.02; P = 0.67). No obvious heterogeneity was detected (I2 = 0.00%, P = 0.77).
Fig 7. Forest plot for each 5 kg /m2 increase of BMI and risk of cryptorchidism.
Subgroup analysis
Because moderate heterogeneity was detected among the studies of alcohol drinking and diabetes, subgroup analyses on geographical region (European, Asian, America, Africa), study design (cohort, case—control, nested case-control), adjusted/crude ORs, and positive/negative results were done (Table 2). For alcohol drinking, high heterogeneity was found in European studies (I2 = 54.60%, P = 0.02), cohort studies (I2 = 69.80%, P < 0.01), studies adjusted for variables (I2 = 77.40%, P < 0.01), and studies reporting positive results (I2 = 93.10%, P < 0.01). For maternal diabetes, high heterogeneity was found in European studies (I2 = 67.50%, P = 0.02), studies adjusted for variables (I2 = 72.30%, P = 0.01), and studies reporting positive results (I2 = 90.20%, P < 0.01).
Table 2. The results of subgroup analysis of diabetes and drinking.
| Diabetes | Drinking | |||||
|---|---|---|---|---|---|---|
| Geographical region | I2 | P-value& | ORs(95%CI) | I2 | P-value& | ORs(95%CI) |
| European | 67.50% | 0.02 | 1.61(0.70, 3.67) | 54.60% | 0.02 | 0.96(0.83, 1.11) |
| America | 0.00% | 0.42 | 1.07(0.98, 1.18) | 0.00% | 0.90 | 1.00(0.86, 1.17) |
| Asian | – | – | – | 26.00% | 0.25 | 0.74(0.20, 2.73) |
| Africa | – | – | 3.20(0.71, 14.36) | – | – | – |
| Study design | ||||||
| Cohort | 48.90% | 0.10 | 1.07(0.85, 1.36) | 69.80% | < 0.01 | 0.98(0.84, 1.14) |
| Case-control | 66.40% | 0.02 | 1.25(0.89, 1.76) | 0.00% | 0.68 | 0.93(0.78, 1.11) |
| Nested-CC$ | 0.00% | 0.41 | 1.85(0.83, 3.93) | – | – | 1.19(0.55, 2.58) |
| Adjusted/crude ORs | ||||||
| Adjusted | 72.30% | 0.01 | 1.19(0.88, 1.60) | 77.40% | < 0.01 | 0.98(0.80, 1.21) |
| Crude | 45.30% | 0.08 | 1.25(0.94, 1.26) | 0.00% | 0.91 | 0.97(0.85, 1.09) |
| Positive/negative results | ||||||
| Positive | 90.20% | < 0.01 | 0.87(0.1, 7.50) | 93.10% | < 0.01 | 0.99(0.73, 1.36) |
| Negative | 30.10% | 0.17 | 1.12(0.98, 1.29) | 0.00% | 0.83 | 0.96(0.86, 1.06) |
P-value& is the P value of heterogeneity;
Nested-CC$ are nested case-control studies;
–: there is no or only one study in the group.
I2: the heterogeneity within subgroups, which varied from 0 to 100% and was described as low (0–40%), moderate (30–60%), substantial (50–90%), and considerable (75–100%) [23].
ORs: The odds ratio in each subgroup.
Sensitivity analysis
Sensitivity analysis was conducted to evaluate the robustness of the results because some studies reported only RRs or HRs, and others had not adjusted for confounding variables. We found three articles [27, 33, 45], significantly influenced the diabetes results. Two of which were conducted in America and one in British. When one of them was omitted from the analysis the risk represented by diabetes was no longer significant. Another article [34], with an OR of 1.17 (95%CI: 1.03–1.34), influences the results of alcohol drinking obviously. When omitted it, the pooled ORs reached to statistical significance (OR = 0.90, 95%CI: 0.83–0.96). These results may be explained in part by ethnic or clinical diversity (i.e., the severity of diabetes). Sensitivity analysis did not result in any substantial change in the meta-analysis results on the influence of smoking and pre-pregnancy obesity.
Publication bias
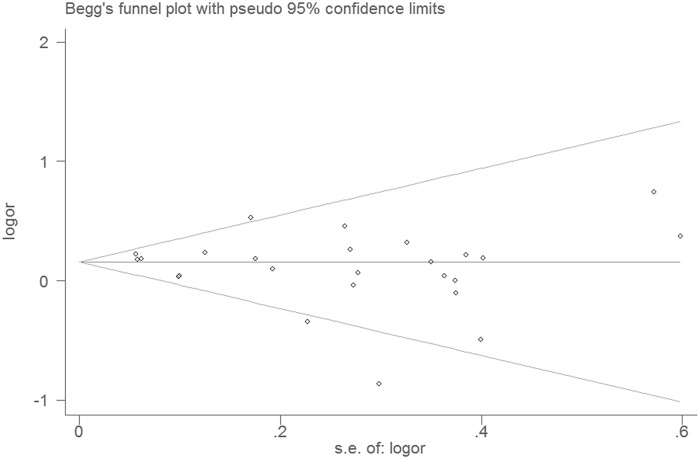
Publication bias was evaluated by Egger’s test (P1) and Begg’s test (P2), which yielded no significant results for maternal gestational diabetes (P1 = 0.26, P2 = 0.47), smoking (P1 = 0.17, P2 = 0.30), drinking (P1 = 0.74, P2 = 0.51),or pre-pregnancy obesity (P1 = 0.52, P2 = 0.75). The results suggested no obvious publication bias in present meta-analysis. Fig. 8 shows the Begg’s test plot for studies that reported on maternal gestational smoking.
Fig 8. Begg’s test of studies reporting on maternal gestational smoking and risk of cryptorchidism.
Discussion
The present meta-analysis confirmed that maternal gestational smoking was associated with increased risk of cryptorchidism in newborn males. Sensitivity analysis supported the robustness of this result. This finding is consistent with two previous meta-analyses of maternal smoking and cryptorchidism. One [17] analyzed 13 studies by pooling the effect size but without adjusting any data or conducting additional analyses (e.g., heterogeneity testing) and without reporting fixed- or random-effect models. The second study [18], which was well designed but only included 18 studies, reported a summary OR (1.13, 95%CI: 1.02–1.25) similar to that seen here. Our updated meta-analysis, included 25 studies, had a rigorous design, and followed a widely accepted methodology, all of which support the validity of the results.
In this analysis, maternal gestational diabetes was linked with marginal increased risk of cryptorchidism, but the result should be interpreted with caution. Moderate heterogeneity was detected among the selected studies although a major source of heterogeneity was revealed by subgroup analysis. We also note that instability in sensitivity analysis can reduce the credibility of the results. After omitting one of the above three studies from the analysis, the effect of maternal gestational diabetes was no longer significant.
Alcohol drinking, overall, was not associated with increased risk of cryptorchidism, but high levels of alcohol drinking should be noted and discouraged. Previous studies [10, 34] reported an increased risk of cryptorchidism when mothers were heavy drinkers. The ORs of our additional meta-analysis including 6 of 15 studies showed that five or more drinks /week was associated with increased risk of cryptorchidism. But this result was statistically non-significant and was based on small numbers which needed further investigation. Moderate alcohol drinking may reduce the cryptorchidism risk. Our additional meta-analysis showed that 1 to 5 drinks /week was associated decreased risk of cryptorchidism. The unstable results of sensitivity analysis support this result. When omitted one article [34], the pooled ORs reached to statistic significance and showed reduced risk (OR = 0.90, 95%CI: 0.83–0.96) of cryptorchidism. These different effects by consumption level of alcohol on cryptorchidism were consistent with findings of study [51] of maternal alcohol drinking and risk of fetal growth and preterm birth. Further studies were needed to verify it.
The relationship between maternal pre-pregnancy obesity and the risk of cryptorchidism has been controversial. In our meta-analysis, we did not find evidence of such a relationship, and the sensitivity analysis indicates that our results are robust. We believe that the evidence of the studies evaluated here is not sufficient to confirm any relationship between pre-pregnancy obesity and the risk of cryptorchidism. In addition, no dose—response relationship was found between maternal pre-pregnancy BMI and the risk of cryptorchidism, which also supports this conclusion.
The descent of the testes normally occurs in two stages; a transabdominal stage at 7 to 15 weeks [52] and a transinguinal stage [6] at 20 to 35 weeks of pregnancy [52, 53]. Insulin-like peptide (INSL3), anti-Mullerian hormone (AMH), human chorionic gonadotropin (HCG), androgens and their receptor genes are all involved in these events [6, 53, 54, 55]. Any factors that can influence the levels of these hormones may affect descent of the testes. In our meta-analysis, maternal gestational smoking was found to be the only factor with clear evidence for significantly increasing the risk of cryptorchidism. Although the mechanism underlying this relationship is not known, previous studies may provide some clues. Previous studies [56, 57] found that maternal gestational smoking is associated with reduced levels of intact HCG and desert hedgehog (DHH) signaling in newborn males. HCG is needed for testicular descent and is used as a hormone treatment for cryptorchidism [6]. The effect of maternal gestational smoking on cryptorchidism risk may be mediated by reduction in HCG levels. Although the results for the effect of maternal diabetes were unstable, previous studies observed decreased serum sex hormone binding globulin (SHBG) levels [58–59] and increased insulin levels [60, 61] in umbilical cord blood in pregnant women with diabetes. Decreased SHBG levels may result in an increase of the ratio of non-SHBG-bound levels of estradiol and testosterone [62], which may then interfere with the descent of the testis. Whether down regulated expression of DHH or increased insulin levels in umbilical cord blood can cause cryptorchidism in newborn males is unclear. Further study is necessary.
Strengths and limitations
Our meta-analysis followed a strict protocol, and the results provide an objective assessment of the relationships between maternal exposure to potential risk factors and cryptorchidism. Significant publication bias was not detected among the selected studies, adding to the strength of the results. Sensitivity analyses helped to avoid interference from confounding factors and to reduce reporting bias. Finally, additional meta-analyses (e.g., dose-response) allowed for investigation of possible relationships between the extent of exposure and the degree of risk.
This meta-analysis also has several limitations. Moderate heterogeneity was detected in studies of alcohol drinking and diabetes and sensitivity analysis revealed the result of alcohol drinking and diabetes was unstable. Although some variability is inevitable, this may influence the reliability of our results. In addition, at least half the studies did not adjust for confounding variables. Although we conducted subgroup meta-analyses to avoid bias, this may still affect our results. In addition, one [63] article did not find any relationships between maternal smoking and drinking with risk of cryptorchidism. Because this might have affected our results, we tried to contact the corresponding author for additional data, but with no response. Fourth, the Newcastle-Ottawa Scale [64] or other scales provided standard items for quality assessment; however, in our meta-analysis we included case-control, cohort, and nested case-control studies and did not assessed the quality of each study since there is not a standard specific to the assessment of nested case-control studies. Moreover, the studies we included usually contain multiple risk factors and some studies only adjusted confounders for the main risk factors (such as smoking) but not minor risk factors (such as diabetes). In this situation, the quality assessment tools are unusable.
Conclusion
Smoking may be a risk factor for cryptorchidism; both parents should avoid fetal exposure to cigarette smoke. Maternal gestational diabetes may be (a) risk factor, but needs further research for confirmation. Drinking alcohol during pregnancy is not associated with increased risk of cryptorchidism, but five or more drinks a week may increase risk while one to five drinks a week may reduce the risk. No significant associations were found between maternal pre-pregnancy obesity or overweight and the risk of cryptorchidism.
Supporting Information
(XLSX)
(DOC)
Data Availability
All relevant data are included within the paper and its Supporting Information files.
Funding Statement
These authors have no support or funding to report.
References
- 1. Wagner-Mahler K, Kurzenne J- Y, Delattre I, Bérard E, Mas J- C, Bornebush L, et al. Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. Int J Androl. 2011; 34(5):e499–510. 10.1111/j.1365-2605.2011.01211.x [DOI] [PubMed] [Google Scholar]
- 2. Zakaria M, Azab S, El baz M, Fawaz L, Bahagat A. Cryptorchidism in Egyptian neonates. J Pediatr Urol. 2013; 9(6):815–819. 10.1016/j.jpurol.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 3. Comploj E, Pycha A. Diagnosis and Management of Cryptorchidism. European Urology Supplements. 2012; 11(2):2–9. [Google Scholar]
- 4. Wenzler DL, Bloom DA, Park JM. What is the rate of spontaneous testicular descent in infants with cryptorchidism? J Urol. 2004; 171(2):849–51. [DOI] [PubMed] [Google Scholar]
- 5. Rubenwolf P, Stein R. Diagnosis and management of the undescended testis—an update in the light of the current guidelines. Aktuelle Urol. 2013; 44(6):445–51. 10.1055/s-0033-1358664 [DOI] [PubMed] [Google Scholar]
- 6. Abacı A, Çatlı G, Anık A, Böber E. Epidemiology, classification and management of undescended testes: does medication have value in its treatment? J Clin Res Pediatr Endocrinol. 2013; 5(2):65–72. 10.4274/Jcrpe.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huff DS, Fenig DM, Canning DA, Carr MG, Zderic SA, Snyder HM, et al. Abnormal germ cell development in cryptorchidism. Horm Res. 2001; 55:11–17. [DOI] [PubMed] [Google Scholar]
- 8. Cook MB, Akre O, Forman D, Madigan MP, Richiardi L, McGlynn KA. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer—experiences of the son. Int J Epidemiol. 2010; 39(6):1605–18. 10.1093/ije/dyq120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferlin A, Zuccarello D, Zuccarello B, Chirico MR, Zanon GF, Foresta C. Genetic alterations associated with cryptorchidism. JAMA. 2008; 300(19):2271–6. 10.1001/jama.2008.668 [DOI] [PubMed] [Google Scholar]
- 10. Carbone P, Giordano F, Nori F, Mantovani A, Taruscio D, Lauria L, et al. The possible role of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: a population-based case-control study in rural Sicily. Int J Androl. 2007; 30(1):3–13. [DOI] [PubMed] [Google Scholar]
- 11. Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, Schmidt IM, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004; 363(9417):1264–9. [DOI] [PubMed] [Google Scholar]
- 12. Mongraw-Chaffin ML, Cohn BA, Cohen RD, Christianson RE. Maternal smoking, alcohol consumption, and caffeine consumption during pregnancy in relation to a son’s risk of persistent cryptorchidism: a prospective study in the Child Health and Development Studies cohort, 1959–1967. Am J Epidemiol. 2008; 167(3):257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brouwers MM, de Bruijne LM, de Gier RP, Zielhuis GA, Feitz WF, Roelevelda N, et al. Risk factors for undescended testis. J Pediatr Urol. 2012; 8(1):59–66. 10.1016/j.jpurol.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 14. Strandberg-Larsen K, Jensen MS, Ramlau-Hansen CH, Grønbaek M, Olsen J. Alcohol binge drinking during pregnancy and cryptorchidism. Hum Reprod. 2009; 24(12):3211–9. 10.1093/humrep/dep325 [DOI] [PubMed] [Google Scholar]
- 15. Virtanen HE, Tapanainen AE, Kaleva MM, Suomi AM, Main KM, Skakkebaek NE, et al. Mild gestational diabetes as a risk factor for congenital cryptorchidism. J Clin Endocrinol Metab. 2006; 91(12):4862–5. [DOI] [PubMed] [Google Scholar]
- 16. Adams SV, Hastert TA, Huang Y, Starr JR. No association between maternal pre-pregnancy obesity and risk of hypospadias or cryptorchidism in male newborns. Birth Defects Res A Clin Mol Teratol. 2011; 91(4):241–8. 10.1002/bdra.20805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Håkonsen LB, Ernst A, Ramlau-Hansen CH. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl. 2014; 16(1):39–49. 10.4103/1008-682X.122351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hackshaw Allan, Rodeck Charles, Boniface Sadie. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update. 2011; 17(5):589–604. 10.1093/humupd/dmr022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 21; 6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998; 280: 1690–1691. [DOI] [PubMed] [Google Scholar]
- 21. Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004; 160(4):301–5. [DOI] [PubMed] [Google Scholar]
- 22. Jones ME, Swerdlow AJ, Griffith M, Goldacre MJ. Prenatal risk factors for cryptorchidism: a record linkage study. Paediatr Perinat Epidemiol. 1998; 12(4):383–96. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. Accessed 2014 Feb 18.)
- 24. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. The Stata Journal. 2006; 6(1): 40–57. [Google Scholar]
- 25. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993; 4: 218 [DOI] [PubMed] [Google Scholar]
- 26. Larsson SC, Orsini N. Coffee consumption and Risk of stroke: A dose-response Meta-analysis of prospective studies. American Journal of Epidemiology. 2011; 174:993–1001. 10.1093/aje/kwr226 [DOI] [PubMed] [Google Scholar]
- 27. McGlynn KA, Graubard BI, Klebanoff MA, Longnecker MP. Risk factors for cryptorchism among populations at differing risks of testicular cancer. Int J Epidemiol. 2006; 35(3):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jensen MS, Toft G, Thulstrup AM, Bonde JP, Olsen J. Cryptorchidism according to maternal gestational smoking. Epidemiology Mar. 2007; 18(2):220–5. [DOI] [PubMed] [Google Scholar]
- 29. Kurahashi N, Kasai S, Shibata T, Kakizaki H, Nonomura K, Sata F, et al. Parental and neonatal risk factors for cryptorchidism. Med SciMonit. 2005; 11(6):CR274–283. [PubMed] [Google Scholar]
- 30. Berkowitz GS, Lapinski RH. Risk factors for cryptorchidism: a nested case-control study. Paediatr Perinat Epidemiol. 1996; 10(1):39–51. [DOI] [PubMed] [Google Scholar]
- 31. Mori M, Davies TW, Tsukamoto T, Kumamoto Y, Fukuda K. Maternal and other factors of cryptorchidism—a case-control study in Japan. Kurume Med J. 1992; 39(2):53–60. [DOI] [PubMed] [Google Scholar]
- 32. Agopian AJ, Langlois PH, Ramakrishnan A, Canfield MA. Epidemiologic features of male genital malformations and subtypes in Texas. Am J Med Genet A. 2014; 164A(4):943–9. 10.1002/ajmg.a.36389 [DOI] [PubMed] [Google Scholar]
- 33. Trabert B, Chodick G, Shalev V, Sella T, Longnecker MP, McGlynn KA, et al. Gestational diabetes and the risk of cryptorchidism and hypospadias. Epidemiology. 2014; 25(1):152–3. 10.1097/EDE.0000000000000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Damgaard IN, Jensen TK, Petersen JH, Skakkebaek NE, Toppari J, Main KM. Cryptorchidism and maternal alcohol consumption during pregnancy. Environ Health Perspect. 2007; 115(2):272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernandez MF, Olmos B, Granada A, López-Espinosa MJ, Molina-Molina JM, Fernandez JM, et al. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: a nested case-control study. Environ Health Perspect. 2007; 115 Suppl 1:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giordano F, Carbone P, Nori F, Mantovani A, Taruscio D, Figà-Talamanca I. Maternal diet and the risk of hypospadias and cryptorchidism in the offspring. Paediatr Perinat Epidemiol. 2008; 22(3):249–60. 10.1111/j.1365-3016.2007.00918.x [DOI] [PubMed] [Google Scholar]
- 37. Jensen MS, Bonde JP, Olsen J. Prenatal alcohol drinking and cryptorchidism. ActaPaediatr. 2007; 96(11):1681–5. [DOI] [PubMed] [Google Scholar]
- 38. Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect. 2004; 112(15):1570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biggs ML, Baer A, Critchlow CW. Maternal, delivery, and perinatal characteristics associated with cryptorchidism: a population-based case-control study among births in Washington State. Epidemiology. 2002; 13(2):197–204. [DOI] [PubMed] [Google Scholar]
- 40. Berkowitz GS, Lapinski RH, Godbold JH, Dolgin SE, Holzman IR. Maternal and neonatal risk factors for cryptorchidism. Epidemiology. 1995; 6(2):127–31. [DOI] [PubMed] [Google Scholar]
- 41. Damgaard IN, Jensen TK, Nordic Cryptorchidism Study Group, Petersen JH, Skakkebaek NE, Toppari J, et al. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PLOS ONE. 2008; 3(8):e3051 10.1371/journal.pone.0003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akre O, Lipworth L, Cnattingius S, Sparén P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999; 10(4):364–9. [PubMed] [Google Scholar]
- 43. Beard CM, Melton LJ 3rd, O’Fallon WM, Noller KL, Benson RC. Cryptorchism and maternal estrogen exposure. Am J Epidemiol. 1984; 120(5):707–16. [DOI] [PubMed] [Google Scholar]
- 44. Preiksa RT, Zilaitiene B, Matulevicius V, Skakkebaek NE, Petersen JH, Jørgensen N, et al. Higher than expected prevalence of congenital cryptorchidism in Lithuania: a study of 1204 boys at birth and 1 year follow-up. Hum Reprod. 2005; 20(7):1928–32. [DOI] [PubMed] [Google Scholar]
- 45. Davies TW, Williams DR, Whitaker RH. Risk factors for undescended testis. Int J Epidemiol. 1996; 15(2):197–201. [DOI] [PubMed] [Google Scholar]
- 46. Shiono PH, Klebanoff MA, Berendes HW. Congenital malformations and maternal smoking during pregnancy. Teratology. 1986; 34(1):65–71. [DOI] [PubMed] [Google Scholar]
- 47. McBride ML, Van den Steen N, Lamb CW, Gallagher RP. Maternal and gestational factors in cryptorchidism. Int J Epidemiol. 1991; 20(4):964–70. [DOI] [PubMed] [Google Scholar]
- 48. Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. 2007; 115(10):1519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Main KM. PBDEs and Cryptorchidism: Main et al. Respond. Environmental Health Perspectives. 2008; 116(5): A194–5. 10.1289/ehp.11052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner-Mahler K, Kurzenne JY, Delattre I, Bérard E, Mas JC, Bornebush L, et al. Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. Int J Androl. 2011; 34(5 Pt 2): e499–510. 10.1111/j.1365-2605.2011.01211.x [DOI] [PubMed] [Google Scholar]
- 51. O’Leary CM, Nassar N, Kurinczuk JJ, Bower C. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG. 2009; 116(3):390–400. 10.1111/j.1471-0528.2008.02058.x [DOI] [PubMed] [Google Scholar]
- 52. Ritzén EM, Bergh A, Bjerknes R, Christiansen P, Cortes D, Haugen SE, et al. Nordic consensus on treatment of undescended testes. ActaPaediatr. 2007; 96:638–643. [DOI] [PubMed] [Google Scholar]
- 53. Hutson JM, Hasthorpe S, Heyns CF. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr Rev. 1997; 18:259–80. [DOI] [PubMed] [Google Scholar]
- 54. Khatwa UA, Menon PS. Management of undescended testis. Indian J Pediatr. 2000; 67:449–454. [DOI] [PubMed] [Google Scholar]
- 55. Hutson J, Hasthorpe S. Testicular descent and cryptorchidism: The state of the art in 2004. J Pediatr Surg. 2005; 40:297–302. [DOI] [PubMed] [Google Scholar]
- 56. Fraser A, McNally W, Sattar N, Anderson EL, Lashen H, Fleming R, et al. Prenatal exposures and anti-Mullerian hormone in female adolescents: the Avon Longitudinal Study of Parents and Children. Am J Epidemiol. 2013; 178(9):1414–23. 10.1093/aje/kwt137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fowler PA, Cassie S, Rhind SM, Brewer MJ, Collinson JM, Lea RG, et al. Maternal smoking during pregnancy specifically reduces human fetal desert hedgehog gene expression during testis development. J Clin Endocrinol Metab. 2008; 93(2):619–26. [DOI] [PubMed] [Google Scholar]
- 58. Kopp HP, Festa A, Krugluger W, Schernthaner G. Low levels of Sex Hormone-Binding Globulin predict insulin requirement in patients with gestational diabetes mellitus. Exp Clin Endocrinol Diabetes. 2001; 109:365–369. [DOI] [PubMed] [Google Scholar]
- 59. Thadhani R, Wolf M, Hsu-Blatman K, Sandler L, Nathan D, Ecker JL. First-trimester sex hormone binding globulin and subsequent gestational diabetes mellitus. Am J Obstet Gynecol. 2003; 189:171–176. [DOI] [PubMed] [Google Scholar]
- 60. Roach VJ, Fung H, Cockram CS, Lau TK, Rogers MS. Evaluation of glucose intolerance in pregnancy using biochemical markers of fetal hyperinsulinemia. Gynecol Obstet Invest. 1998; 45:174–176. [DOI] [PubMed] [Google Scholar]
- 61. Magee MS, Walden CE, Benedetti TJ, Knopp RH. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA. 1993; 269:609–615. [PubMed] [Google Scholar]
- 62. Simmons D. Interrelation between umbilical cord serum sex hormones, sex hormone-binding globulin, insulin-like growth factor I, and insulin in neonates from normal pregnancies and pregnancies complicated by diabetes. J Clin Endocrinol Metab. 1995; 80:2217–2221. [DOI] [PubMed] [Google Scholar]
- 63. Gaspari L, Paris F, Jandel C, Kalfa N, Orsini M, Daurès JP, et al. Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: a nested case-control study. Hum Reprod. 2011; 26(11):3155–62. 10.1093/humrep/der283 [DOI] [PubMed] [Google Scholar]
- 64.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 2014 Feb 18.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOC)
Data Availability Statement
All relevant data are included within the paper and its Supporting Information files.