Abstract
Adjunctive dexamethasone saves lives in the treatment of tuberculous meningitis but this response is influenced by the patient’s LTA4H genotype. Despite less certain benefit, adjunctive dexamethasone is also frequently used in the treatment of pyogenic bacterial meningitis, but the influence of LTA4H genotype on outcomes has not been previously investigated. We genotyped the LTA4H promoter region SNP (rs17525495) in 390 bacterial meningitis patients and 751 population controls. rs17525495 was associated with susceptibility to bacteriologically confirmed bacterial meningitis (P = 0.01, OR 1.27 95% confidence interval [CI] 1.05–1.54) but did not influence clinical presentation, disease severity or survival following dexamethasone treatment.
Introduction
Vaccination against some of the causative agents of bacterial meningitis has reduced the burden of this disease in high-income countries [1]. Despite these advances in prevention, the burden of this life-threatening disease remains highest in resource-limited countries resulting in approximately 1.2 million cases of bacterial meningitis globally every year. To reduce the high mortality and morbidity of bacterial meningitis in developing countries, rapid diagnosis and initiation of effective antibiotics is crucial to avoid the devastating consequences of delayed treatment. Equally, strategies to control the inflammatory response in the subarachnoid space may also reduce mortality and decrease the rate of neurological sequelae that occurs in approximately 50% of survivors [2].
The treatment of bacterial meningitis has been the subject of a number of randomised controlled trials (RCT). The interventions investigated have included continuous antibiotic infusion, the optimal duration of antibiotic therapy, and adjunctive treatments such as glycerol and dexamethasone [3,4]. A RCT of adjunctive dexamethasone in Vietnam found dexamethasone was associated with increased survival, but only in patients with microbiologically confirmed bacterial meningitis [5]. A more recent meta-analysis of data from all published RCTs concluded that adjunctive corticosteroids reduces hearing loss and neurological sequelae following bacterial meningitis, but probably has no effect on mortality [4].
Leukotriene A4 hydrolase, encoded by LTA4H, catalyses the production of leukotriene B4 (LTB4), an eicosanoid that has potent chemoattractant and pro-inflammatory properties [6]. Recently we, and others reported that a SNP rs17525495 in the LTA4H promoter, regulating LTA4H transcription, determined inflammatory cell recruitment, patient survival and response to dexamethasone treatment in Vietnamese adults with tuberculous meningitis (TBM) [7]. Tobin et al [7,8] additionally showed that the balance of pro- and anti-inflammatory pathways (LTB4 and lipoxin A4, respectively), regulated by LTA4H, played a significant role in controlling bacterial growth and susceptibility to mycobacterial infections. In TBM patients receiving adjunctive dexamethasone, only individuals with the high expression LTA4H genotype, and therefore excess TNF and inflammation, benefitted from anti-inflammatory therapy [7,9]. This was one of the first examples where host genotype-specific therapies could influence survival from an infectious disease. Since the inflammatory network regulated by LTA4H may be fundamental to the host inflammatory response to diverse infections, we hypothesized that outcome of dexamethasone treatment in bacterial meningitis is dependent on LTA4H genotype.
Methods
Study subjects
Patients with suspected bacterial meningitis (N = 435) were recruited into a randomised controlled trial of adjunctive dexamethasone treatment in Ho Chi Minh City (HCMC), Vietnam [5]. Study patients were >14 years old, had clinical evidence of meningitis (headache, fever, vomiting and neck stiffness) and at least one of the following; bacteria in cerebrospinal fluid (CSF) detected by Gram’s or acridine orange stain; positive CSF fluid latex agglutination; pathogenic bacteria cultured from blood or CSF; or a clinical history of illness >7 days, with cloudy CSF, >60% neutrophils by white cell count, and CSF to blood glucose ratio <50%. Patients were randomly assigned to receive intravenous dexamethasone sodium phosphate, 0.4 mg per kg body weight, every 12 hours for 4 days, or placebo. The study medication was given 15 minutes before the administration of antibiotics, although some patients may have had prior antibiotic treatment. Upon discharge, patients were classified “definite bacterial meningitis” if bacteria were detected by CSF stain or CSF blood culture. “Probable meningitis” was defined as patients that were not microbiologically confirmed with no alternative diagnosis.
The population control individuals (N = 751) were cord blood control samples collected from babies born in 2003 at Hung Vuong Obstetric Hospital, HCMC, Vietnam, as described previously [10]
Verbal consent was obtained from all subjects with bacterial meningitis, or their parent/guardian/relative in the case of severe cases where the patient was unable to consider consent independently, i.e. unconscious. Obtaining verbal informed consent was confirmed by the assignment of a study identifier and completion of the study enrollment form. This consent procedure reflected current practise at the Hospital for Tropical Diseases and in Viet Nam during the study period (1996–2005). In Viet Nam, patients over 14 years of age are considered adults and treated within the adult care system. These patients have the autonomy to give independent consent for medical procedures, and for clinical research when approved by an Ethics Committee. This consent process, including recruitment of patients over 15 years of age to give independent consent to participate in this trial, was approved by the Scientific and Ethical Committee of the Hospital for Tropical Diseases, Ho Chi Minh City, in line with the standard health care system. The original clinical study describing these patients and detailing this approved consent process was published in 2007[5].
For cord blood control samples, written informed consent was obtained from the mother. Ethical approval for cord blood control collection was granted by the scientific and ethical committees at the Hospital for Tropical Diseases, Ho Chi Minh City and Hung Vuong Hospital, Ho Chi Minh City. The clinical study was also approved by the Oxford Tropical Research Ethics Committee, United Kingdom.
DNA extraction and quantification
Genomic DNA from bacterial meningitis and cord blood controls was extracted from between 1–5ml of venous blood collected in tubes containing EDTA anti-coagulant. DNA was extracted by using the Nucleon BACC2 Genomic DNA extraction kits (GE Healthcare) or the blood maxi kit from Qiagen (Lewes, UK). DNA concentration was determined by Nanodrop.
Genotyping
rs17525495 was genotyped by Taqman using a pre-designed assay kits (Applied Biosystems) according to the suppliers instructions (http://tools.lifetechnologies.com/content/sfs/manuals/TaqMan_SNP_Genotyping_Assays_man.pdf). This was performed using a LightCycler 480 Probes Master kit on the LightCycler 480 real-time PCR system (Roche) according to the suppliers protocol (https://pim-eservices.roche.com/LifeScience/Document/4dd0e207-97ed-e311-98a1-00215a9b0ba8).
Clinical severity and inflammation indices
All clinical data were obtained from the previously published study [5]. CSF concentrations of TNF-α were measured in a sub-group of patients with microbiologically confirmed bacterial meningitis patients, as has been reported previously [11].
Data Analysis
Genotypic deviations from Hardy-Weinberg equilibrium (HWE) were assessed using a chi-square statistical test. Univariate analysis was performed for categorical variables with Pearson’s χ 2 test to assess associations between disease phenotype and allele or genotype frequencies. Logistic regression of the case control association analysis allowed the odds ratios for the genotypes to be estimated. In this approach we modelled the SNP of interest assuming several related genotypic mechanisms (additive, recessive and general models). For more details of the general model, see Dunstan et al [10]. No corrections for multiple testing were performed.
When investigating severity and inflammation indices of bacterial meningitis patients by genotype, categorical variables were summarised by absolute count (%) and compared by the χ2 test, whereas continuous data were summarised by median (IQR) and compared by the Kruskall-Wallis test. One-month mortality among patients not treated and treated with dexamethasone adjunctive therapy, and stratified by rs17525495 genotype, were summarized with Kaplan-Meier curves. Comparisons between genotypes were based on Cox regression models adjusted for the randomized treatment group; the hypothesis that the effect of dexamethasone on mortality depends on the genotype was assessed with an interaction test. In addition to one-month mortality, we also analyzed the composite endpoint of death or severe neurological sequelae or bilateral severe deafness at 1 month [4] using logistic regression. All analyses were performed using the statistical package R v3.0.1 [12].
Results and Discussion
Clinical characteristics of cohort
435 Vietnamese adults with suspected bacterial meningitis were randomly allocated adjunctive dexamethasone or placebo in addition to standard antimicrobial therapy [5]. 423 were subsequently diagnosed as having either microbiologically confirmed bacterial meningitis (n = 300; 69%) or probable bacterial meningitis (n = 123; 28%) [5]. Only one of these patients was infected with HIV (419 tested). 12 adults (3%) had an alternative diagnosis and were excluded from the current study. In addition, DNA or genotype data was unavailable from 33 patients leaving 390 patients to be analysed in the genetics study. These patients were adults with a median (interquartile range [IQR]) age of 42 (29–55) years, of which 106 (27%) were female. Within the control group 49% of subjects were female. Additional molecular diagnostics implemented after the initial RCT resulted in 312 (80%) patients with a confirmed diagnosis of bacterial meningitis and the remaining 78 (20%) had “probable meningitis”. The most commonly identified bacterial agent in the patients with microbiologically confirmed bacterial meningitis was Streptococcus suis [123/312 (39%)] [5]. Other agents identified were Streptococcus pneumoniae [75/312 (24%)], Neisseria meningitidis [26/312 (8%)], Haemophilus influenzae [8/312 (3%)], aerobic Gram negative bacilli [18/312 (6%)] and others [62/312 (20%) which comprised a mix of Streptococcus spp., Staphylococcus aureus, coagulase-negative staphylococcus, Klebsiella spp., Escherichia coli and other gram negative bacteria] [5].
The effect of LTA4H genotype on susceptibility to bacterial meningitis
We genotyped the LTA4H promoter SNP rs17525495, which has been previously associated with TBM, in 390 bacterial meningitis cases and 751 population controls. rs17525495 was in Hardy Weinberg Equilibrium in controls (P = 0.57) The frequency of the minor allele in Vietnamese controls was 0.33, whereas it is 0.04 in Caucasians (CEU), 0.29 in Chinese and Japanese (CHB+JPT) and 0.12 in Nigerians (YRI) (http://www.ncbi.nlm.nih.gov/SNP/). Table 1 shows that rs17525495 was associated with susceptibility to bacterial meningitis, both in patients with microbiologically confirmed bacterial meningitis and in those in whom the diagnosis was likely but unproven. In the additive model, rs17525495 was associated with bacteriologically confirmed bacterial meningitis (P = 0.01, OR 1.27, 95% confidence interval [CI] 1.05–1.54), with the recessive model of inheritance showing an increased risk of bacterial meningitis for TT homozygotes (P = 0.008, OR 1.65, 95% CI 1.15–2.39). rs17525495 was not significantly associated with BM under the heterozygote advantage model (P = 0.91, OR 0.99, 95% CI 0.77–1.26; data not shown).
Table 1. rs17525495 is associated with susceptibility to bacterial meningitis.
| subject | allele | genotype | freq | P | P | P | HWE j | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| group | freq a C b | CC/CT c /TT | CC/CT/TT | (add d ) | OR (95% CI) e | (gen f ) | OR (95% CI) g | OR (95% CI) h | (rec i ) | OR (95% CI) | control P |
| control | 0.67 | 339/326/86 | 0.45/0.43/0.11 | 0.57 | |||||||
| BM all | 0.61 | 155/168/67 | 0.40/0.43/0.17 | 0.01 | 1.26 (1.06–1.50) | 0.02 | 1.13(0.86–1.47) | 1.70 (1.18–2.47) | 0.008 | 1.60 (1.13–2.27) | 0.07 |
| BM def k | 0.61 | 124/133/55 | 0.40/0.43/0.18 | 0.01 | 1.27 (1.05–1.54) | 0.02 | 1.12(0.84–1.49) | 1.75 (1.18–2.60) | 0.008 | 1.65 (1.15–2.39) | 0.07 |
afrequency
bcytosine
cthymine
dadditive model
eodds ratio (95% confidence interval)
fgeneral model; a genotypic model where one genotype group (e.g. CC group) is baseline
gestimates of odds for general model, comparing baseline (CC) to CT
hestimates of odds for general model comparing baseline (CC) to TT
irecessive model
jHardy Weinberg Equilibrium
kdefinite. NB: data for dominant and heterozygote advantage model were not significant and are not shown here.
Studies in zebra fish and humans with mycobacterial infection have suggested a human model of heterozygous advantage, with increased disease susceptibility experienced by those with either minor or major allele homozygosity [8]. Our current findings suggest only the TT homozygotes, with a presumed hyper-inflammatory phenotype, have increased susceptibility to bacterial meningitis. To date, mechanisms of host genetic susceptibility to the commonest causes of bacterial meningitis (S. pneumoniae and N. meningitidis) have generally evoked a failure of immune response. For example, there is a well-documented association between genetically determined deficiencies in the terminal complement components (C5–9) and meningococcal meningitis [13], and deficiencies in the soluble pattern recognition molecule mannose binding lectin have recently been associated with increased susceptibility to bacterial meningitis, especially pneumococcal meningitis [14]. Therefore, the association between an LTA4H hyper-inflammatory phenotype and the development of bacterial meningitis may represent a novel mechanism of susceptibility which needs further investigation in other pyogenic bacterial infections and confirmation in different populations.
The effect of LTA4H genotype on presentation and response to treatment of bacterial meningitis
To determine if LTA4H genotype had any effect on the clinical parameters of bacterial meningitis we investigated baseline markers of clinical severity and the CSF inflammatory indices of the patients, stratified by rs17525495 genotype. LTA4H genotype was not associated with any significant difference in disease severity before the start of treatment, or difference in CSF inflammatory indices, regardless of whether the diagnosis was confirmed or probable (Table 2).
Table 2. Clinical severity and CSF inflammation indices of bacterial meningitis patients by rs17525495 genotype on study recruitment.
| total | TT | TT Summary | CT | CT Summary | CC | CC Summary | Test | |
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | n | statistic a | n | statistic | n | statistic | statistic b |
| all BM | ||||||||
| Glascow coma score | 390 | 67 | 13 (10–15) | 168 | 13 (9.75–15) | 154 | 13 (9.35–15) | 0.99 |
| coma group C | 390 | |||||||
| - normal | 67 | 22 (33) | 168 | 58 (35) | 154 | 54 (35) | 0.91 | |
| - mild impairment | 23 (34) | 62 (37) | 49 (32) | |||||
| - moderate impairment | 14 (21) | 34 (20) | 32 (21) | |||||
| - severe impairment | 8 (12) | 14 (8) | 19 (12) | |||||
| opening pressure (cmHgCSF) | 306 | 48 | 21 (14–43) | 137 | 22 (16–50) | 121 | 25 (14–46) | 0.75 |
| CSF white cell count / mm3 | 389 | 66 | 4505 (2025–9042.5) | 168 | 2500 (1095–6788.5) | 155 | 3280 (825–8600) | 0.13 |
| CSF Neutrophil percentage | 386 | 66 | 90.5 (78.25–95) | 166 | 85 (77–92) | 154 | 90 (78–94) | 0.07 |
| CSF total number of Neutrophil / mm3 | 386 | 66 | 3733.3 (1571.5–7635) | 166 | 2184.7 (922.2–5764.5) | 154 | 2819.2 (666.9–7530.2) | 0.19 |
| Proportion of glucose in the CSF | 387 | 67 | 17 (8.5–34.5) | 166 | 25.5 (12–44.75) | 154 | 27 (11–46) | 0.13 |
| Albumin in CSF (mg/dl) | 382 | 66 | 265 (170–400) | 163 | 239 (143.5–406) | 153 | 228 (147–385) | 0.47 |
| definite BM | ||||||||
| Glascow coma score | 312 | 55 | 13 (9–15) | 133 | 13 (9–15) | 124 | 13 (9–15) | 0.8134 |
| coma group | 312 | |||||||
| - normal | 55 | 16 (29) | 133 | 44 (33) | 124 | 40 (32) | 0.80 | |
| - mild impairment | 19 (35) | 50 (38) | 40 (32) | |||||
| - moderate impairment | 12 (22) | 29 (22) | 29 (23) | |||||
| - severe impairment | 8 (15) | 10 (8) | 15 (12) | |||||
| opening pressure (cmHgCSF) | 245 | 38 | 25 (14.2–49.7) | 109 | 22 (17–50) | 98 | 25 (16.2–46) | 0.99 |
| CSF white cell count / mm3 | 311 | 54 | 4255 (1880–8952.5) | 133 | 2620 (1100–6790) | 124 | 3625 (847.5–9060.5) | 0.31 |
| CSF Neutrophil percentage | 308 | 54 | 91 (79.25–97.75) | 131 | 87 (78.5–93) | 123 | 90 (78–95) | 0.09 |
| CSF total number of Neutrophil / mm3 | 308 | 54 | 3530.1 (1571.5–7635) | 131 | 2352 (927–6054.5) | 123 | 3465 (685.4–8087.8) | 0.37 |
| Proportion of glucose in the CSF | 310 | 55 | 19 (9.5–34.5) | 132 | 26.5 (10–46) | 123 | 23 (9–46) | 0.13 |
| Albumin in CSF (mg/dl) | 305 | 54 | 270 (170–396.5) | 128 | 265 (148.5–428.5) | 123 | 248 (160.5–430) | 0.93 |
| TNF-alpha in CSF (pg/ml) | 126 | 23 | 812.95 (42.44–3550.55) | 52 | 91.12 (30.28–897.32) | 51 | 205.64 (37.68–3312.95) | 0.15 |
aSummary statistic is absolute count (%) for categorical variables and median (IQR) for continuous data
btest statistic for categorical variables is the χ 2 test (6 df), and for continuous variables is the Kruskall-Wallis test
C Glasgow coma score groups were defined as ‘normal’ if the score was 15/15, ‘mild impairment’ if the score was 13 or 14, ‘moderate impairment’ if the score was 11 or 12, and ‘severe impairment’ if the score was <11.
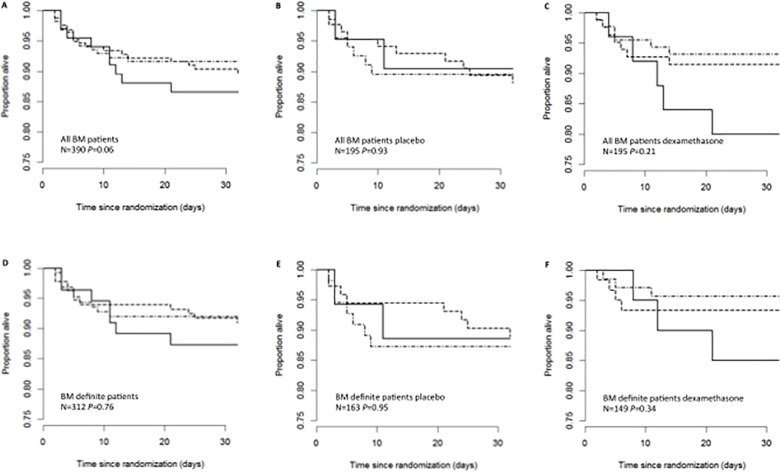
Thirty-nine patients died during the one month of follow-up. Survival curves stratified by rs17525495 genotype are displayed in Fig. 1 and corresponding Cox regression outputs are in S1 Table. No significant differences in survival between the three genotypes groups were found (all P>0.20). Moreover, there was no evidence that response to dexamethasone differed by genotype (interaction tests: P = 0.32 (all BM) and P = 0.41(definite BM)). There was also no evidence for an effect of genotype on the composite endpoint of death or severe neurological sequelae or bilateral severe deafness at 1 month. (S2 Table)
Fig 1. LTA4H genotypes, dexamethasone and survival in bacterial meningitis patients.
Kaplan-Meier estimates of survival among BM patients stratified by their rs17525495 genotype in, (A) all BM patients (bacterially confirmed and probable), (B) all BM patients who received placebo, (C) all BM patients who received dexamethasone (D) definite BM patients (bacterially confirmed), (E) definite BM patients who received placebo and (F) definite BM patients who received dexamethasone. Solid line denotes TT, dash line denotes CT, and dot-dash line denotes CC genotype group. Comparisons of survival between genotypes were based on Cox regression models adjusted for the randomized treatment group
Here, we show that only TT homozygous individuals, with a predicted pro-inflammatory phenotype, are more susceptible to bacterial meningitis, but, unlike TBM, we found no evidence that LTA4H genotype influences response to treatment with dexamethasone. There may be a number of explanations for this observation, including differences in the pathophysiology of tuberculous and bacterial meningitis and differences in the duration of dexamethasone therapy given for each disease (4 days for bacterial meningitis versus 8 weeks for TBM). Also, adjunctive dexamethasone improves survival from TBM [13] but not bacterial meningitis [4], suggesting dexamethasone acts on different, and disease-specific, inflammatory pathways. Furthermore, a limitation of the study is that the observed number of deaths was low, thus power to detect differences are limited. Additionally, it is possible that the pathogenesis and mechanisms of bacterial meningitis caused by different pathogens (ie. S.suis and S.pneumoniae) could differ and affect our observed results. It is a further limitation of our study that we were unable to perform analysis by infecting organism due to small sample sizes and reduced power to detect associations. As S.suis only rarely causes bacterial meningitis in other settings our results should also be interpreted with caution in other countries.
Comparison of the clinical presentation of tuberculous and bacterial meningitis illustrates some of the key differences in pathophysiology. TBM presents with many days or even weeks of symptoms and the inflammation in the CSF is characterised by a moderate increase in white cells, the majority of which are mononuclear [13]. In contrast, pyogenic bacterial meningitis present acutely, with hours rather than days of symptoms, and with large numbers of neutrophils in both peripheral blood and CSF [15]. These clinical differences point to fundamental differences in the host response to infection with the respective pathogens. Macrophages are the cells primarily infected by M. tuberculosis, but they are also responsible for its control through the development of granuloma. In contrast, neutrophils are the key effector cells in pyogenic bacterial infections. Tobin et al showed that the LTA4H genotype influences macrophage response to mycobacterial infection [8]. Neutrophil recruitment may be influenced by LTA4H genotype by diverting eicosanoid synthesis to production of either the pro-inflammatory, neutrophil chemoattractant, LTB4, or the anti-inflammatory lipoxin A4. But in the model studied, these responses were driven by initial macrophage infection and there is no basis for believing a pyogenic bacterial infection would engage the same pathways. Lastly, it is possible that the failure to observe any effect of dexamethasone on survival from bacterial meningitis, or any interaction of its effect with LTA4H genotype, may be because it is only given for 4 days. This explanation seems less likely, given data from animal models have suggested early initial control of the inflammatory response by corticosteroids is sufficient to alter disease pathophysiology and improve outcome from bacterial meningitis [16].
LTA4H genotype is associated with susceptibility to bacterial meningitis in the Vietnamese. However we have no evidence to indicate LTA4H genotype is a critical determinant of bacterial meningitis outcome in the response to dexamethasone therapy.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
We would like to thank all the Vietnamese individuals who agreed to provide samples for this study. We acknowledge the work of the clinical staff from the Hospital of Tropical Diseases, HCMC, Viet Nam, who initially diagnosed and studied the patients with bacterial meningitis. We would like to thank the clinical staff from Hung Vuong Obstetric Hospital for the collection of the cord blood controls.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded through the Wellcome Trust Major Overseas Program in Vietnam (089276/Z/09/Z) to JJF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McIntyre PB, O'Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012; 380: 1703–1711. 10.1016/S0140-6736(12)61187-8 [DOI] [PubMed] [Google Scholar]
- 2. Kasanmoentalib ES, Brouwer MC, van de Beek D. Update on bacterial meningitis: epidemiology, trials and genetic association studies. Current opinion in neurology. 2013; 26: 282–288. 10.1097/WCO.0b013e328360415c [DOI] [PubMed] [Google Scholar]
- 3. Molyneux E, Nizami SQ, Saha S, Huu KT, Azam M, Bhutta ZA, et al. 5 versus 10 days of treatment with ceftriaxone for bacterial meningitis in children: a double-blind randomised equivalence study. Lancet. 2011; 377: 1837–1845. 10.1016/S0140-6736(11)60580-1 [DOI] [PubMed] [Google Scholar]
- 4. van de Beek D, Farrar JJ, de Gans J, Mai NT, Molyneux EM, Peltola H, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet neurology. 2010; 9: 254–263. 10.1016/S1474-4422(10)70023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen TH, Tran TH, Thwaites G, Ly VC, Dinh XS, Ho Dang TN, et al. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. The New England journal of medicine. 2007; 357: 2431–2440. [DOI] [PubMed] [Google Scholar]
- 6. Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annual review of immunology. 2007; 25: 101–137. [DOI] [PubMed] [Google Scholar]
- 7. Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012; 148: 434–446. 10.1016/j.cell.2011.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tobin DM, Vary JC Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010; 140: 717–730. 10.1016/j.cell.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. The New England journal of medicine. 2004; 351: 1741–1751. [DOI] [PubMed] [Google Scholar]
- 10. Dunstan SJ, Rockett KA, Quyen NT, Teo YY, Thai CQ, Hang NT, et al. Variation in human genes encoding adhesion and proinflammatory molecules are associated with severe malaria in the Vietnamese. Genes and immunity. 2012; 13: 503–508. 10.1038/gene.2012.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mai NT, Tuan TV, Wolbers M, Hoang DM, Nga TV, Chau TT, et al. Immunological and biochemical correlates of adjunctive dexamethasone in Vietnamese adults with bacterial meningitis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009; 49: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team. R: A language and environment for statistical computing; R Project for Statistical Computing website. Available: http://www.R-project.org/ Accessed 27 Jan 2014.
- 13. Brouwer MC, de Gans J, Heckenberg SG, Zwinderman AH, van der Poll T, van de Beek D. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. The Lancet infectious diseases. 2009; 9: 31–44. 10.1016/S1473-3099(08)70261-5 [DOI] [PubMed] [Google Scholar]
- 14. Brouwer MC, Baas F, van der Ende A, van de Beek D. Genetic variation and cerebrospinal fluid levels of mannose binding lectin in pneumococcal meningitis patients. PloS one. 2013; 8: e65151 10.1371/journal.pone.0065151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thwaites GE, Chau TT, Stepniewska K, Phu NH, Chuong LV, Sinh DX, et al. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet. 2002; 360: 1287–1292. [DOI] [PubMed] [Google Scholar]
- 16. Lutsar I, Friedland IR, Jafri HS, Wubbel L, Ahmed A, Trujillo M, et al. Factors influencing the anti-inflammatory effect of dexamethasone therapy in experimental pneumococcal meningitis. The Journal of antimicrobial chemotherapy. 2003; 52: 651–655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.