Abstract
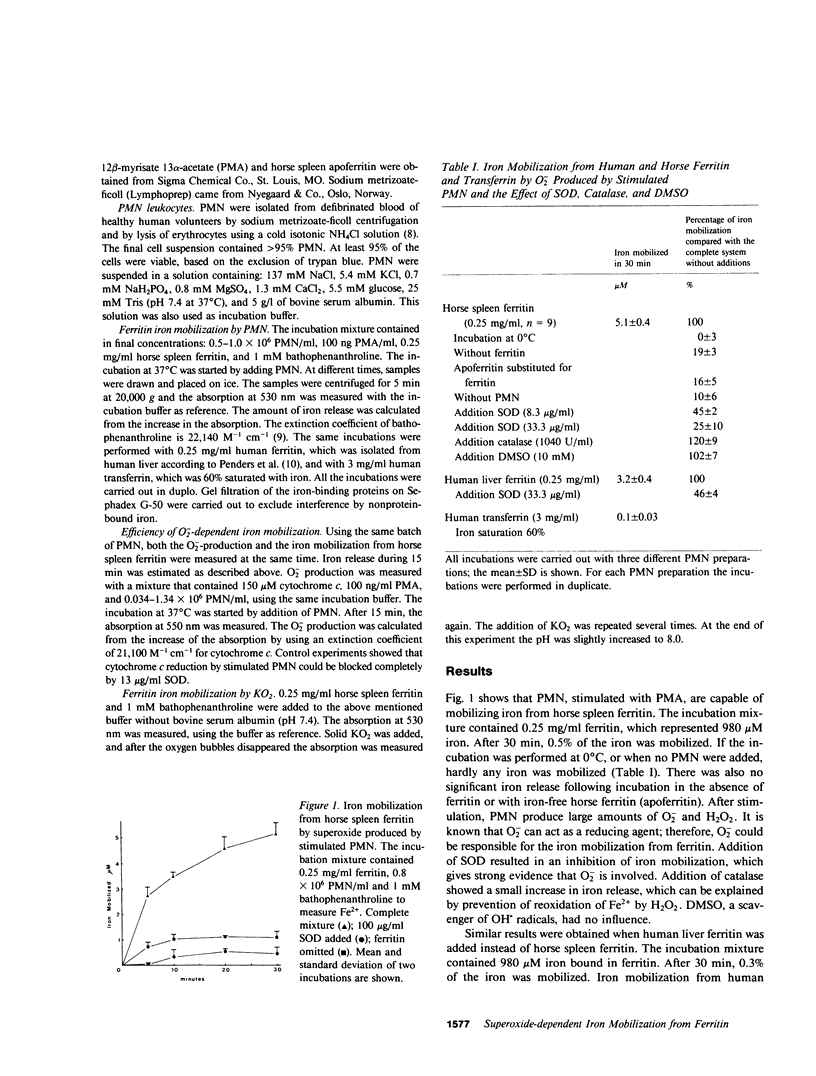
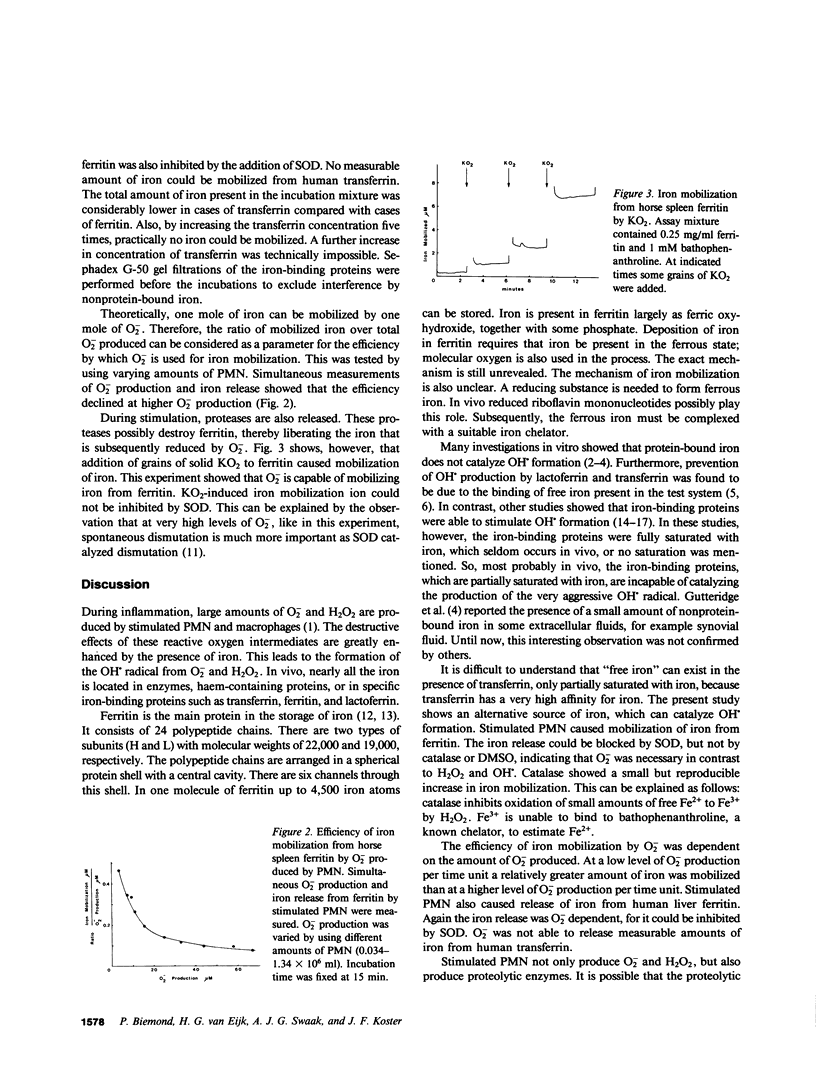
During inflammation, the superoxide anion (O-2) and hydrogen peroxide (H2O2) are produced by stimulated polymorphonuclear leukocytes and macrophages. The toxic effects of these reactive oxygen intermediates increase when traces of iron are present, because iron catalyzes the formation of the hydroxyl radical (OH.). Partially saturated iron-binding proteins, such as transferrin and ferritin, are unable to catalyze OH. formation in vitro. Mobilization of iron from these proteins is necessary for iron stimulation of OH. formation. This paper reports that stimulated polymorphonuclear leukocytes mobilize iron from human and horse ferritin, but not from human transferrin. Iron release from ferritin depends on O-2 because it can be prevented by the addition of superoxide dismutase. Catalase and dimethylsulfoxide have no inhibitory effect on iron mobilization. The efficiency of the iron release increases at low levels of O-2 production. Only O-2 produced by granulocytes is sufficient for iron mobilization, because solid potassium superoxide is also able to release iron from ferritin. We propose that this reaction may potentiate the formation of the OH. radical in inflammatory states.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Thornalley P. J. Enhanced production of hydroxyl radicals by the xanthine-xanthine oxidase reaction in the presence of lactoferrin. Biochim Biophys Acta. 1982 Mar 15;715(1):116–120. doi: 10.1016/0304-4165(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Bacon P. A., Eastham E. J., Brigham K. Synovial fluid ferritin in rheumatoid arthritis. Br Med J. 1980 Sep 13;281(6242):715–716. doi: 10.1136/bmj.281.6242.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. Superoxide radical and superoxide dismutases: threat and defense. Acta Physiol Scand Suppl. 1980;492:9–18. [PubMed] [Google Scholar]
- Crichton R. R. Structure and function of ferritin. Angew Chem Int Ed Engl. 1973;12(1):57–65. doi: 10.1002/anie.197300571. [DOI] [PubMed] [Google Scholar]
- Duggan D. E., Streeter K. B. Inhibition of ferritin reduction by pyrazolo(3,4d)pyrimidines. Arch Biochem Biophys. 1973 May;156(1):66–70. doi: 10.1016/0003-9861(73)90341-x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Paterson S. K., Segal A. W., Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981 Oct 1;199(1):259–261. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Jones T., Spencer R., Walsh C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogues. Biochemistry. 1978 Sep 19;17(19):4011–4017. doi: 10.1021/bi00612a021. [DOI] [PubMed] [Google Scholar]
- MAZUR A., GREEN S., SAHA A., CARLETON A. Mechanism of release of ferritin iron in vivo by xanthine oxidase. J Clin Invest. 1958 Dec;37(12):1809–1817. doi: 10.1172/JCI103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Motohashi N., Mori I. Superoxide-dependent formation of hydroxyl radical catalyzed by transferrin. FEBS Lett. 1983 Jun 27;157(1):197–199. doi: 10.1016/0014-5793(83)81144-2. [DOI] [PubMed] [Google Scholar]
- Muirden K. D. Ferritin in synovial cells in patients with rheumatoid arthritis. Ann Rheum Dis. 1966 Sep;25(5):387–401. doi: 10.1136/ard.25.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders T. J., de Rooij-Dijk H. H., Leijnse B. Rapid isolation of ferritin by means of ultracentrifugation. Biochim Biophys Acta. 1968 Dec 3;168(3):588–590. doi: 10.1016/0005-2795(68)90198-0. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med. 1974 Sep;47(3):223–233. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Winterbourn C. C. Hydroxyl radical production in body fluids. Roles of metal ions, ascorbate and superoxide. Biochem J. 1981 Jul 15;198(1):125–131. doi: 10.1042/bj1980125. [DOI] [PMC free article] [PubMed] [Google Scholar]


