Abstract
The hyperparathyroidism characteristic of patients with moderate renal insufficiency could be caused by decreases in the plasma concentration of ionized calcium (Ca++) evoked by: (a) recurring increases in the plasma concentration of inorganic phosphorus that may be detectable only in the post-prandial period; (b) a reversible, phosphorus-mediated suppression of renal 25-hydroxyvitamin D-1 alpha-hydroxylase that decreases the plasma concentration of 1,25-dihydroxyvitamin D (1,25-(OH)2D) enough to decrease both gut absorption and bone resorption of Ca++; (c) both of these. In a group of eight children with moderate renal insufficiency, mean glomerular filtration rate (GFR) 45 +/- 4 (SE) ml/min per 1.73 M2, ages 6-17 yr, we tested these hypotheses by determining the effect of short term (5 d) restriction and supplementation of dietary intake of phosphorus on the plasma concentration of 1,25-(OH)2D, the serum concentrations of immunoreactive parathyroid hormone (iPTH) and phosphorus, and the fractional renal excretion of phosphorus ( FEPi ). When dietary phosphorus was normal, 1.2 g/d, the serum concentrations of phosphorus throughout the day were not greater than those of normal control children, and the serum concentrations of carboxyl-terminal iPTH (C-iPTH) were greater, 59 +/- 9 vs. 17 +/- 3 mu leq/ml, and unchanging; the serum concentration of intact-iPTH was also greater, 198 +/- 14 vs. 119 +/- 8 pg/ml. The plasma concentration of 1,25-(OH)2D was lower than that of age-matched controls, 27 +/- 3 vs. 36 +/- 2 pg/ml (P less than 0.01). When dietary phosphorus was restricted to 0.35 g/d, the plasma concentration of 1,25-(OH)2D increased by 60% to a mean value not different from that of normal controls, while serum concentrations of C-iPTH and intact-iPTH decreased by 25%, the latter concentration to a mean value not different from that of controls. FEPi decreased from 31 to 9%. When dietary phosphorus was supplemented to 2.4 g/d, the plasma concentration of 1,25-(OH)2D decreased 32%, while those of C-iPTH and intact-iPTH increased by 131 and 45%, respectively; FEPi increased from 27 to 53%. Plasma concentrations of 25-hydroxyvitamin D remained normal and unchanged, and GFR did not change when dietary phosphorus was manipulated. The data demonstrate that in children with moderate renal insufficiency: (a) A normal dietary intake of phosphorus in attended by a decreased circulating concentration of 1,25-(OH)2D and an increased concentration of iPTH, but not by recurring increases in the serum concentration of phosphorus at any time of the day; (b) Dietary phosphorus is, however, a major determinant of the circulating concentrations of both 1,25-(OH)2D and iPTH, which vary inversely and directly, respectively, with dietary intake of phosphorus, and increase and decrease, respectively, to normal values when phosphorus is restricted for 5 d; (c) Restriction and supplementation of dietary phosphorus induces changes in the serum concentration of iPTH that correlate strongly but inversely with those induced in the plasma concentration of 1,25-(OH)2D (r = -0.88, P < 0.001); and (d) The physiologic responsiveness of the renal tubule to changes in dietary phosphorus is to a substantial extent intact. The data provide support for the second hypothesis stated.
Full text
PDF

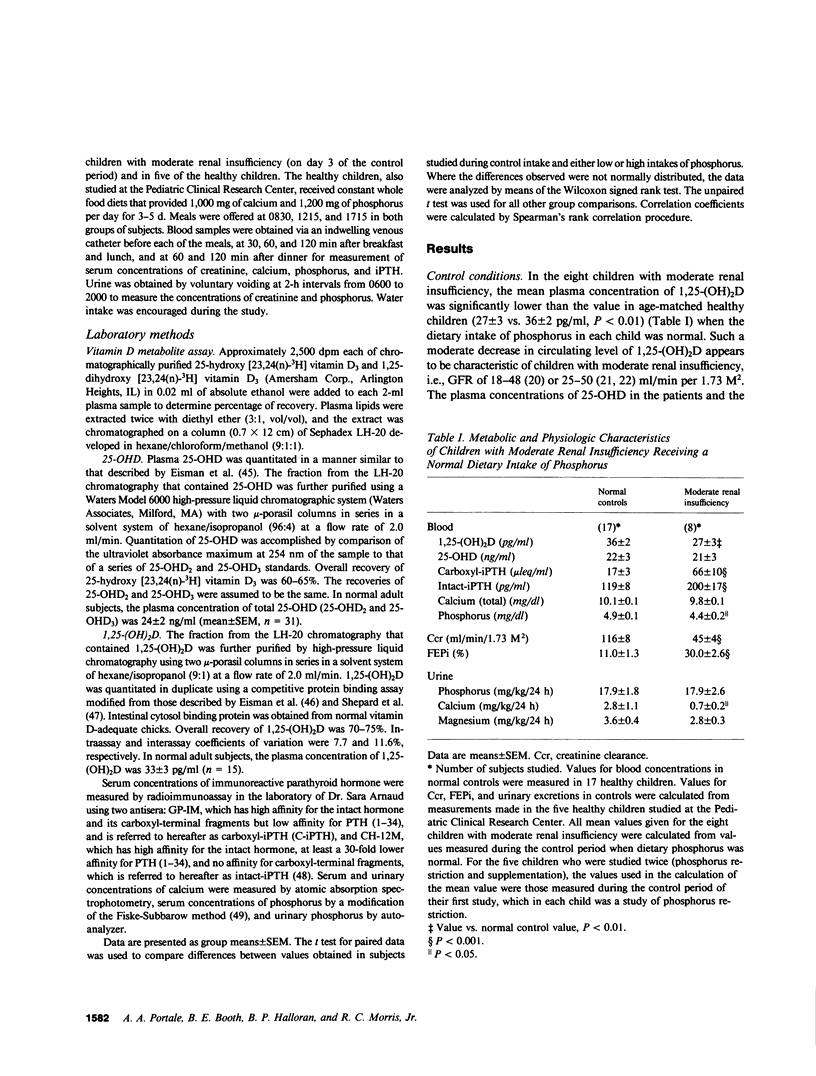
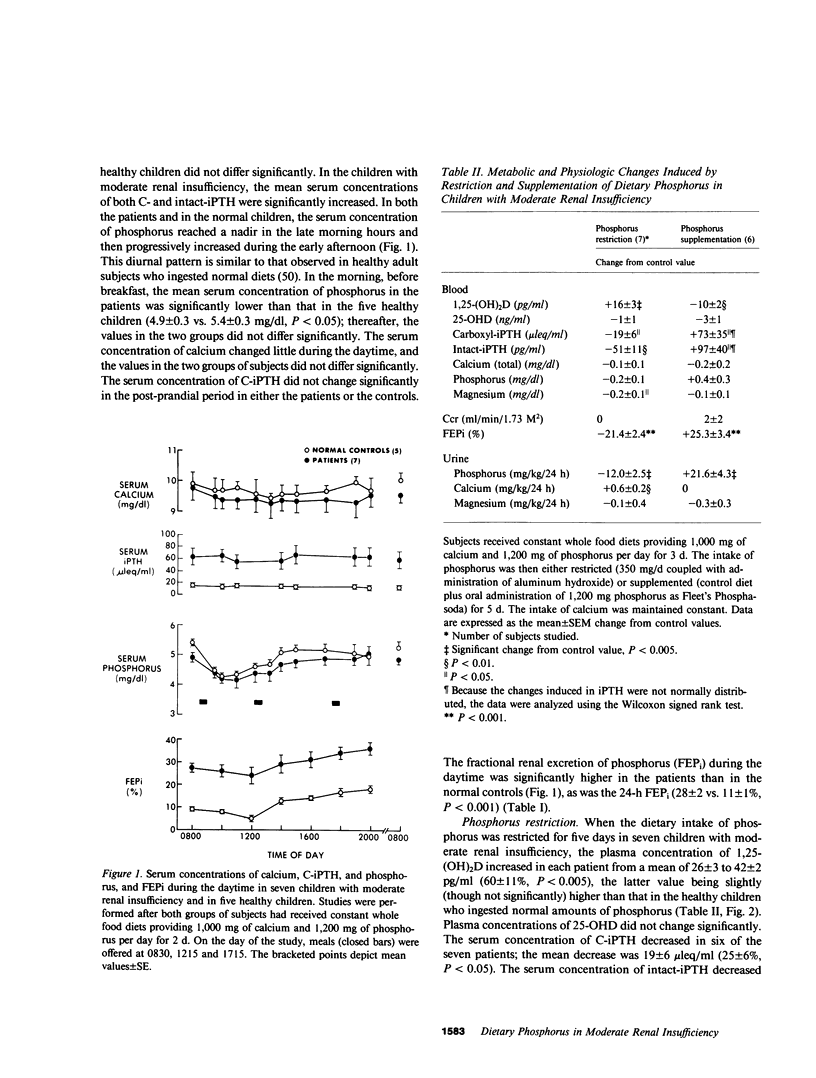
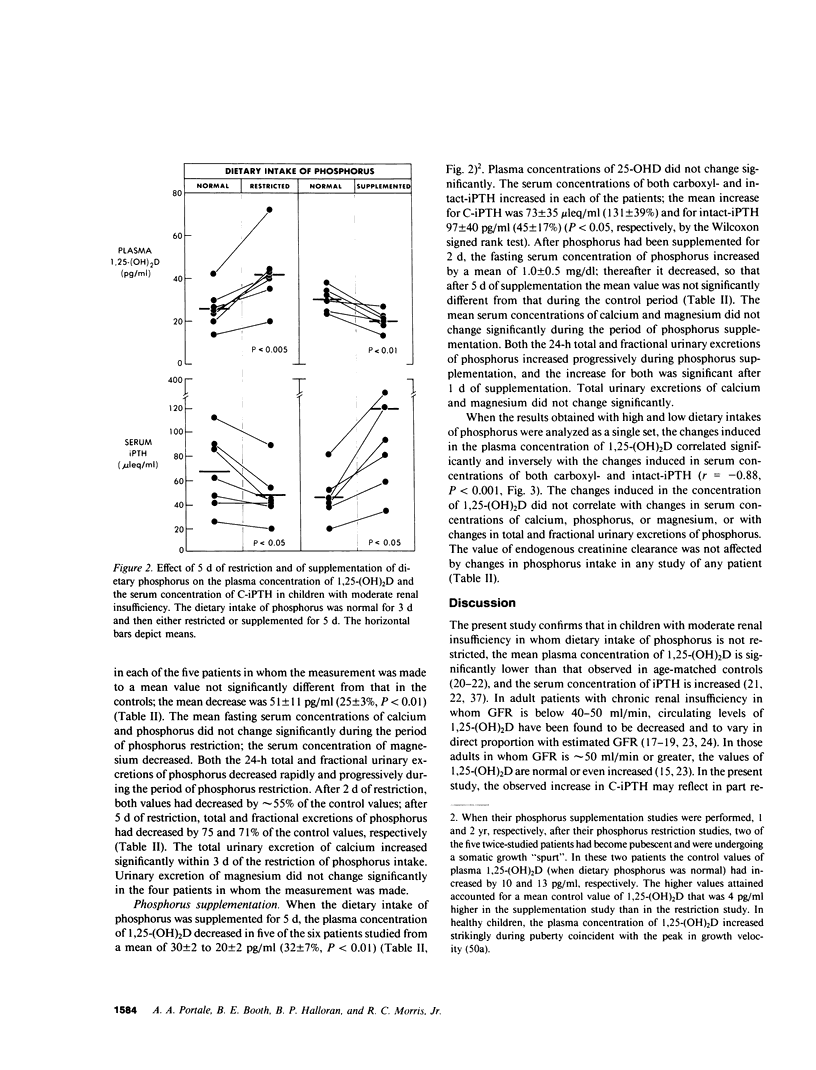
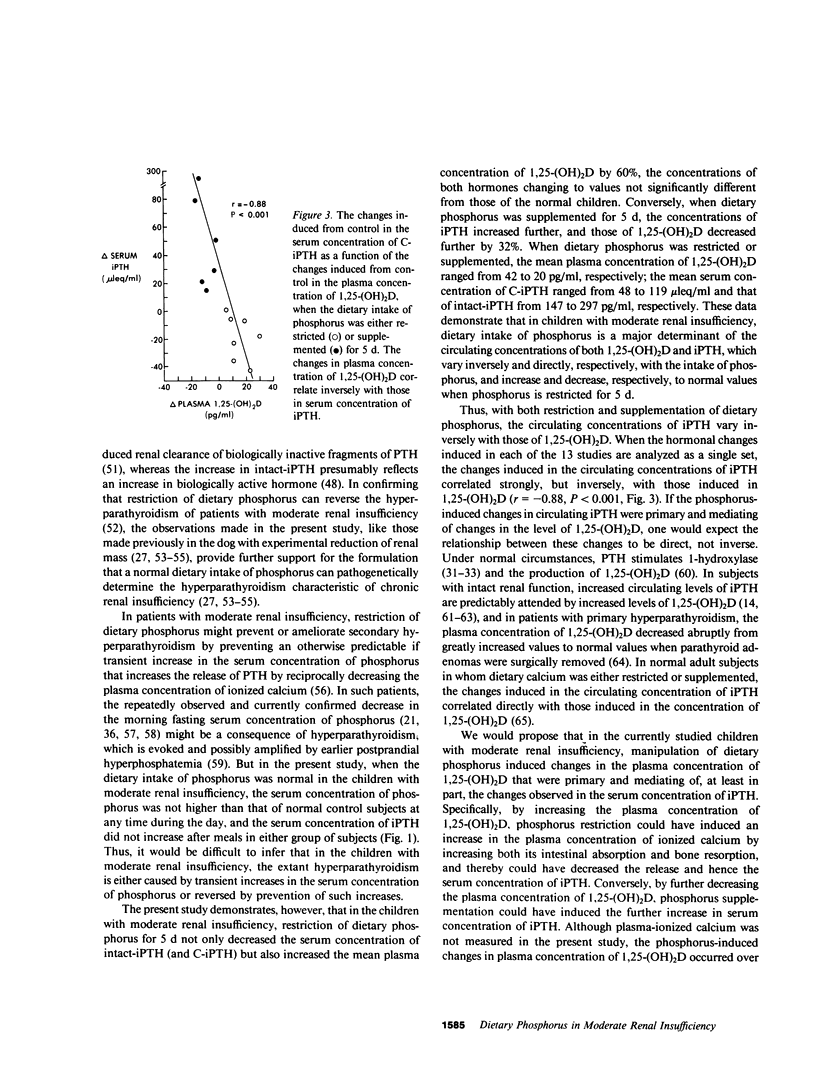




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams N. D., Gray R. W., Lemann J., Jr The effects of oral CaCO3 loading and dietary calcium deprivation on plasma 1,25-dihydroxyvitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 1979 Jun;48(6):1008–1016. doi: 10.1210/jcem-48-6-1008. [DOI] [PubMed] [Google Scholar]
- Akiba T., Endou H., Koseki C., Sakai F., Horiuchi N., Suda T. Localization of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the mammalian kidney. Biochem Biophys Res Commun. 1980 May 14;94(1):313–318. doi: 10.1016/s0006-291x(80)80222-1. [DOI] [PubMed] [Google Scholar]
- Aksnes L., Aarskog D. Plasma concentrations of vitamin D metabolites in puberty: effect of sexual maturation and implications for growth. J Clin Endocrinol Metab. 1982 Jul;55(1):94–101. doi: 10.1210/jcem-55-1-94. [DOI] [PubMed] [Google Scholar]
- Amiel C., Kuntziger H., Richet G. Micropuncture study of handling of phosphate by proximal and distal nephron in normal and parathyroidectomized rat. Evidence for distal reabsorption. Pflugers Arch. 1970;317(2):93–109. doi: 10.1007/BF00592495. [DOI] [PubMed] [Google Scholar]
- Arnaud C. D. Hyperparathyroidism and renal failure. Kidney Int. 1973 Aug;4(2):89–95. doi: 10.1038/ki.1973.87. [DOI] [PubMed] [Google Scholar]
- Baxter L. A., DeLuca H. F. Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. J Biol Chem. 1976 May 25;251(10):3158–3161. [PubMed] [Google Scholar]
- Bellorin-Font E., Martin K. J., Freitag J. J., Anderson C., Sicard G., Slatopolsky E., Klahr S. Altered adenylate cyclase kinetics in hyperfunctioning human parathyroid glands. J Clin Endocrinol Metab. 1981 Mar;52(3):499–507. doi: 10.1210/jcem-52-3-499. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Canfield R. E., Jacobs T. P., Polay J. S., D'Adamo A. P., Eisman J. A., DeLuca H. F. Response of 1alpha,25-dihydroxyvitamin D3 to hypocalcemia in human subjects. N Engl J Med. 1978 Aug 31;299(9):437–441. doi: 10.1056/NEJM197808312990902. [DOI] [PubMed] [Google Scholar]
- Booth B. E., Tsai H. C., Morris R. C., Jr Parathyroidectomy reduces 25-hydroxyvitamin D3-1 alpha-hydroxylase activity in the hypocalcemic vitamin D-deficient chick. J Clin Invest. 1977 Dec;60(6):1314–1320. doi: 10.1172/JCI108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle I. T., Miravet L., Gray R. W., Holick M. F., Deluca H. F. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology. 1972 Mar;90(3):605–608. doi: 10.1210/endo-90-3-605. [DOI] [PubMed] [Google Scholar]
- Breslau N. A., McGuire J. L., Zerwekh J. E., Pak C. Y. The role of dietary sodium on renal excretion and intestinal absorption of calcium and on vitamin D metabolism. J Clin Endocrinol Metab. 1982 Aug;55(2):369–373. doi: 10.1210/jcem-55-2-369. [DOI] [PubMed] [Google Scholar]
- Brickman A. S., Coburn J. W., Friedman G. R., Okamura W. H., Massry S. G., Norman A. W. Comparison of effects of 1 alpha-hydroxy-vitamin D3 and 1,25-dihydroxy-vitamin D3 in man. J Clin Invest. 1976 Jun;57(6):1540–1547. doi: 10.1172/JCI108424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A. S., Coburn J. W., Massry S. G., Norman A. W. 1,25 Dihydroxy-vitamin D3 in normal man and patients with renal failure. Ann Intern Med. 1974 Feb;80(2):161–168. doi: 10.7326/0003-4819-80-2-161. [DOI] [PubMed] [Google Scholar]
- Broadus A. E., Magee J. S., Mallette L. E., Horst R. L., Lang R., Jensen P. S., Gertner J. M., Baron R. A detailed evaluation of oral phosphate therapy in selected patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1983 May;56(5):953–961. doi: 10.1210/jcem-56-5-953. [DOI] [PubMed] [Google Scholar]
- Brown E. M., Wilson R. E., Eastman R. C., Pallotta J., Marynick S. P. Abnormal regulation of parathyroid hormone release by calcium in secondary hyperparathyroidism due to chronic renal failure. J Clin Endocrinol Metab. 1982 Jan;54(1):172–179. doi: 10.1210/jcem-54-1-172. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler D. H., Bressler R., Haussler M. R. Radioreceptor assay for 1 alpha,25-dihydroxyvitamin D3. Science. 1974 Mar 15;183(4129):1089–1091. doi: 10.1126/science.183.4129.1089. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Chan M., Ferriere C., Roberts K. D. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978 Nov 16;276(5685):287–289. doi: 10.1038/276287a0. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Castillo L., Korycka-Dahl M., DeLuca H. F. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974 Aug;104(8):1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- Chesney R. W., Hamstra A. J., Mazess R. B., Rose P., DeLuca H. F. Circulating vitamin D metabolite concentrations in childhood renal diseases. Kidney Int. 1982 Jan;21(1):65–69. doi: 10.1038/ki.1982.9. [DOI] [PubMed] [Google Scholar]
- Chesney R. W., Rosen J. F., Hamstra A. J., DeLuca H. F. Serum 1,25-dihydroxyvitamin D levels in normal children and in vitamin D disorders. Am J Dis Child. 1980 Feb;134(2):135–139. doi: 10.1001/archpedi.1980.02130140009004. [DOI] [PubMed] [Google Scholar]
- Cheung A. K., Manolagas S. C., Catherwood B. D., Mosely C. A., Jr, Mitas J. A., 2nd, Blantz R. C., Deftos L. J. Determinants of serum 1,25(OH)2D levels in renal disease. Kidney Int. 1983 Jul;24(1):104–109. doi: 10.1038/ki.1983.131. [DOI] [PubMed] [Google Scholar]
- Christiansen C., Christensen M. S., Melsen F., Rødbro P., DeLuca H. F. Mineral metabolism in chronic renal failure with special reference to serum concentrations of 1.25(OH)2D and 24.25(OH)2D. Clin Nephrol. 1981 Jan;15(1):18–22. [PubMed] [Google Scholar]
- Coburn J. W., Koppel M. H., Brickman A. S., Massry S. G. Study of intestinal absorption of calcium in patients with renal failure. Kidney Int. 1973 Apr;3(4):264–272. doi: 10.1038/ki.1973.40. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Stead W. W., Myers J. L. Renal handling of phosphate and calcium. Annu Rev Physiol. 1979;41:257–271. doi: 10.1146/annurev.ph.41.030179.001353. [DOI] [PubMed] [Google Scholar]
- Dominguez J. H., Gray R. W., Lemann J., Jr Dietary phosphate deprivation in women and men: effects on mineral and acid balances, parathyroid hormone and the metabolism of 25-OH-vitamin D. J Clin Endocrinol Metab. 1976 Nov;43(5):1056–1068. doi: 10.1210/jcem-43-5-1056. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Hamstra A. J., Kream B. E., DeLuca H. F. 1,25-Dihydroxyvitamin D in biological fluids: a simplified and sensitive assay. Science. 1976 Sep 10;193(4257):1021–1023. doi: 10.1126/science.1085035. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Hamstra A. J., Kream B. E., DeLuca H. F. A sensitive, precise, and convenient method for determination of 1,25-dihydroxyvitamin D in human plasma. Arch Biochem Biophys. 1976 Sep;176(1):235–243. doi: 10.1016/0003-9861(76)90161-2. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Shepard R. M., DeLuca H. F. Determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma using high-pressure liquid chromatography. Anal Biochem. 1977 May 15;80(1):298–305. doi: 10.1016/0003-2697(77)90648-0. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Wark J. D., Prince R. L., Moseley J. M. Modulation of plasma 1,25-dihydroxyvitamin D in man by stimulation and suppression tests. Lancet. 1979 Nov 3;2(8149):931–933. doi: 10.1016/s0140-6736(79)92624-2. [DOI] [PubMed] [Google Scholar]
- Fotino S. Phosphate excretion in chronic renal failure: evidence for a mechanism other than circulating parathyroid hormone. Clin Nephrol. 1977 Dec;8(6):499–503. [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Freitag J., Martin K. J., Hruska K. A., Anderson C., Conrades M., Ladenson J., Klahr S., Slatopolsky E. Impaired parathyroid hormone metabolism in patients with chronic renal failure. N Engl J Med. 1978 Jan 5;298(1):29–32. doi: 10.1056/NEJM197801052980107. [DOI] [PubMed] [Google Scholar]
- Friis T., Hahnemann S., Weeke E. Serum calcium and serum phsophorus in uraemia during administration of osdium phytate and aluminium hydroxide. Acta Med Scand. 1968 Jun;183(6):497–505. doi: 10.1111/j.0954-6820.1968.tb10513.x. [DOI] [PubMed] [Google Scholar]
- Gallagher J. C., Riggs B. L., Jerpbak C. M., Arnaud C. D. The effect of age on serum immunoreactive parathyroid hormone in normal and osteoporotic women. J Lab Clin Med. 1980 Mar;95(3):373–385. [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. W., Napoli J. L. Dietary phosphate deprivation increases 1,25-dihyroxyvitamin D3 synthesis in rat kidney in vitro. J Biol Chem. 1983 Jan 25;258(2):1152–1155. [PubMed] [Google Scholar]
- Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972 Dec 10;247(23):7528–7532. [PubMed] [Google Scholar]
- Gray R. W., Wilz D. R., Caldas A. E., Lemann J., Jr The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 1977 Aug;45(2):299–306. doi: 10.1210/jcem-45-2-299. [DOI] [PubMed] [Google Scholar]
- Gray R., Boyle I., DeLuca H. F. Vitamin D metabolism: the role of kidney tissue. Science. 1971 Jun 18;172(3989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Baylink D. J., Hughes M. R., Brumbaugh P. F., Wergedal J. E., Shen F. H., Nielsen R. L., Counts S. J., Bursac K. M., McCain T. A. The assay of 1alpha,25-dihydroxyvitamin D3: physiologic and pathologic modulation of circulating hormone levels. Clin Endocrinol (Oxf) 1976;5 (Suppl):151S–165S. doi: 10.1111/j.1365-2265.1976.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med. 1977 Nov 3;297(18):974–983. doi: 10.1056/NEJM197711032971804. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Midgett R. J., Norman A. W. Regulation of 25-hydroxyvitamin D3-1-hydroxylase in vivo. J Biol Chem. 1974 Dec 10;249(23):7584–7592. [PubMed] [Google Scholar]
- Holick M. F., Garabedian M., DeLuca H. F. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science. 1972 Jun 9;176(4039):1146–1147. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Juttmann J. R., Buurman C. J., De Kam E., Visser T. J., Birkenhäger J. C. Serum concentrations of metabolites of vitamin D in patients with chronic renal failure (CRF). Consequences for the treatment with 1-alpha-hydroxy-derivatives. Clin Endocrinol (Oxf) 1981 Mar;14(3):225–236. doi: 10.1111/j.1365-2265.1981.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. A., Canterbury J. M., Bourgoignie J. J., Veliz G., Gavellas G., Reiss E., Bricker N. S. Reversal of hyperparathyroidism in response to dietary phosphorus restriction in the uremic dog. Kidney Int. 1979 Jan;15(1):43–48. doi: 10.1038/ki.1979.6. [DOI] [PubMed] [Google Scholar]
- Kaplan R. A., Haussler M. R., Deftos L. J., Bone H., Pak C. Y. The role of 1 alpha, 25-dihydroxyvitamin D in the mediation of intestinal hyperabsorption of calcium in primary hyperparathyroidism and absorptive hypercalciuria. J Clin Invest. 1977 May;59(5):756–760. doi: 10.1172/JCI108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Localization of 25-hydroxyvitamin D3 1 alpha-hydroxylase and 24-hydroxylase along the rat nephron. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llach F., Massry S. G., Singer F. R., Kurokawa K., Kaye J. H., Coburn J. W. Skeletal resistance to endogenous parathyroid hormone in pateints with early renal failure. A possible cause for secondary hyperparathyroidism. J Clin Endocrinol Metab. 1975 Aug;41(2):339–345. doi: 10.1210/jcem-41-2-339. [DOI] [PubMed] [Google Scholar]
- Lund B., Sørensen O. H., Lund B., Bishop J. E., Norman A. W. Stimulation of 1,25-dihydroxyvitamin D production by parathyroid hormone and hypocalcemia in man. J Clin Endocrinol Metab. 1980 Mar;50(3):480–484. doi: 10.1210/jcem-50-3-480. [DOI] [PubMed] [Google Scholar]
- Madsen S., Olgaard K., Ladefoged J. Suppressive effect of 1,25-dihydroxyvitamin D3 on circulating parathyroid hormone in acute renal failure. J Clin Endocrinol Metab. 1981 Oct;53(4):823–827. doi: 10.1210/jcem-53-4-823. [DOI] [PubMed] [Google Scholar]
- Markowitz M., Rotkin L., Rosen J. F. Circadian rhythms of blood minerals in humans. Science. 1981 Aug 7;213(4508):672–674. doi: 10.1126/science.7256269. [DOI] [PubMed] [Google Scholar]
- Mason R. S., Lissner D., Wilkinson M., Posen S. Vitamin D metabolites and their relationship to azotaemic osteodystrophy. Clin Endocrinol (Oxf) 1980 Oct;13(4):375–385. doi: 10.1111/j.1365-2265.1980.tb03399.x. [DOI] [PubMed] [Google Scholar]
- Mawer E. B., Taylor C. M., Backhouse J., Lumb G. A., Stanbury S. W. Failure of formation of 1,25-dihydroxycholecalciferol in chronic renal insufficiency. Lancet. 1973 Mar 24;1(7804):626–628. doi: 10.1016/s0140-6736(73)92197-1. [DOI] [PubMed] [Google Scholar]
- Midgett R. J., Spielvogel A. M., Coburn J. W., Norman A. W. Studies on calciferol metabolism. VII. The renal production of the biologically active form of vitamin D, 1,25-dihydroxycholecalciferol; species, tissue and subcellular distribution. J Clin Endocrinol Metab. 1973 Jun;36(6):1153–1161. doi: 10.1210/jcem-36-6-1153. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Wong R. G. Biological activity of the vitamin D metabolite 1,25-dihydroxycholecalciferol in chickens and rats. J Nutr. 1972 Dec;102(12):1709–1718. doi: 10.1093/jn/102.12.1709. [DOI] [PubMed] [Google Scholar]
- Norman M. E., Mazur A. T., Borden S., 4th, Gruskin A., Anast C., Baron R., Rasmussen H. Early diagnosis of juvenile renal osteodystrophy. J Pediatr. 1980 Aug;97(2):226–232. doi: 10.1016/s0022-3476(80)80479-3. [DOI] [PubMed] [Google Scholar]
- Oldham S. B., Smith R., Hartenbower D. L., Henry H. L., Norman A. W., Coburn J. W. The acute effects of 1,25-dihydroxycholecalciferol on serum immunoreactive parathyroid hormone in the dog. Endocrinology. 1979 Jan;104(1):248–254. doi: 10.1210/endo-104-1-248. [DOI] [PubMed] [Google Scholar]
- Piel C. F., Roof B. S., Avioli L. V. Metabolism of tritiated 25-hydroxycholecalciferol in chronically uremic children before and after successful renal homotransplantation. J Clin Endocrinol Metab. 1973 Dec;37(6):944–948. doi: 10.1210/jcem-37-6-944. [DOI] [PubMed] [Google Scholar]
- Portale A. A., Booth B. E., Tsai H. C., Morris R. C., Jr Reduced plasma concentration of 1,25-dihydroxyvitamin D in children with moderate renal insufficiency. Kidney Int. 1982 Apr;21(4):627–632. doi: 10.1038/ki.1982.70. [DOI] [PubMed] [Google Scholar]
- Rader J. I., Baylink D. J., Hughes M. R., Safilian E. F., Haussler M. R. Calcium and phosphorus deficiency in rats: effects on PTH and 1,25-dihydroxyvitamin D3. Am J Physiol. 1979 Feb;236(2):E118–E122. doi: 10.1152/ajpendo.1979.236.2.E118. [DOI] [PubMed] [Google Scholar]
- Reiss E., Canterbury J. M., Egdahl R. H. Experience with a radioimmunoassay of parathyroid hormone in human sera. Trans Assoc Am Physicians. 1968;81:104–115. [PubMed] [Google Scholar]
- Rutherford W. E., Bordier P., Marie P., Hruska K., Harter H., Greenwalt A., Blondin J., Haddad J., Bricker N., Slatopolsky E. Phosphate control and 25-hydroxycholecalciferol administration in preventing experimental renal osteodystrophy in the dog. J Clin Invest. 1977 Aug;60(2):332–341. doi: 10.1172/JCI108781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRICKLER J. C., THOMPSON D. D., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF INORGANIC PHOSPHATE EXCRETION IN THE RAT. J Clin Invest. 1964 Aug;43:1596–1607. doi: 10.1172/JCI105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard R. M., Horst R. L., Hamstra A. J., DeLuca H. F. Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55–69. doi: 10.1042/bj1820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Bricker N. S. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney Int. 1973 Aug;4(2):141–145. doi: 10.1038/ki.1973.92. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Caglar S., Gradowska L., Canterbury J., Reiss E., Bricker N. S. On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using "proportional reduction" of dietary phosphorus intake. Kidney Int. 1972 Sep;2(3):147–151. doi: 10.1038/ki.1972.84. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Caglar S., Pennell J. P., Taggart D. D., Canterbury J. M., Reiss E., Bricker N. S. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971 Mar;50(3):492–499. doi: 10.1172/JCI106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Rutherford W. E., Hruska K., Martin K., Klahr S. How important is phosphate in the pathogenesis of renal osteodystrophy? Arch Intern Med. 1978 May 15;138(Spec No):848–852. [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Taylor A., Norman M. E. Interrelationship of serum 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D in juvenile renal osteodystrophy after therapy with 25-hydroxyvitamin D3. Metab Bone Dis Relat Res. 1982;4(4):255–261. doi: 10.1016/0221-8747(82)90036-4. [DOI] [PubMed] [Google Scholar]
- Van Den Berg C. J., Kumar R., Wilson D. M., Heath H., 3rd, Smith L. H. Orthophosphate therapy decreases urinary calcium excretion and serum 1,25-dihydroxyvitamin D concentrations in idiopathic hypercalciuria. J Clin Endocrinol Metab. 1980 Nov;51(5):998–1001. doi: 10.1210/jcem-51-5-998. [DOI] [PubMed] [Google Scholar]
- van Stone J. C., Frank D. E., Bradford W. R. The effect of decreased renal function with and without reduction in renal mass on 1,25-dihydroxycholecalciferol production in rats. J Lab Clin Med. 1977 Jun;89(6):1168–1174. [PubMed] [Google Scholar]



