Abstract
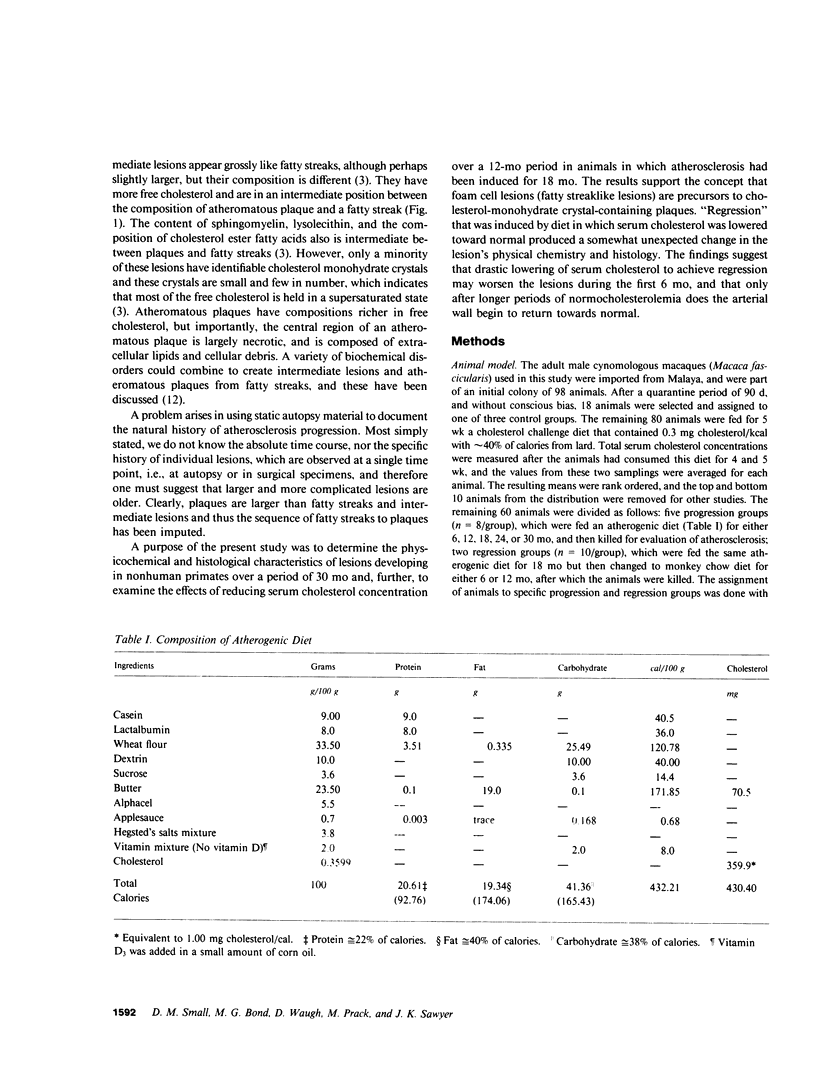
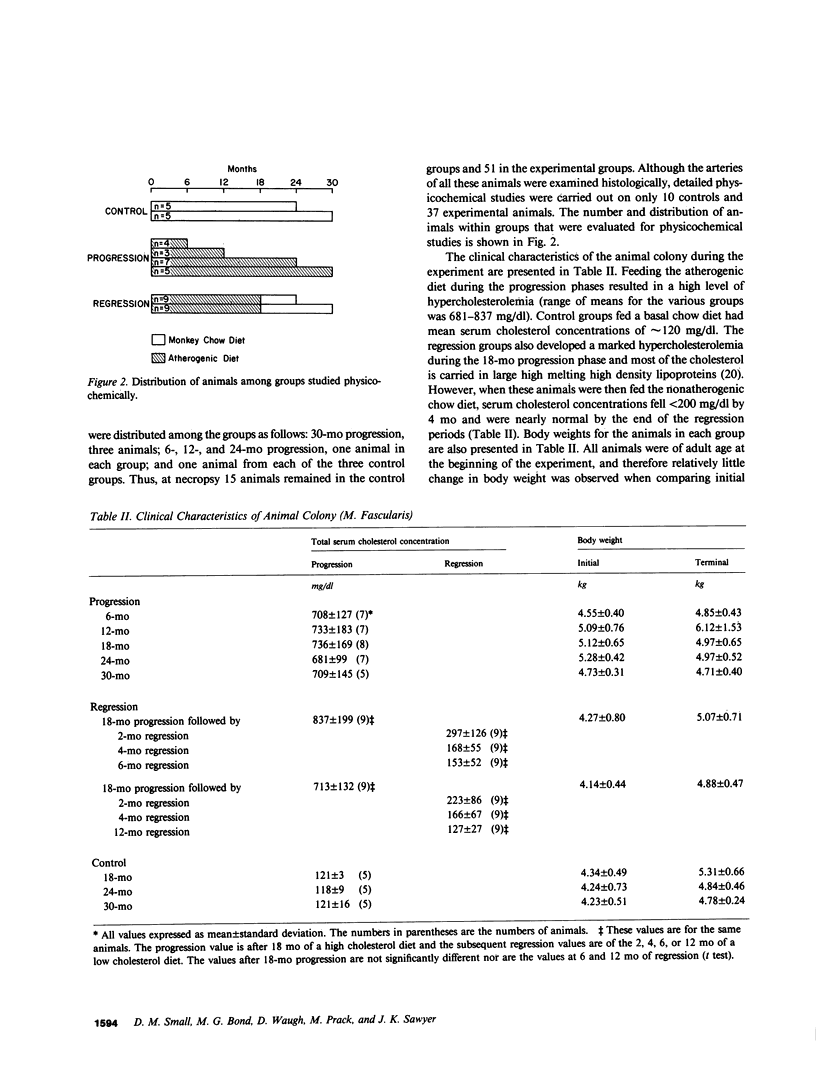
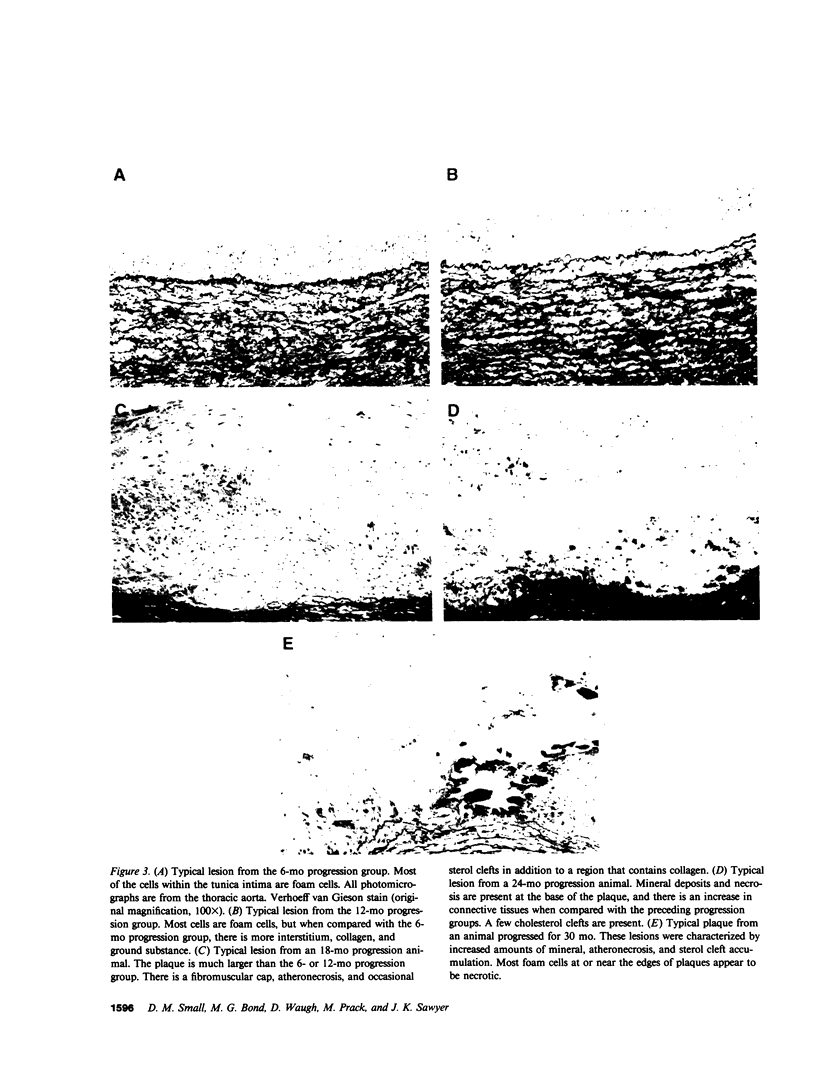
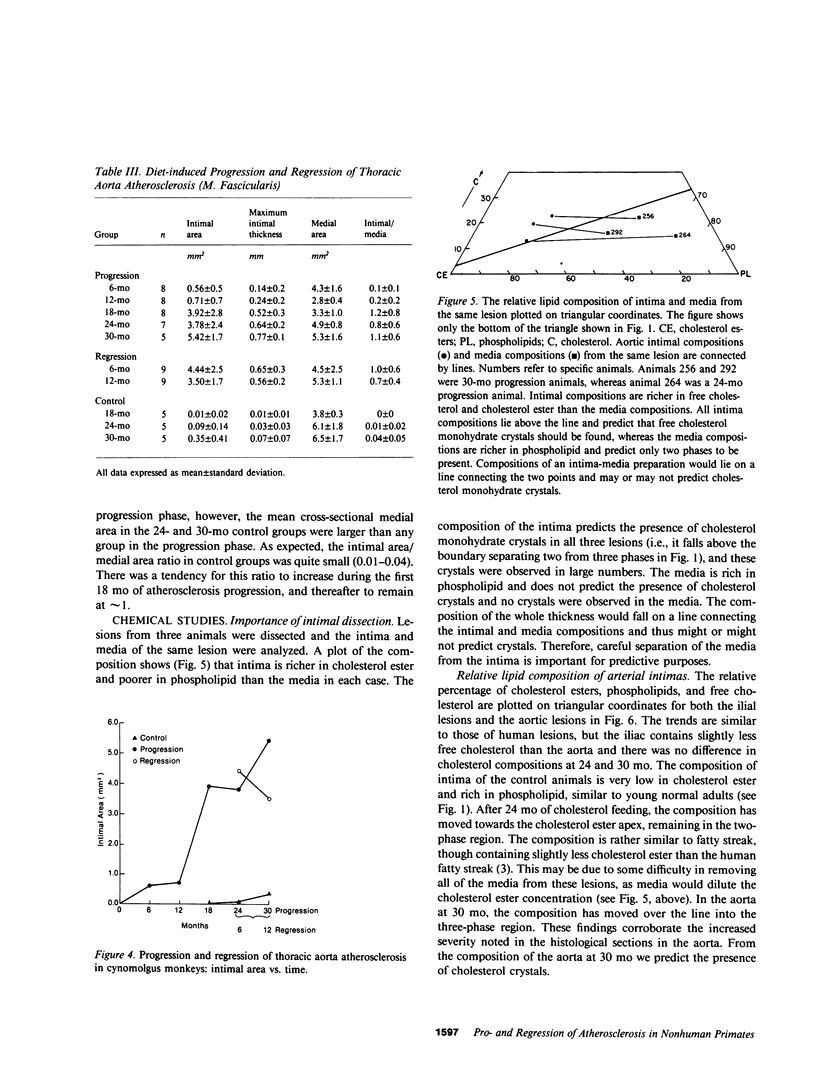
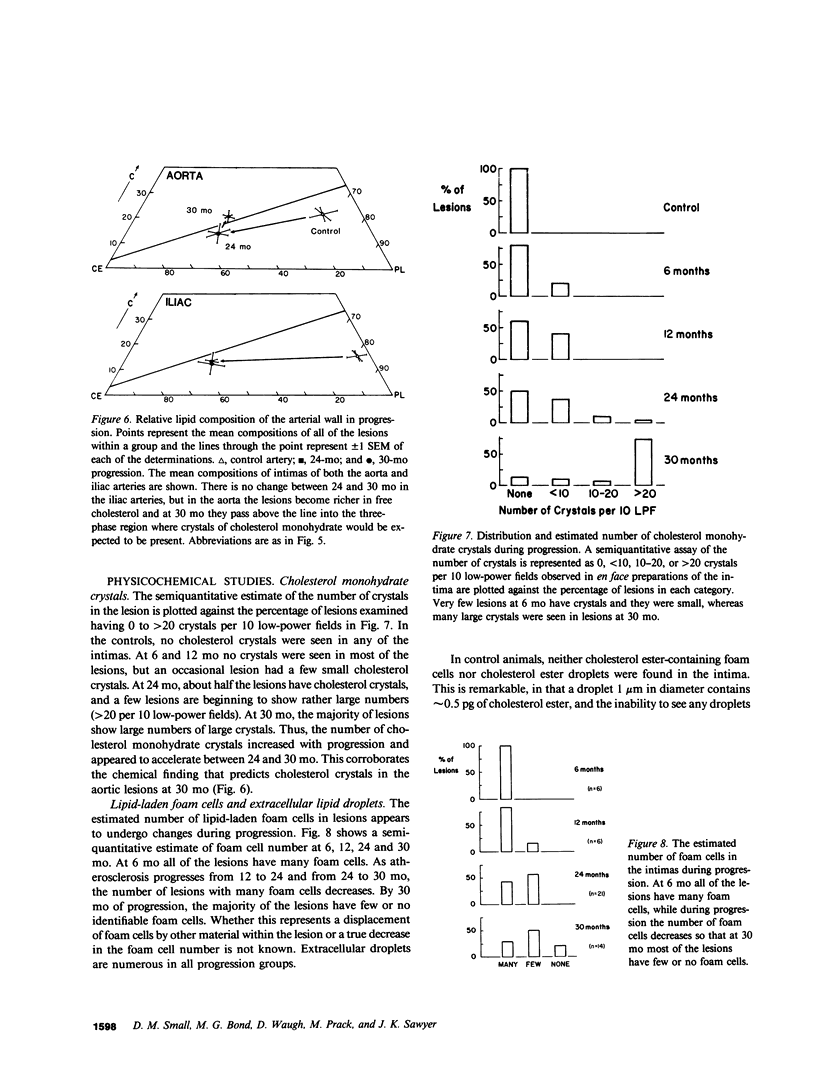
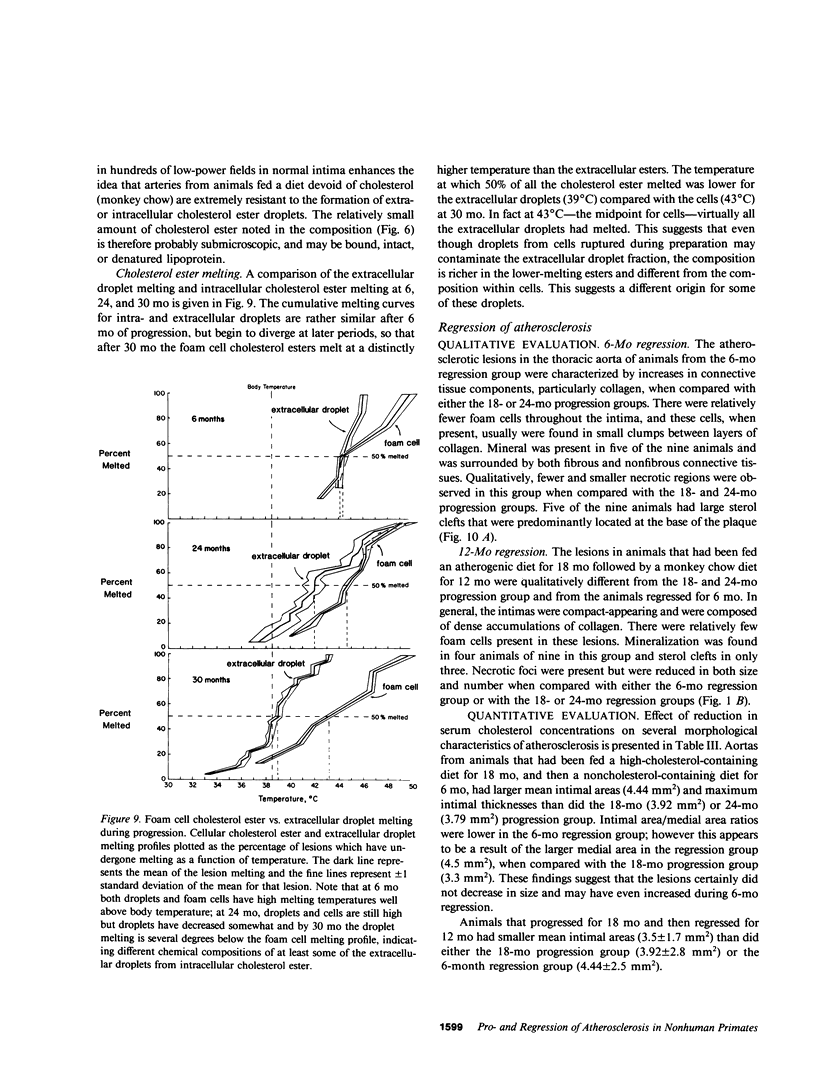
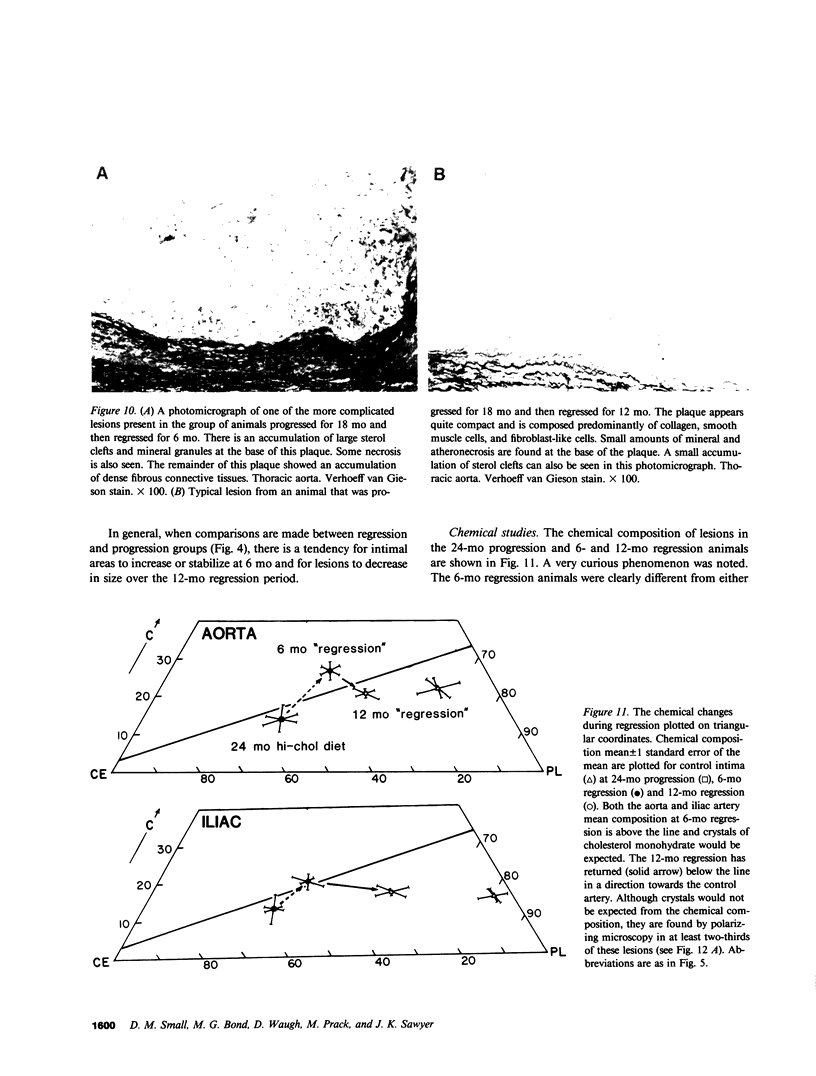
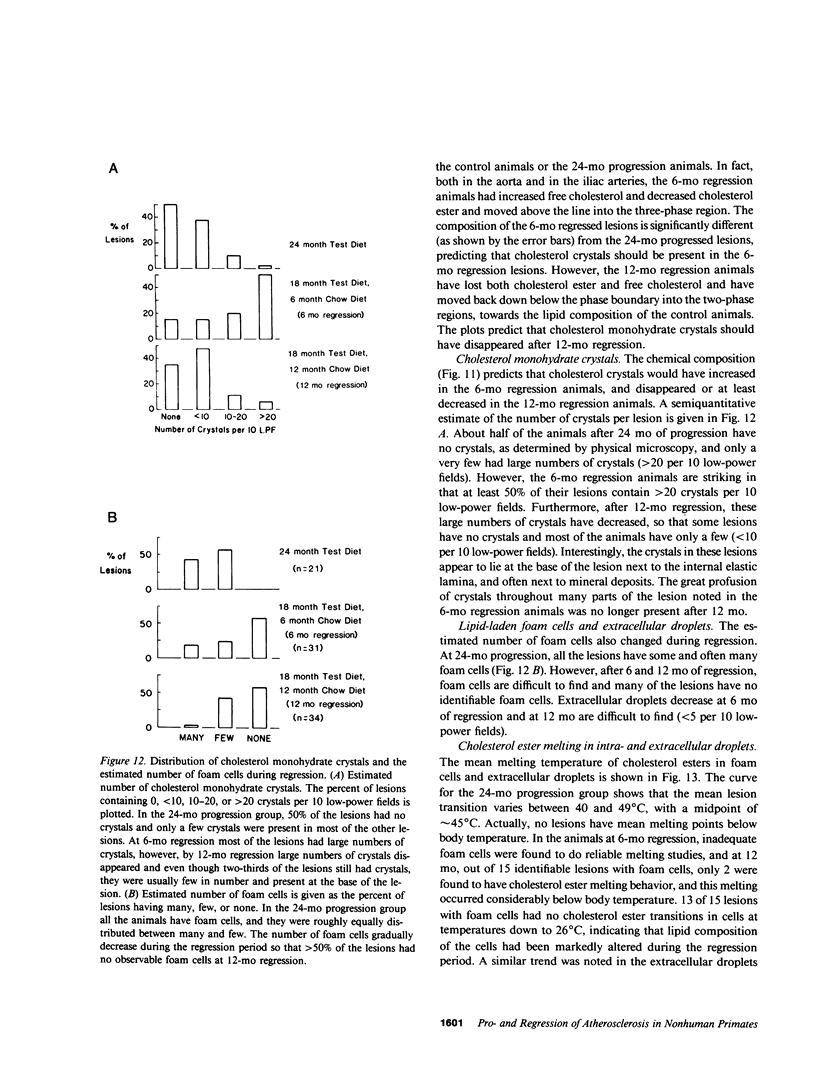
To identify the temporal changes occurring during progression and regression of atherosclerosis in nonhuman primates, we have studied the physicochemical and histological characteristics of arterial wall lesions during a 30-mo progression period of diet-induced hypercholesterolemia and during a 12-mo period of regression. Three groups of cynomolgous monkeys (Macaca fascicularis) were studied. Control groups were fed a basal chow diet for 18, 24, and 30 mo and were compared with progression groups that were fed a high-cholesterol-containing diet for up to 30 mo. Regression groups were fed a high-cholesterol diet for 18 mo to induce atherosclerosis and then fed monkey chow for up to 12 mo. The progression group monkeys were killed at 6, 12, 18, 24, and 30 mo, and the regression animals were killed at 24 and 30 mo (i.e., after 6 and 12 mo of being fed a noncholesterol-containing chow diet). Histology and morphometry, physical microscopy for cholesterol monohydrate crystals, foam cell and droplet melting points and chemical composition studies were completed on a large number of individual arterial lesions. Control animals had very little cholesterol ester, rare foam cells, and no extracellular cholesterol ester droplets or cholesterol crystals. During progression, the arteries first increased cholesterol ester content to produce high melting (approximately 45 degrees C) foam cell-rich lesions essentially devoid of cholesterol crystals. With time, the number of cholesterol crystals increased so that by 30 mo large numbers were present. Foam cells decreased with time but their melting temperature remained high while that of extracellular droplets fell to approximately 38 degrees C. Between 18 and 30 mo necrosis appeared and worsened. After 6-mo regression, unexpected changes occurred in the lesions. Compared with 24-mo progression, the chemical composition showed a relative increase in free cholesterol, a decrease in cholesterol ester and microscopy revealed large numbers of cholesterol crystals. Concomitantly, foam cells decreased and the melting temperature of both intra- and extracellular cholesterol ester markedly decreased. After 12-mo regression cholesterol decreased, cholesterol crystals and necrosis diminished and collagen appeared increased. Thus, during progression there is initially an increase in the number of foam cells containing very high-melting intracellular cholesterol ester droplets. By 30 mo, cholesterol crystals and necrosis dominate and high-melting foam cells appear only at lesion margins, suggesting that the initial process continues at the lesion edge. The lower melting point of extracellular esters indicates a lipid composition different from intracellular droplets. Thus, the changes observed in these animals generally reflect those predicted for progression of human atherosclerosis. During the initial 6 mo of regression, necrosis remains, the number of foam cell decreases, and cholesterol ester content decreases; however the relative proportion of free cholesterol content increases, and large numbers of cholesterol content are formed. Thus, large and rapid decreases in serum cholesterol concentration to produce regression in fact may result in the precipitation of cholesterol monohydrate and an apparent worsening of the lesions. More prolonged regression (12-mo) tends to return the lipid composition of the artery wall towards normal, partially reduces cholesterol crystals, and results in an improved but scarred intima.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. L., Megan M. B. Lipid depletion in atheromatous coronary arteries in rhesus monkeys after regression diets. Circ Res. 1972 Jun;30(6):675–680. doi: 10.1161/01.res.30.6.675. [DOI] [PubMed] [Google Scholar]
- Bond M. G., Adams M. R., Bullock B. C. Complicating factors in evaluating coronary artery atherosclerosis. Artery. 1981;9(1):21–29. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Clarkson T. B., Bond M. G., Bullock B. C., Marzetta C. A. A study of atherosclerosis regression in Macaca mulatta. IV. Changes in coronary arteries from animals with atherosclerosis induced for 19 months and then regressed for 24 or 48 months at plasma cholesterol concentrations of 300 or 200 mg/dl. Exp Mol Pathol. 1981 Jun;34(3):345–368. doi: 10.1016/0014-4800(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Clarkson T. B., Lehner N. D., Wagner W. D., St Clair R. W., Bond M. G., Bullock B. C. A study of atherosclerosis regression in Macaca mulatta. I. Design of experiment and lesion induction. Exp Mol Pathol. 1979 Jun;30(3):360–385. doi: 10.1016/0014-4800(79)90090-x. [DOI] [PubMed] [Google Scholar]
- DePalma R. G., Bellon E. M., Koletsky S., Schneider D. L. Atherosclerotic plaque regression in rhesus monkeys induced by bile acid sequestrant. Exp Mol Pathol. 1979 Dec;31(3):423–439. doi: 10.1016/0014-4800(79)90042-x. [DOI] [PubMed] [Google Scholar]
- Downing D. T. Photodensitometry in the thin-layer chromatographic analysis of neutral lipids. J Chromatogr. 1968 Nov 5;38(1):91–99. doi: 10.1016/0021-9673(68)85011-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hata Y., Insull W., Jr Significance of cholesterol esters as liquid crystal in human atherosclerosis. Jpn Circ J. 1973 Mar;37(3):269–275. doi: 10.1253/jcj.37.269. [DOI] [PubMed] [Google Scholar]
- Hollander W., Paddock J., Colombo M. Lipoproteins in human atherosclerotic vessels. I. Biochemical properties of arterial low density lipoproteins, very low density lipoproteins, and high density lipoproteins. Exp Mol Pathol. 1979 Apr;30(2):144–171. doi: 10.1016/0014-4800(79)90051-0. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. S., Small D. M., Brook J. G., Lees R. S. The storage lipids in Tangier disease. A physical chemical study. J Clin Invest. 1977 Jun;59(6):1045–1054. doi: 10.1172/JCI108727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. S., Small D. M. Isolation and and partial characterization of the lipid phases of human atherosclerotic plaques. J Biol Chem. 1980 Oct 25;255(20):9753–9759. [PubMed] [Google Scholar]
- Katz S. S., Small D. M., Smith F. R., Dell R. B., Goodman D. S. Cholesterol turnover in lipid phases of human atherosclerotic plaque. J Lipid Res. 1982 Jul;23(5):733–737. [PubMed] [Google Scholar]
- Katz S. S. The lipids or grossly normal human aortic intima from birth to old age. J Biol Chem. 1981 Dec 10;256(23):12275–12280. [PubMed] [Google Scholar]
- Kokatnur M. G., Malcom G. T., Eggen D. A., Strong J. P. Depletion of aortic free and ester cholesterol by dietary means in rhesus monkeys with fatty streaks. Atherosclerosis. 1975 Mar-Apr;21(2):195–203. doi: 10.1016/0021-9150(75)90080-5. [DOI] [PubMed] [Google Scholar]
- Lang P. D., Insull W., Jr Lipid droplets in atherosclerotic fatty streaks of human aorta. J Clin Invest. 1970 Aug;49(8):1479–1488. doi: 10.1172/JCI106365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis C. R., Shipley G. G., Small D. M. The phase behavior of hydrated cholesterol. J Lipid Res. 1979 May;20(4):525–535. [PubMed] [Google Scholar]
- Panganamala R. V., Geer J. C., Sharma H. M., Cornwell D. G. The gross and histologic appearance and the lipid composition of normal intima and lesions from human coronary arteries and aorta. Atherosclerosis. 1974 Jul-Aug;20(1):93–104. doi: 10.1016/0021-9150(74)90083-5. [DOI] [PubMed] [Google Scholar]
- Small D. M. Cellular mechanisms for lipid deposition in atherosclerosis (first of two parts). N Engl J Med. 1977 Oct 20;297(16):873–877. doi: 10.1056/NEJM197710202971608. [DOI] [PubMed] [Google Scholar]
- Small D. M. Cholesterol nucleation and growth in gallstone formation. N Engl J Med. 1980 Jun 5;302(23):1305–1307. doi: 10.1056/NEJM198006053022309. [DOI] [PubMed] [Google Scholar]
- Small D. M., Shipley G. G. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974 Jul 19;185(4147):222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Slater R. S. The microdissection of large atherosclerotic plaques to give morphologically and topographically defined fractions for analysis. 1. The lipids in the isolated fractions. Atherosclerosis. 1972 Jan-Feb;15(1):37–56. doi: 10.1016/0021-9150(72)90036-6. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. R., Patton D., Radhakrishnamurthy B., Foster T. A., Malinow M. R., McLaughlin P., Berenson G. S. Lipid changes in atherosclerotic aortas of Macaca fascicularis after various regression regimens. Atherosclerosis. 1980 Dec;37(4):591–601. doi: 10.1016/0021-9150(80)90066-0. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Small D. M., Atkinson D., Rudel L. L. Studies on the structure of low density lipoproteins isolated from Macaca fascicularis fed an atherogenic diet. J Clin Invest. 1978 Dec;62(6):1354–1363. doi: 10.1172/JCI109256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W. D., St Clair R. W., Clarkson T. B. A study of atherosclerosis regression in Macaca mulatta. II. Chemical changes in arteries from animals with atherosclerosis induced for 19 months then regressed for 24 months at plasma cholesterol concentrations of 300 or 200 mg/dl. Exp Mol Pathol. 1980 Apr;32(2):162–174. doi: 10.1016/0014-4800(80)90052-0. [DOI] [PubMed] [Google Scholar]
- Wagner W. D., St Clair R. W., Clarkson T. B., Connor J. R. A study of atherosclerosis regression in Macaca mulatta: III. Chemical changes in arteries from animals with atherosclerosis induced for 19 months and regressed for 48 months at plasma cholesterol concentrations of 300 or 200 mg/dl. Am J Pathol. 1980 Sep;100(3):633–650. [PMC free article] [PubMed] [Google Scholar]
- Wissler R. W., Vesselinovitch D. Studies of regression of advanced atherosclerosis in experimental animals and man. Ann N Y Acad Sci. 1976;275:363–378. doi: 10.1111/j.1749-6632.1976.tb43368.x. [DOI] [PubMed] [Google Scholar]









