Abstract
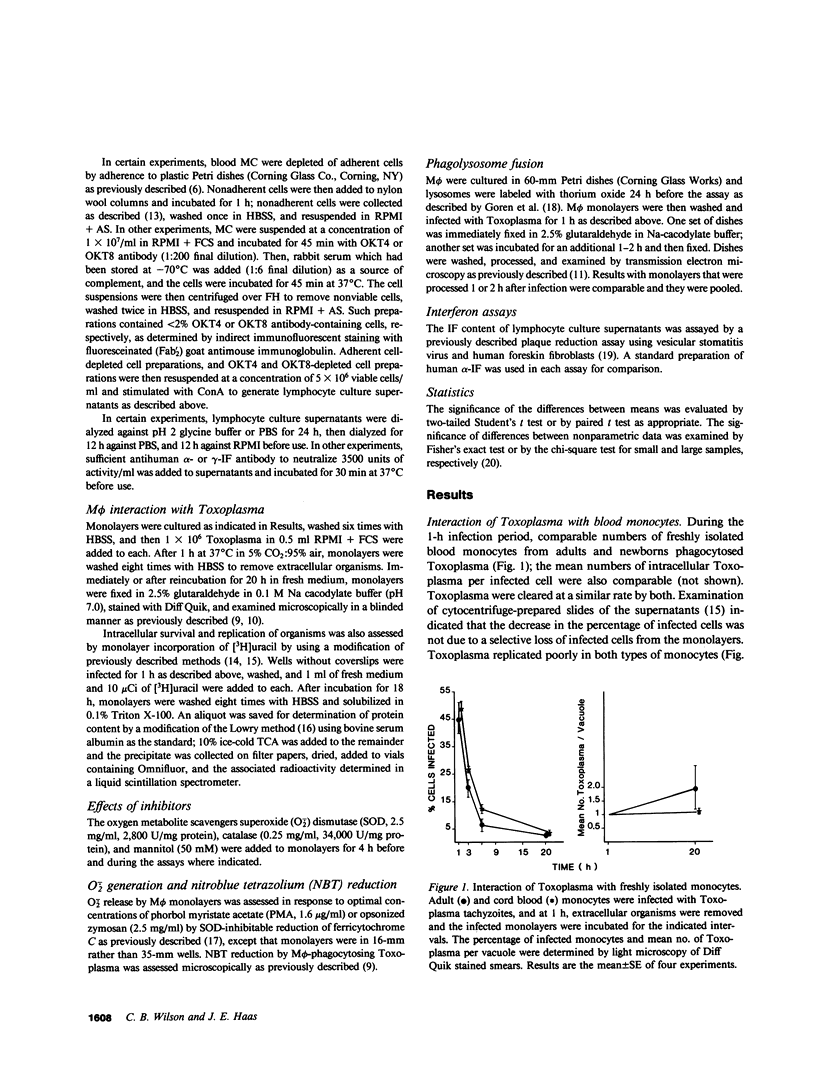
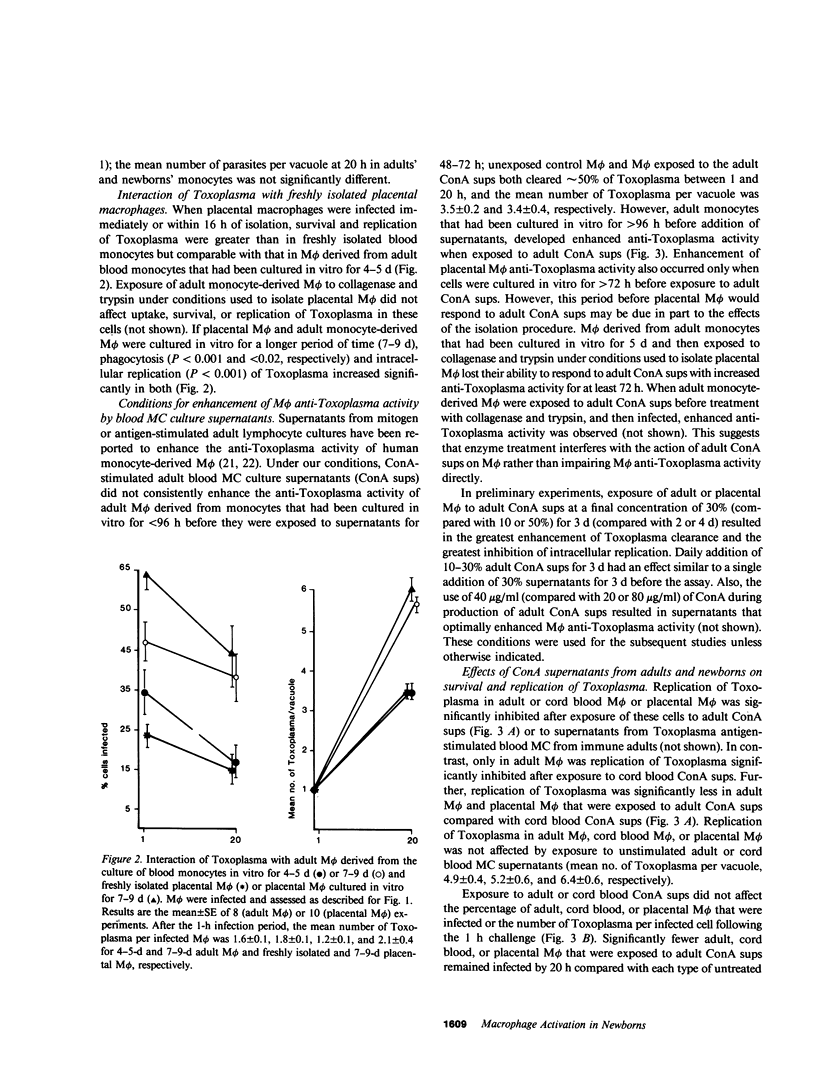
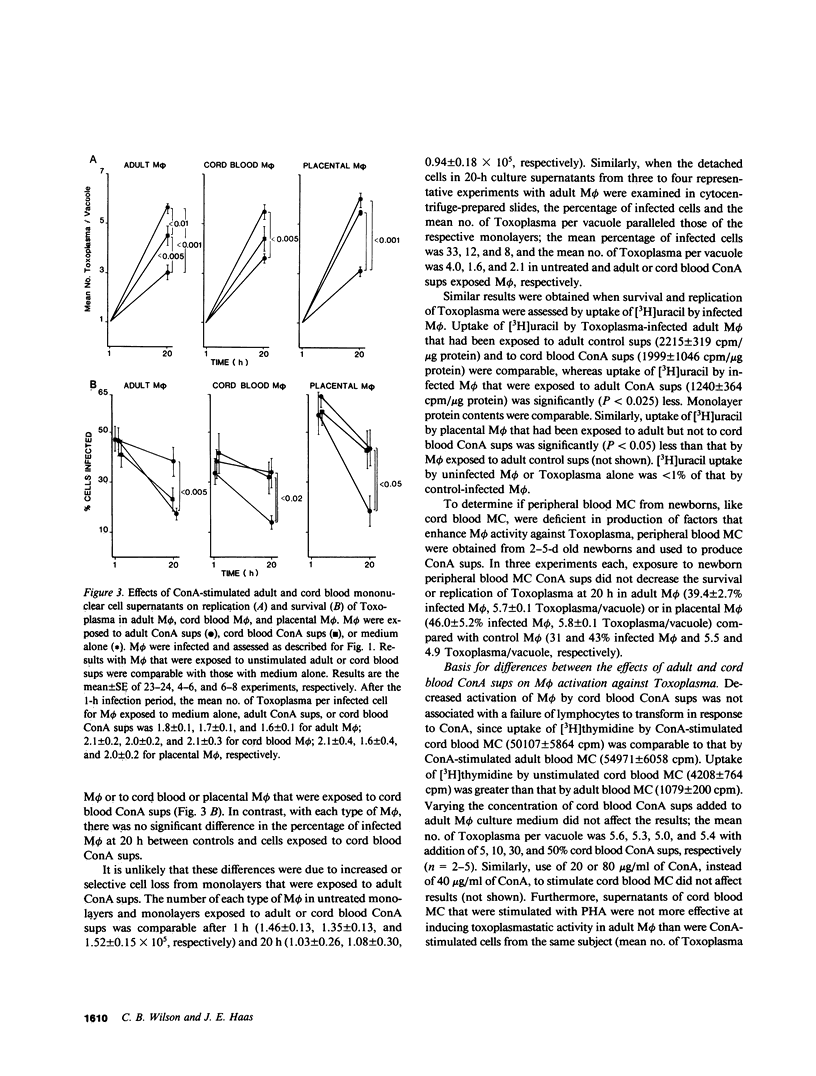
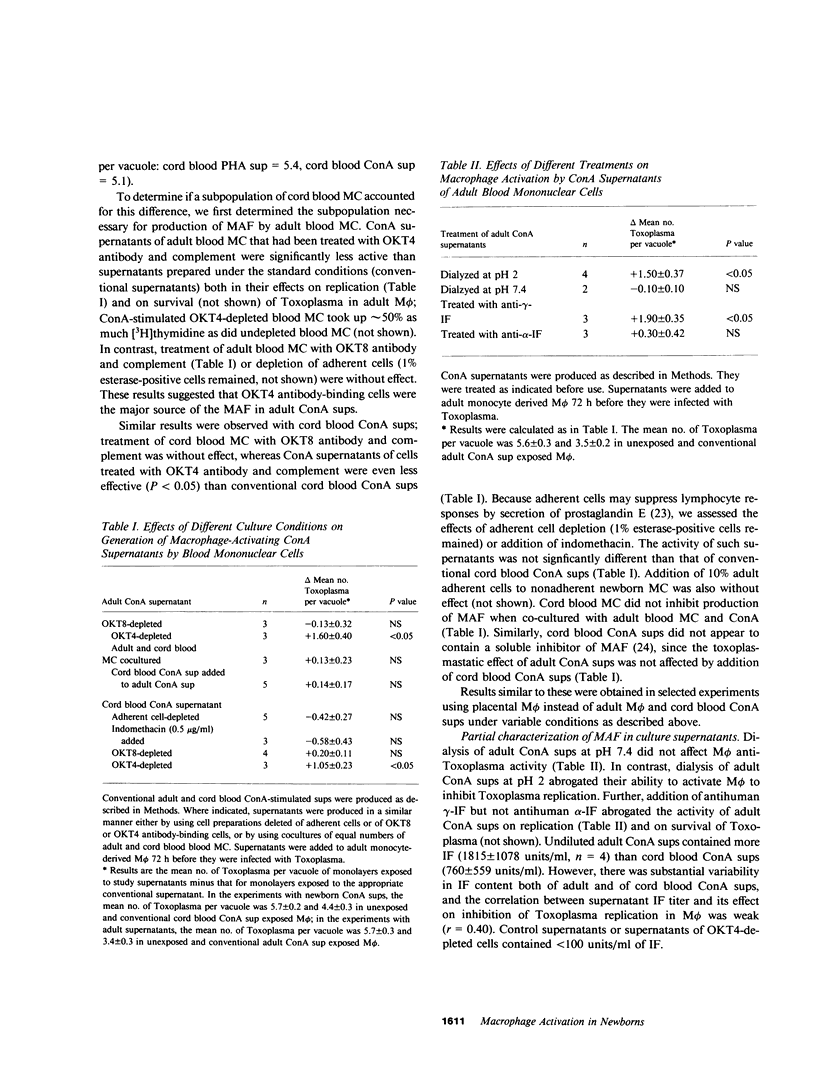
Mononuclear phagocytes, particularly macrophages (M phi) that have been activated by lymphokines, are the principal defense against intracellular pathogens such as Toxoplasma gondii. To determine reasons for the newborns' susceptibility to Toxoplasma infection, we compared: the interaction of Toxoplasma with newborns' mononuclear phagocytes (blood monocytes and two types of newborn M phi, those derived from blood monocytes or from placental tissue) with adults' blood monocytes and monocyte-derived M phi and the production of M phi-activating lymphokines (MAF) by Concanavalin A (ConA)-stimulated newborn and adult blood mononuclear cells (MC). Newborn and adult monocytes killed Toxoplasma with equal efficiency. Similarly, survival and replication of Toxoplasma were comparable in control newborn and adult M phi. Exposure to adult ConA supernatants significantly decreased the survival and replication of Toxoplasma both in adult and newborn M phi. In contrast, exposure to cord blood ConA supernatants failed to affect the survival or the replication of Toxoplasma in newborn M phi and decreased the replication but not the survival of Toxoplasma in adult M phi. Exposure to ConA supernatants of peripheral blood MC from 2-5-d old newborns failed to affect survival or replication of Toxoplasma in newborn or adult M phi. Thus, both generation of MAF by newborn blood MC and response to newborn MAF by newborn M phi were impaired. Generation of MAF by adult blood mononuclear cells was not inhibited by cord blood MC nor was generation of MAF by cord blood MC increased by depletion of OKT8 antibody-binding cells, by depletion of adherent cells with or without addition of adult adherent cells, or by addition of indomethacin. Depletion of OKT4 antibody-binding cells abrogated the generation of MAF both by adult and cord blood MC. The activity of adult ConA supernatants was abrogated by dialysis at pH 2 or by addition of anti-gamma-interferon but not anti-alpha-interferon antibody. However, the correlation between antiviral interferon activity and anti-Toxoplasma activity was weak (r = 0.40). Enhanced M phi anti-Toxoplasma activity was not associated with detectably enhanced superoxide anion generation, nitroblue tetrazolium reduction, or phagolysosome fusion, and was not inhibited by catalase, superoxide dismutase, or mannitol. These results indicate that generation of and response to MAF is decreased in cells from human newborns and that gamma-interferon may be the major MAF under these conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahronheim G. A. Toxoplasma gondii: human interferon studies by plaque assay. Proc Soc Exp Biol Med. 1979 Sep;161(4):522–526. doi: 10.3181/00379727-161-40588. [DOI] [PubMed] [Google Scholar]
- Anderson S. E., Bautista S., Remington J. S. Induction of resistance to Toxoplasma gondii in human macrophages by soluble lymphocyte products. J Immunol. 1976 Aug;117(2):381–387. [PubMed] [Google Scholar]
- Berman J. D., Johnson W. D., Jr Monocyte function in human neonates. Infect Immun. 1978 Mar;19(3):898–902. doi: 10.1128/iai.19.3.898-902.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Chinchilla M., Frenkel J. K. Mediation of immunity to intracellular infection (Toxoplasma and Besnoitia) within somatic cells. Infect Immun. 1978 Mar;19(3):999–1012. doi: 10.1128/iai.19.3.999-1012.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Shparber M., Kent E. F., Jr, Wolff S. M. Production of leukocytic pyrogen from phagocytes of neonates. J Infect Dis. 1981 Oct;144(4):337–343. doi: 10.1093/infdis/144.4.337. [DOI] [PubMed] [Google Scholar]
- Dretschmer R. R., Stewardson R. B., Papierniak C. K., Gotoff S. P. Chemotactic and bactericidal capacities of human newborn monocytes. J Immunol. 1976 Oct;117(4):1303–1307. [PubMed] [Google Scholar]
- Drew W. L., Mintz L., Hoo R., Finley T. N. Growth of herpes simplex and cytomegalovirus in cultured human alveolar macrophages. Am Rev Respir Dis. 1979 Feb;119(2):287–291. doi: 10.1164/arrd.1979.119.2.287. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Eife R. F., Eife G., August C. S., Kuhre W. L., Staehr-Johansen K. Lymphotoxin production and blast cell transformation by cord blood lymphocytes: dissociated lymphocyte function in newborn infants. Cell Immunol. 1974 Dec;14(3):435–442. doi: 10.1016/0008-8749(74)90194-4. [DOI] [PubMed] [Google Scholar]
- Frazier J. P., Cleary T. G., Pickering L. K., Kohl S., Ross P. J. Leukocyte function in healthy neonates following vaginal and cesarean section deliveries. J Pediatr. 1982 Aug;101(2):269–272. doi: 10.1016/s0022-3476(82)80138-8. [DOI] [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Aging and the immune response. II. Lymphocyte responsiveness and macrophage activation in Toxoplasma gondii-infected mice. J Immunol. 1978 Mar;120(3):944–949. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T., Levin S., Handzel Z. T. Leucocyte migration inhibition factor (LIF) production by lymphocytes of normal children, newborns, and children with immune deficiency. Clin Exp Immunol. 1976 Jun;24(3):448–454. [PMC free article] [PubMed] [Google Scholar]
- Handzel Z. T., Levin S., Dolphin Z., Schlesinger M., Hahn T., Altman Y., Schechter B., Shneyour A., Trainin N. Immune competence of newborn lymphocytes. Pediatrics. 1980 Mar;65(3):491–496. [PubMed] [Google Scholar]
- Hayward A. R., Merrill D. Requirement for OKT8+ suppressor cell proliferation for suppression by human newborn T cells. Clin Exp Immunol. 1981 Sep;45(3):468–474. [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Jordan M. C., Mar V. L. Spontaneous activation of latent cytomegalovirus from murine spleen explants. Role of lymphocytes and macrophages in release and replication of virus. J Clin Invest. 1982 Oct;70(4):762–768. doi: 10.1172/JCI110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Kleinschmidt W. J., Schultz R. M. Similarities of murine gamma interferon and the lymphokine that renders macrophages cytotoxic. J Interferon Res. 1982;2(2):291–299. doi: 10.1089/jir.1982.2.291. [DOI] [PubMed] [Google Scholar]
- Kohl S., Frazier J. J., Greenberg S. B., Pickering L. K., Loo L. S. Interferon induction of natural killer cytotoxicity in human neonates. J Pediatr. 1981 Mar;98(3):379–384. doi: 10.1016/s0022-3476(81)80699-3. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Weigent D. A., Georgiades J. A., Johnson H. M., Stanton G. J. Antibody to staphylococcal enterotoxin A-induced human immune interferon (IFN gamma). J Immunol. 1981 Apr;126(4):1620–1623. [PubMed] [Google Scholar]
- Locksley R. M., Wilson C. B., Klebanoff S. J. Role for endogenous and acquired peroxidase in the toxoplasmacidal activity of murine and human mononuclear phagocytes. J Clin Invest. 1982 May;69(5):1099–1111. doi: 10.1172/JCI110545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., Kirkpatrick D., Fitzgerald P. A., Ching C. Y., Pahwa R. N., Good R. A., Smithwick E. M. Studies of the cell lineage of the effector cells that spontaneously lyse HSV-1 infected fibroblasts (NK(HSV-1)). J Immunol. 1982 Aug;129(2):824–828. [PubMed] [Google Scholar]
- McLeod R., Remington J. S. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979 May 10;27(1):19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Benjamin W. R., Farrar J. J. Macrophage activation for tumor cytotoxicity: induction of macrophage tumoricidal activity by lymphokines from EL-4, a continuous T cell line. J Immunol. 1982 Dec;129(6):2802–2807. [PubMed] [Google Scholar]
- Meyers J. D., McGuffin R. W., Neiman P. E., Singer J. W., Thomas E. D. Toxicity and efficacy of human leukocyte interferon for treatment of cytomegalovirus pneumonia after marrow transplantation. J Infect Dis. 1980 May;141(5):555–562. doi: 10.1093/infdis/141.5.555. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Byrne G. I., Rothermel C. D., Cartelli D. M. Lymphokine enhances oxygen-independent activity against intracellular pathogens. J Exp Med. 1983 Jul 1;158(1):234–239. doi: 10.1084/jem.158.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. How protozoa evade intracellular killing. Ann Intern Med. 1983 Jun;98(6):1016–1018. doi: 10.7326/0003-4819-98-6-1016. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. IV. Role of endogenous scavengers of oxygen intermediates. J Exp Med. 1980 Dec 1;152(6):1610–1624. doi: 10.1084/jem.152.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. R., Lazary S., Hitzig W. H. Production of migration inhibitory factor and blast cell transformation by cord blood lymphocytes. Int Arch Allergy Appl Immunol. 1976;50(5):593–605. doi: 10.1159/000231563. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Leonard E. J., Meltzer M. S. Macrophages in resistance to rickettsial infections: characterization of lymphokines that induce rickettsiacidal activity in macrophages. J Immunol. 1981 Jan;126(1):204–207. [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Possanza G., Cohen M. C., Yoshida T., Cohen S. Human macrophage migration inhibition factor: evidence for subunit structure. Science. 1979 Jul 20;205(4403):300–301. doi: 10.1126/science.377487. [DOI] [PubMed] [Google Scholar]
- Rodriguez M. A., Bankhurst A. D., Ceuppens J. L., Williams R. C., Jr Characterization of the suppressor cell activity in human cord blood lymphocytes. J Clin Invest. 1981 Dec;68(6):1577–1585. doi: 10.1172/JCI110412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Some physicochemical characteristics of an immune lymphocyte product which inhibits the multiplication of toxoplasma within mouse macrophages. Microbiol Immunol. 1979;23(1):17–30. doi: 10.1111/j.1348-0421.1979.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Stiehm E. R., Winter H. S., Bryson Y. J. Cellular (T cell) immunity in the human newborn. Pediatrics. 1979 Nov;64(5 Pt 2 Suppl):814–821. [PubMed] [Google Scholar]
- Thorley-Lawson D. A. The transformation of adult but not newborn human lymphocytes by Epstein Barr virus and phytohemagglutinin is inhibited by interferon: the early suppression by T cells of Epstein Barr infection is mediated by interferon. J Immunol. 1981 Mar;126(3):829–833. [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- Wilson C. B., Bohnsack J., Weaver W. M. Effects of muramyl dipeptide on superoxide anion release and on anti-microbial activity of human macrophages. Clin Exp Immunol. 1982 Aug;49(2):371–376. [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Haas J. E., Weaver W. M. Isolation, purification and characteristics of mononuclear phagocytes from human placentas. J Immunol Methods. 1983 Feb 11;56(3):305–317. doi: 10.1016/s0022-1759(83)80020-9. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Activity of human blood leukocytes against Toxoplasma gondii. J Infect Dis. 1979 Dec;140(6):890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Effects of monocytes from human neonates on lymphocyte transformation. Clin Exp Immunol. 1979 Jun;36(3):511–520. [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]


