Abstract
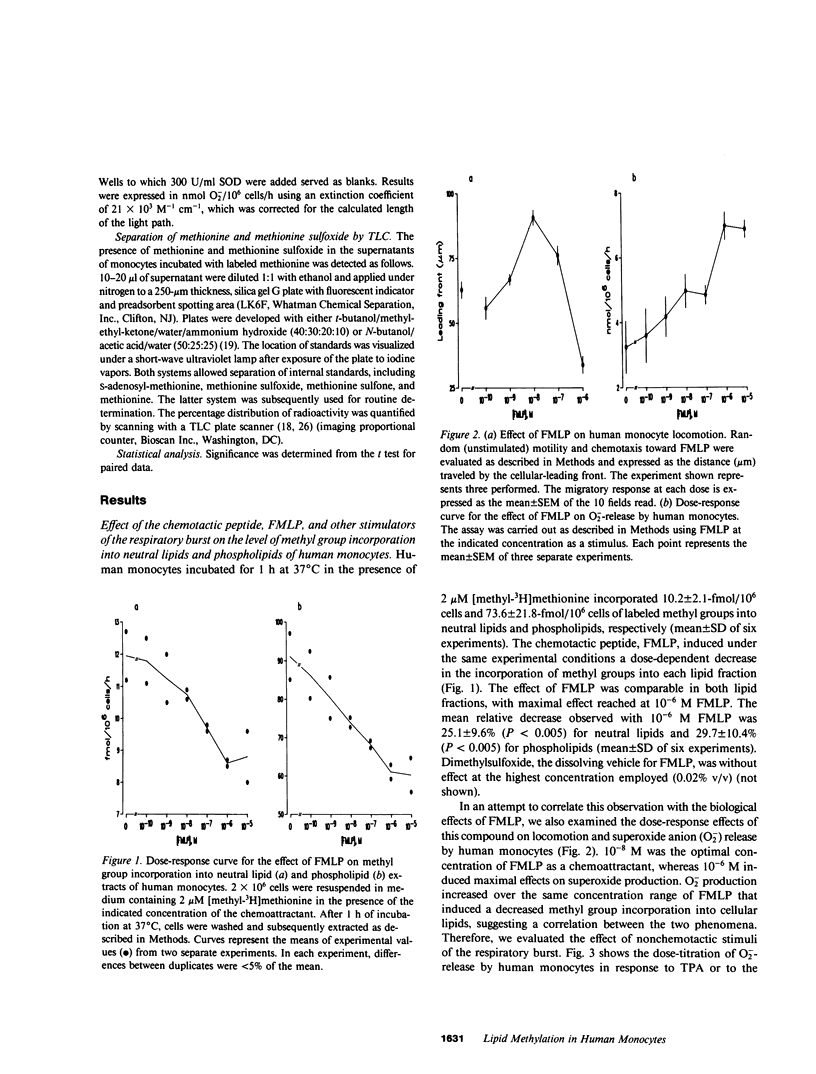
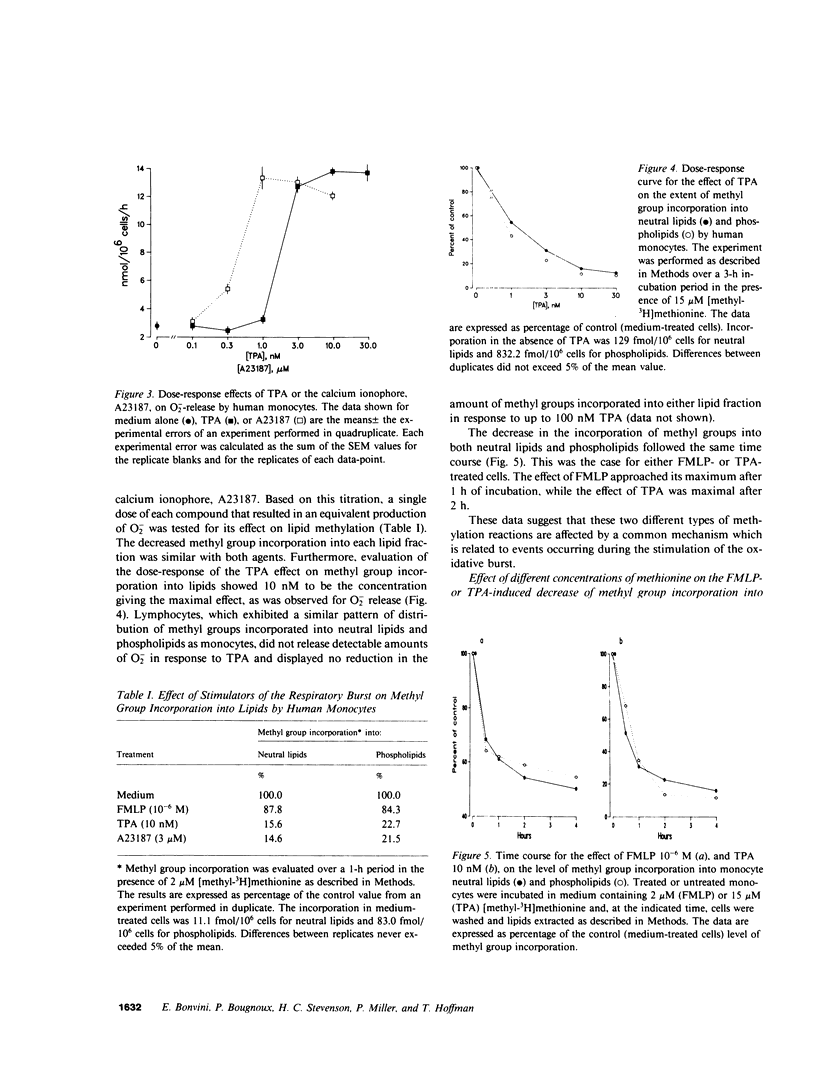
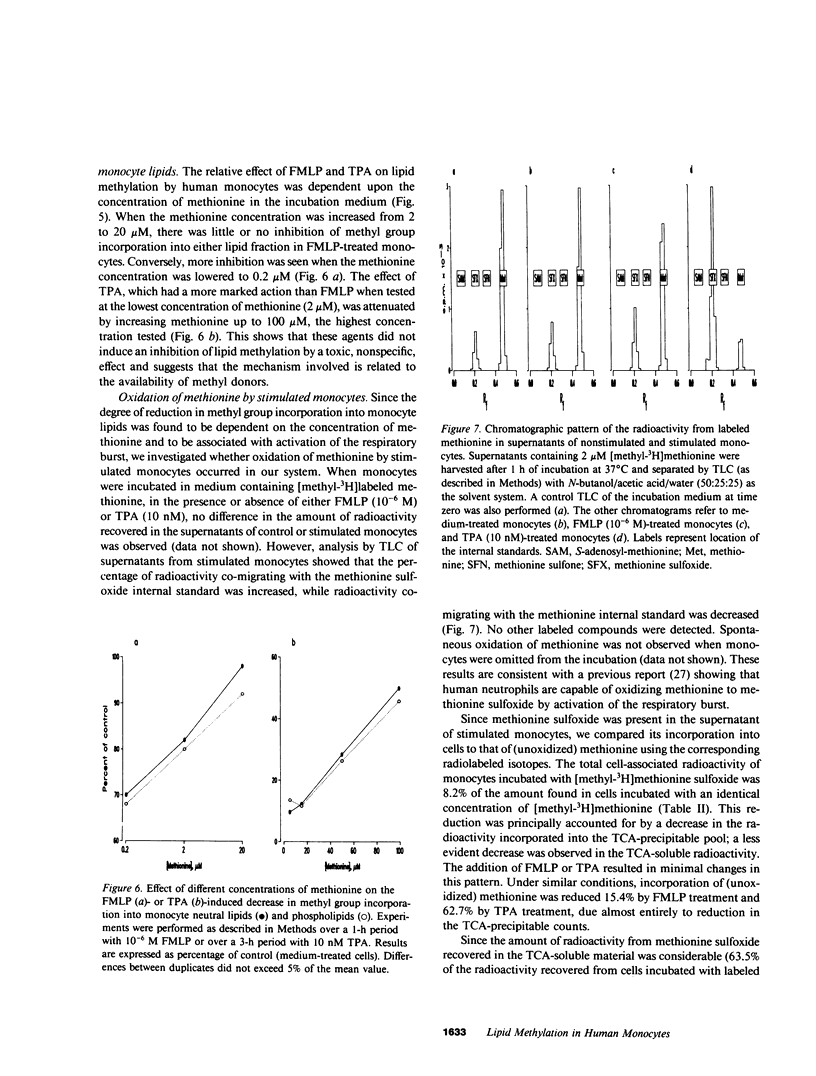
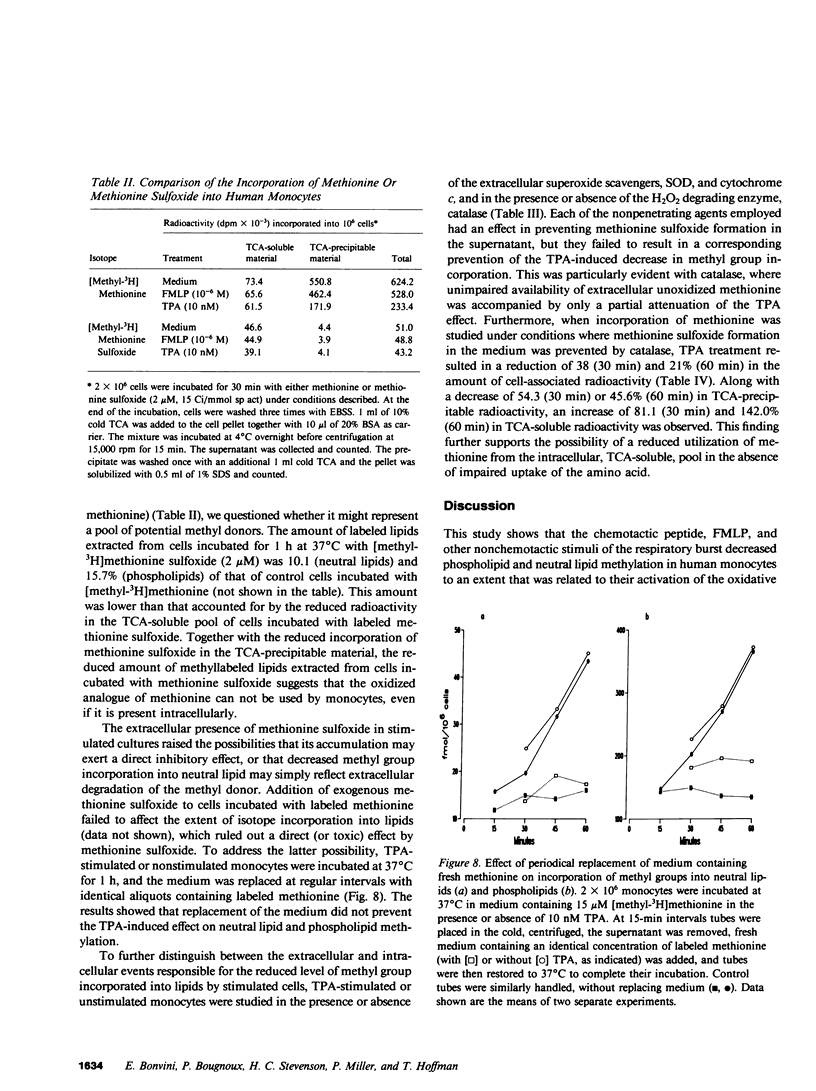
Chemotaxis and generation of the oxidative burst by phagocytes are among the biological functions thought to require methylation reaction(s) for their expression. The present study investigated the effect of different stimuli of the oxidative burst on lipid methylation by human elutriated monocytes as measured by methyl group incorporation from [methyl-3H]methionine into both phospholipid and neutral lipid extracts. Normal monocytes, incubated at 37 degrees C for 1 h with 2 microM methionine, incorporated 10.2-fmol/10(6) cells and 73.6-fmol/10(6) cells of methyl groups into neutral lipids and phospholipids, respectively. Stimulators of the respiratory burst, such as the chemotactic peptide N-formyl-L-methionyl-L-leucyl-L-phenylalanine, the tumor promoter, 12-O-tetradecanoyl phorbol-13-acetate, and the calcium ionophore, A23187, decreased the incorporation of methyl groups into both neutral lipids and phospholipids in a similar manner. Increasing the concentration of methionine in the medium reversed or attenuated the inhibition achieved at lower levels. An inverse relationship existed between the degree of methylation and the extent of stimulation of the oxidative burst, measured as superoxide anion (O-2) release. Stimulated monocytes oxidized methionine to methionine sulfoxide (which cannot act as a methyl-donor), and this was dependent on activation of the respiratory burst. Elimination of the accumulated methionine sulfoxide by replacement of the medium or by prevention of extracellular methionine oxidation by catalase did not effectively restore the normal level of methylation in stimulated cells, and the reduced methylation was not primarily related to a defective methionine uptake by stimulated monocytes. These data suggest that intracellular events related to activation of the respiratory burst are responsible for the decreased lipid methylation in stimulated cells, possibly by their leading to intracellular formation of methionine sulfoxide and by their limiting the availability of methyl-donor. This mechanism may be of potential relevance for the expression of biological functions where methionine-dependent reactions are involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Pike M. C., Snyderman R. The role of transmethylation reactions in regulating the binding of BCG-activated murine macrophages to neoplastic target cells. J Immunol. 1981 Jul;127(1):225–230. [PubMed] [Google Scholar]
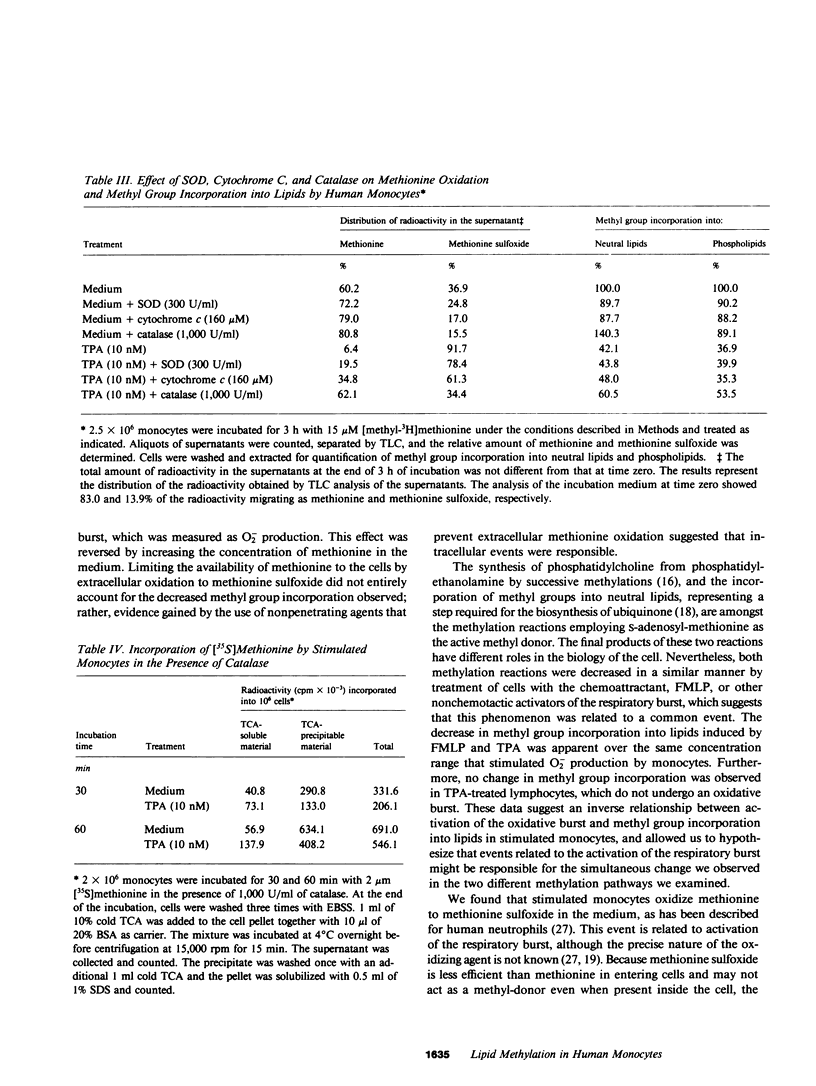
- Aksamit R. R., Falk W., Cantoni G. L. Inhibition of chemotaxis by S-3-deazaadenosylhomocysteine in a mouse macrophage cell line. J Biol Chem. 1982 Jan 25;257(2):621–625. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baird W. M., Diamond L., Borun T. W., Shulman S. Analysis of metabolism of carcinogenic polycyclic hydrocarbons by position-sensing proportional counting of thin-layer chromatograms. Anal Biochem. 1979 Oct 15;99(1):165–169. doi: 10.1016/0003-2697(79)90058-7. [DOI] [PubMed] [Google Scholar]
- Bougnoux P., Bonvini E., Stevenson H. C., Markey S., Zatz M., Hoffman T. Identification of ubiquinone-50 as the major methylated nonpolar lipid in human monocytes. Regulation of its biosynthesis via methionine-dependent pathways and relationship to superoxide production. J Biol Chem. 1983 Apr 10;258(7):4339–4344. [PubMed] [Google Scholar]
- Cantoni G. L. Biological methylation: selected aspects. Annu Rev Biochem. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- Fernstrom J. D., Wurtman R. J., Hammarstrom-Wiklund B., Rand W. M., Munro H. N., Davidson C. S. Diurnal variations in plasma concentrations of tryptophan, tryosine, and other neutral amino acids: effect of dietary protein intake. Am J Clin Nutr. 1979 Sep;32(9):1912–1922. doi: 10.1093/ajcn/32.9.1912. [DOI] [PubMed] [Google Scholar]
- Gil M. G., Alonso F., Sánchez-Crespo M., Mato J. M. Inhibition of phospholipid methyltransferase during zymosan induced secretion of platelet-activating factor in human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1981 Aug 14;101(3):740–748. doi: 10.1016/0006-291x(81)91813-1. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort E. N., Goy M. F., Larsen S. H., Adler J. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3939–3943. doi: 10.1073/pnas.72.10.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine F., Waller J. P., van Rapenbusch R. Studies on methionyl transfer RNA synthetase. 1. Purification and some properties of methionyl transfer RNA synthetase from Escherichia coli K-12. Eur J Biochem. 1968 Apr 3;4(2):213–221. doi: 10.1111/j.1432-1033.1968.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Chiang P. K., Cantoni G. L. The action of the adenosylhomocysteine hydrolase inhibitor, 3-deazaadenosine, on phagocytic function of mouse macrophages and human monocytes. Biochem Biophys Res Commun. 1978 Sep 14;84(1):102–109. doi: 10.1016/0006-291x(78)90269-3. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Early increases in phospholipid methylation are not necessary for the mitogenic stimulation of lymphocytes. J Biol Chem. 1982 Jul 25;257(14):8183–8189. [PubMed] [Google Scholar]
- O'Dea R. F., Viveros O. H., Axelrod J., Aswanikaumar S., Schiffmann E., Corcoran B. A. Raipid stimulation of protein carboxymethylation in leukocytes by a chemotatic peptide. Nature. 1978 Mar 30;272(5652):462–464. doi: 10.1038/272462a0. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylation: chemical, enzymological, and biological significance. Adv Enzymol Relat Areas Mol Biol. 1975;42:227–286. doi: 10.1002/9780470122877.ch5. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Role of transmethylation in the elicitation of an oxidative burst in macrophages. Cell Immunol. 1982 Sep 15;72(2):277–285. doi: 10.1016/0008-8749(82)90475-0. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Kredich N. M., Snyderman R. Phospholipid methylation in macrophages is inhibited by chemotactic factors. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2922–2926. doi: 10.1073/pnas.76.6.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Kredich N. M., Snyderman R. Requirement of S-adenosyl-L-methionine-mediated methylation for human monocyte chemotaxis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3928–3932. doi: 10.1073/pnas.75.8.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Transmethylation reactions are required for initial morphologic and biochemical responses of human monocytes to chemoattractants. J Immunol. 1981 Oct;127(4):1444–1449. [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Transmethylation reactions regulate affinity and functional activity of chemotactic factor receptors on macrophages. Cell. 1982 Jan;28(1):107–114. doi: 10.1016/0092-8674(82)90380-4. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Kredich N. M. Role of transmethylation reactions in cellular motility and phagocytosis. Mol Immunol. 1980 Feb;17(2):209–218. doi: 10.1016/0161-5890(80)90073-5. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. R., Koshland D. E., Jr Identification of a protein methyltransferase as the cheR gene product in the bacterial sensing system. Proc Natl Acad Sci U S A. 1977 Feb;74(2):533–537. doi: 10.1073/pnas.74.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Chen J. W. Oxidation of methionine by human polymorphonuclear leukocytes. J Clin Invest. 1980 May;65(5):1041–1050. doi: 10.1172/JCI109756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F. Myeloperoxidase-mediated oxidation of methionine and amino acid decarboxylation. Infect Immun. 1982 Apr;36(1):136–141. doi: 10.1128/iai.36.1.136-141.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



