Abstract
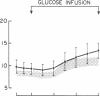
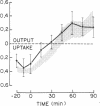
To identify the mechanism(s) of the altered glucoregulatory response to a glucose load in subjects with impaired glucose tolerance, we selectively quantitated the components of net splanchnic glucose balance, i.e., splanchnic glucose uptake and hepatic glucose output, as well as peripheral glucose uptake, by combining [3-3H]glucose infusion with hepatic vein catheterization. After intravenous glucose infusion (6 mg X kg-1 X min-1 for 90 min), blood glucose rose to 172 +/- 7 mg/dl in controls and 232 +/- 13 mg/dl in subjects with impaired glucose tolerance (P less than 0.01). The response of plasma insulin did not differ significantly between the two groups (29 +/- 4 vs. 40 +/- 10 microU/ml at 90 min in control and in glucose intolerant subjects, respectively; P = NS). In both groups, glucose infusion caused the net splanchnic glucose balance to switch from the net output of the basal state to a net glucose uptake. However, this effect was more marked in subjects with impaired glucose tolerance than in control subjects (at 90 min: 2.83 +/- 0.53 vs. 1.60 +/- 0.18 mg X kg-1 X min-1, respectively: P less than 0.05). The different pattern of splanchnic glucose balance was entirely accounted for by a greater rise in splanchnic glucose uptake in the group of glucose intolerants , as the suppression of endogenous glucose output by the glucose load was practically complete in both groups. In contrast, glucose uptake by peripheral tissues increased considerably less in subjects with impaired glucose tolerance than in controls (2.2-2.6 vs 3.6-4.1 mg X kg-1 X min-1, respectively, between 60 and 90 min; P less than 0.01-0.001). Furthermore, a net splanchnic lactate uptake was present in the basal state, which was inhibited by the glucose load and switched to a comparable net lactate output in both groups. These results indicate that the mechanism responsible for the altered glucoregulation in subjects with impaired glucose tolerance resides entirely in the peripheral tissues whose ability to dispose of a glucose load is drastically reduced. On the other hand, no defect is detectable in any of the explored mechanisms regulating splanchnic glucose metabolism during the disposal of an exogenous glucose load.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Felig P., Wahren J., Hendler R. Influence of maturity-onset diabetes on splanchnic glucose balance after oral glucose ingestion. Diabetes. 1978 Feb;27(2):121–126. doi: 10.2337/diab.27.2.121. [DOI] [PubMed] [Google Scholar]
- Ginsberg H., Kimmerling G., Olefsky J. M., Reaven G. M. Demonstration of insulin resistance in untreated adult onset diabetic subjects with fasting hyperglycemia. J Clin Invest. 1975 Mar;55(3):454–461. doi: 10.1172/JCI107951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Liljenquist J. E., Tobin J. D., Sherwin R. S., Watkins P., Andres R., Berman M. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest. 1975 May;55(5):1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz B., Jr Studies on hepatic glucose cycles in normal and methylprednisolone-treated dogs. Metabolism. 1977 Feb;26(2):157–170. doi: 10.1016/0026-0495(77)90051-8. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Peters N., Advani U., Perry G., Rogers J., Brough W. H., Pilkington T. R. Forearm glucose uptake during the oral glucose tolerance test in normal subjects. Diabetes. 1973 Jun;22(6):442–458. doi: 10.2337/diab.22.6.442. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEEVY C. M., MENDENHALL C. L., LESKO W., HOWARD M. M. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962 May;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Inculet R. The effects of ingested and intravenous glucose on forearm uptake of glucose and glucogenic substrate in normal man. Diabetes. 1983 Nov;32(11):977–981. doi: 10.2337/diab.32.11.977. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Reaven G. M., Bernstein R., Davis B., Olefsky J. M. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? Am J Med. 1976 Jan;60(1):80–88. doi: 10.1016/0002-9343(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Mechanism and significance of insulin resistance in non-insulin-dependent diabetes mellitus. Diabetes. 1981 Dec;30(12):990–995. doi: 10.2337/diab.30.12.990. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Saccà L., Vigorito C., Cicala M., Corso G., Sherwin R. S. Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol. 1983 Sep;245(3):E294–E302. doi: 10.1152/ajpendo.1983.245.3.E294. [DOI] [PubMed] [Google Scholar]
- Steele R., Rostami H., Altszuler N. A two-compartment calculator for the dog glucose pool in the nonsteady state. Fed Proc. 1974 Jul;33(7):1869–1876. [PubMed] [Google Scholar]