Abstract
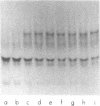
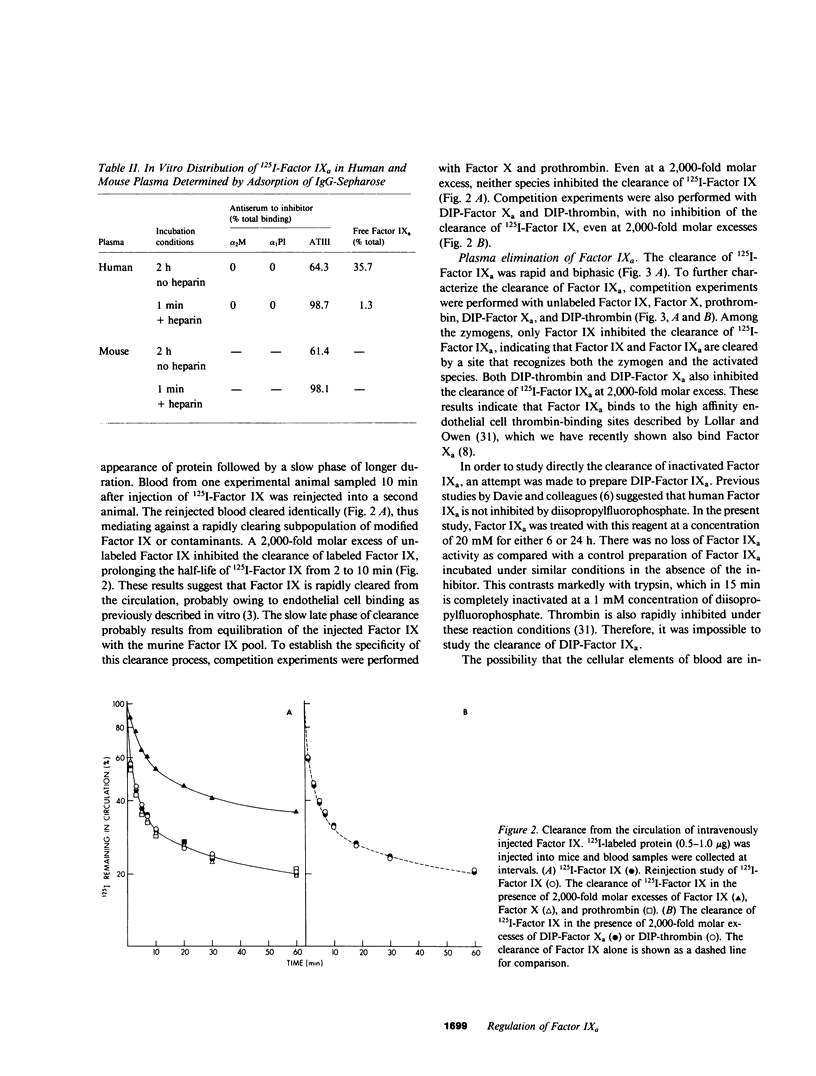
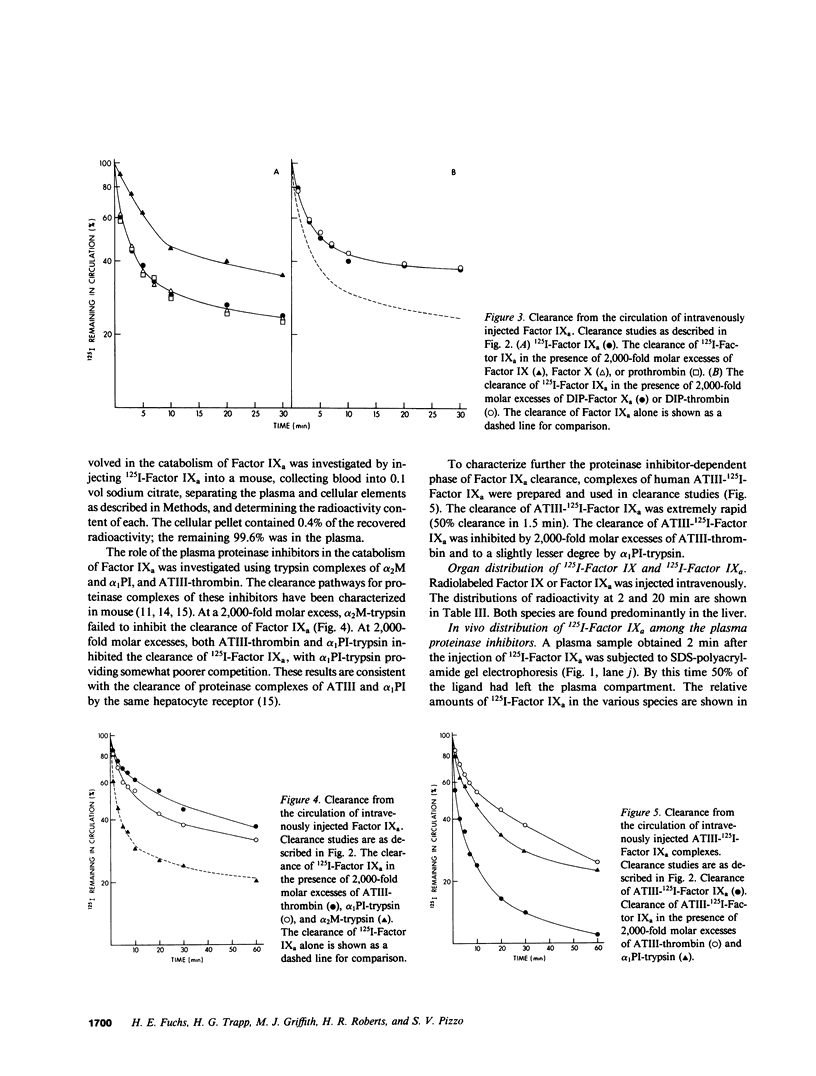
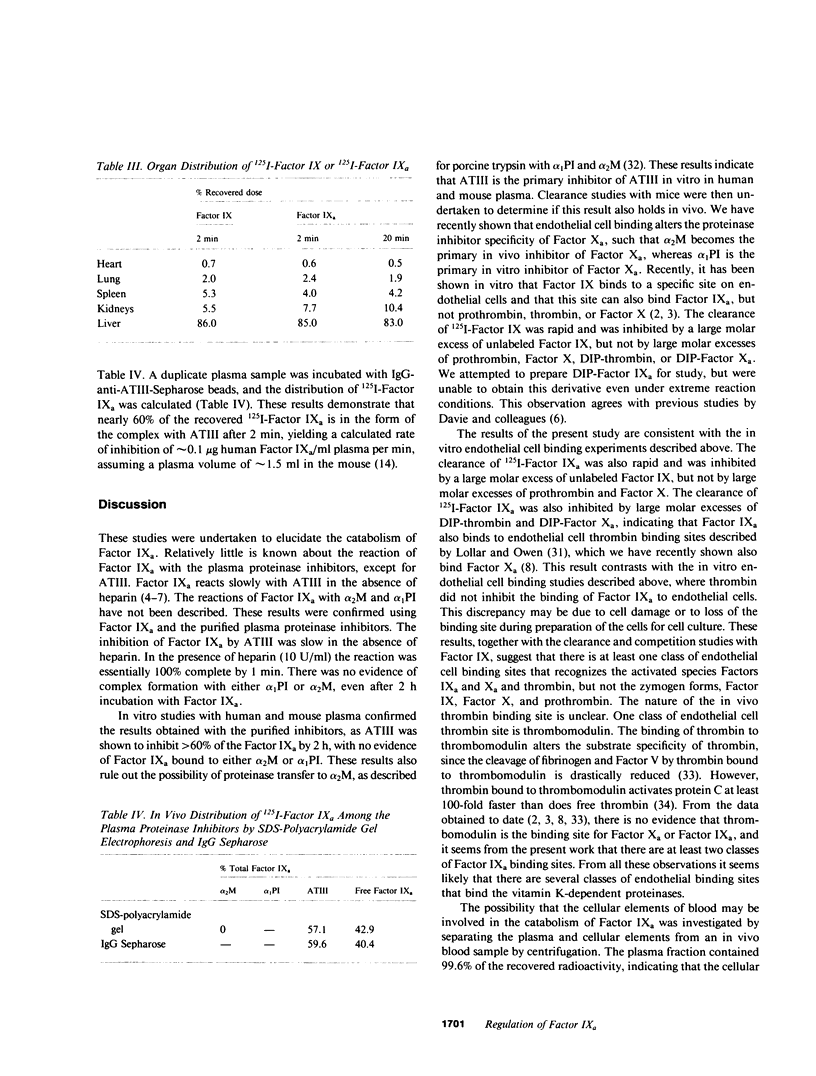
The regulation of human Factor IXa was studied in vitro in human and mouse plasma and in vivo in the mouse. In human plasma, approximately 60% of the 125I-Factor IXa was bound to antithrombin III (ATIII) by 2 h, with no binding to alpha 2-macroglobulin or alpha 1-proteinase inhibitor, as assessed by gel electrophoresis and IgG- antiproteinase inhibitor-Sepharose beads. In the presence of heparin, virtually 100% of the 125I-Factor IXa was bound to ATIII by 1 min. The distribution of 125I-Factor IXa in mouse plasma was similar. The clearance of 125I-Factor IXa was rapid (50% clearance in 2 min) and biphasic and was inhibited by large molar excesses of ATIII-thrombin and alpha 1-proteinase inhibitor-trypsin, but not alpha 2-macro-globulin-trypsin; it was also inhibited by large molar excesses of diisopropylphosphoryl - (DIP-) Factor Xa, DIP-thrombin, and Factor IX, but not by prothrombin or Factor X. The clearance of Factor IX was also rapid (50% clearance in 2.5 min) and was inhibited by a large molar excess of Factor IX, but not by large molar excesses of Factor X, prothrombin, DIP-Factor Xa, or DIP-thrombin. Electrophoresis and IgG- antiproteinase inhibitor-Sepharose bead studies confirmed that by 2 min after injection into the murine circulation, 60% of the 125I-Factor IXa was bound to ATIII. Organ distribution studies with 125I-Factor IXa demonstrated that most of the radioactivity was in the liver. These studies suggest that Factor IXa binds to at least two classes of binding sites on endothelial cells. One site apparently recognizes both Factors IX and IXa, but not Factor X, Factor Xa, prothrombin, or thrombin. The other site recognizes thrombin, Factor Xa, and Factor IXa, but not the zymogen forms of these clotting factors. After this binding, Factor IXa is bound to ATIII and the complex is cleared from the circulation by hepatocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abildgaard U., Lie M., Odegård O. R. Antithrombin (heparin cofactor) assay with "new" chromogenic substrates (S-2238 and Chromozym TH). Thromb Res. 1977 Oct;11(4):549–553. doi: 10.1016/0049-3848(77)90208-0. [DOI] [PubMed] [Google Scholar]
- Beatty K., Travis J., Bieth J. The effect of alpha 2-macroglobulin on the interaction of alpha 1-proteinase inhibitor with porcine trypsin. Biochim Biophys Acta. 1982 Jun 4;704(2):221–226. doi: 10.1016/0167-4838(82)90149-2. [DOI] [PubMed] [Google Scholar]
- Björk I., Jackson C. M., Jörnvall H., Lavine K. K., Nordling K., Salsgiver W. J. The active site of antithrombin. Release of the same proteolytically cleaved form of the inhibitor from complexes with factor IXa, factor Xa, and thrombin. J Biol Chem. 1982 Mar 10;257(5):2406–2411. [PubMed] [Google Scholar]
- Braunstein K. M., Noyes C. M., Griffith M. J., Lundblad R. L., Roberts H. R. Characterization of the defect in activation of factor IX Chapel Hill by human factor XIa. J Clin Invest. 1981 Dec;68(6):1420–1426. doi: 10.1172/JCI110393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Chung K. S., Madar D. A., Goldsmith J. C., Kingdon H. S., Roberts H. R. Purification and characterization of an abnormal factor IX (Christmas factor) molecule. Factor IX Chapel Hill. J Clin Invest. 1978 Nov;62(5):1078–1085. doi: 10.1172/JCI109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Davie E. W. Activation of human factor X (Stuart factor) by a protease from Russell's viper venom. Biochemistry. 1977 Nov 29;16(24):5253–5260. doi: 10.1021/bi00643a015. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L., Harris K. W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982 Jul 25;257(14):7944–7947. [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Fuchs H. E., Pizzo S. V. Regulation of factor Xa in vitro in human and mouse plasma and in vivo in mouse. Role of the endothelium and plasma proteinase inhibitors. J Clin Invest. 1983 Dec;72(6):2041–2049. doi: 10.1172/JCI111169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs H. E., Shifman M. A., Pizzo S. V. In vivo catabolism of alpha 1-proteinase inhibitor-trypsin, antithrombin III-thrombin and alpha 2-macroglobulin-methylamine. Biochim Biophys Acta. 1982 May 27;716(2):151–157. doi: 10.1016/0304-4165(82)90263-x. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Determination of alpha-2-macroglobulin as trypsin-protein esterase. Clin Chim Acta. 1966 Oct;14(4):493–501. doi: 10.1016/0009-8981(66)90037-4. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Balber A. E., Hubbard W. J., Pizzo S. V. Ligand binding, conformational change and plasma elimination of human, mouse and rat alpha-macroglobulin proteinase inhibitors. Biochem J. 1983 Jan 1;209(1):99–105. doi: 10.1042/bj2090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S. L., Einarsson M., Pizzo S. V. Catabolic pathways for streptokinase, plasmin, and streptokinase activator complex in mice. In vivo reaction of plasminogen activator with alpha 2-macroglobulin. J Clin Invest. 1982 Aug;70(2):412–423. doi: 10.1172/JCI110631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. K., Roberts R. C. Physical and chemical properties of human plasma alpha2-macroglobulin. Biochem J. 1978 Jul 1;173(1):27–38. doi: 10.1042/bj1730027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark R. L., Schwartz S. M. Binding of coagulation factors IX and X to the endothelial cell surface. Biochem Biophys Res Commun. 1983 Mar 16;111(2):723–731. doi: 10.1016/0006-291x(83)90365-0. [DOI] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Fujikawa K., Schmer G., Davie E. W. Inhibition of bovine factor IXa and factor Xabeta by antithrombin III. Biochemistry. 1976 Jan 27;15(2):373–377. doi: 10.1021/bi00647a021. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Schmer G., Hermodson M. A., Teller D. C., Davie E. W. Characterization of human, bovine, and horse antithrombin III. Biochemistry. 1976 Jan 27;15(2):368–373. doi: 10.1021/bi00647a020. [DOI] [PubMed] [Google Scholar]
- Lollar P., Owen W. G. Clearance of thrombin from circulation in rabbits by high-affinity binding sites on endothelium. Possible role in the inactivation of thrombin by antithrombin III. J Clin Invest. 1980 Dec;66(6):1222–1230. doi: 10.1172/JCI109973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measurement of alpha 1-antitrypsin in serum, by immunodiffusion and by enzymatic assay. Clin Chem. 1974 Mar;20(3):396–399. [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Pannell R., Johnson D., Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974 Dec 17;13(26):5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. S., McKenna P. W., Rosenberg R. D. Inhibition of human factor IXa by human antithrombin. J Biol Chem. 1975 Dec 10;250(23):8883–8888. [PubMed] [Google Scholar]
- Shifman M. A., Pizzo S. V. The in vivo metabolism of antithrombin III and antithrombin III complexes. J Biol Chem. 1982 Mar 25;257(6):3243–3248. [PubMed] [Google Scholar]
- Stern D. M., Drillings M., Nossel H. L., Hurlet-Jensen A., LaGamma K. S., Owen J. Binding of factors IX and IXa to cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4119–4123. doi: 10.1073/pnas.80.13.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara H., Sinohara H. Mouse plasma trypsin inhibitors. Isolation and characterization of alpha-1-antitrypsin and contrapsin, a novel trypsin inhibitor. J Biol Chem. 1982 Mar 10;257(5):2438–2446. [PubMed] [Google Scholar]
- Thompson A. R. High affinity binding of human and bovine thrombins to p-chlorobenzylamido-epsilon-aminocaproyl agarose. Biochim Biophys Acta. 1976 Jan 23;422(1):200–209. doi: 10.1016/0005-2744(76)90019-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]