Abstract
Objective
Providers recommend waiting to transplant patients with end-stage renal disease (ESRD) secondary to lupus nephritis (LN), to allow for quiescence of systemic lupus erythematosus (SLE)-related immune activity. However, these recommendations are not standardized, and we sought to examine whether duration of time to transplant was associated with risk of graft failure in U.S. LN-ESRD patients.
Methods
Using national ESRD surveillance data (United States Renal Data System), we identified 4743 U.S. patients with LN-ESRD who received a first transplant on or after 1/1/00 (follow-up through 9/30/11). The association of wait time (time from ESRD start to transplant) with graft failure was assessed with Cox proportional hazards models, with splines of the exposure to allow for non-linearity of the association and with adjustment for potential confounding demographic, clinical, and transplant factors.
Results
White LN-ESRD patients who were transplanted later (vs. <3 months on dialysis) were at increased risk of graft failure [adjusted HR (95% confidence interval): 3–12 months, 1.23 (0.93–1.63); 12–24 months, 1.37 (0.92–2.06); 24–36 months, 1.34 (0.92–1.97); and >36 months, 1.98 (1.31–2.99)]. However, no such association was seen among black recipients [3–12 months, 1.07 (0.79–1.45); 12–24 months, 1.01 (0.64–1.60); 24–36 months, 0.78 (0.51–1.18); and >36 months, 0.74 (0.48–1.13)].
Conclusion
While future studies are needed to examine the potential confounding effect of clinically recognized SLE activity on the observed associations, these results suggest that longer wait times to transplant may be associated with equivalent or worse, not better, graft outcomes among LN-ESRD patients.
Kidney transplantation has long been considered a viable option for most patients with end-stage renal disease (ESRD) due to systemic lupus erythematosus (SLE) and associated lupus nephritis (LN) (1). However, many U.S. providers suggest waiting to transplant patients until SLE is quiescent, as indicated by clinical signs such as low steroid requirement and normal complement levels, and rheumatologists and nephrologists often suggest waiting 3 months (2, 3) to 1 year (4, 5), respectively, after the start of ESRD, to allow for this quiescence. These recommendations—which appear to be based upon weak and contradictory evidence of patterns of immune activity in LN-ESRD patients (5)—are not standard and conflict with evidence from the overall ESRD population, in whom longer duration of ESRD prior to transplant is associated with worse transplantation outcomes (6). If these recommendations to wait are not associated with improved graft outcomes, transplantation in LN-ESRD patients may often be delayed unnecessarily, potentially leading to fewer transplantations or worse outcomes. Further, such consequences may be worse for certain subgroups, such as poor (7–9) and black (9) patients, who generally have worse graft outcomes than their wealthier and white counterparts.
A recent single-center study of Taiwanese LN-ESRD patients challenges recommendations for delaying transplantation, with findings suggesting that patients with longer dialysis time prior to transplant had worse graft outcomes (10). To our knowledge, there is no similar evidence addressing whether longer time to transplant is associated with worse kidney transplant outcomes among U.S. LN-ESRD patients. Further, the degree to which these associations may be modified by sociodemographic characteristics is not known. We address these questions using national surveillance data on ESRD patients to estimate the association of time from start of ESRD to kidney transplant with subsequent graft failure in U.S. LN-ESRD patients and to examine whether sociodemographic factors modify these associations.
Patients and Methods
Study Population and Data Sources
We examined U.S. patients with LN-ESRD who received a kidney transplant on or after 1/1/00 (follow-up through 9/30/11) using United States Renal Data System (USRDS) data (11). Use of these data, which include administrative data supplied by the Centers for Medicare & Medicaid Services (CMS) and the United Network for Organ Sharing (UNOS) on all U.S. patients treated for ESRD, was approved by the Emory Institutional Review Board. Follow-up in the USRDS is nearly complete due to universal coverage of ESRD-related services (11). We obtained primary attributed cause of ESRD, sociodemographics, and clinical factors from the CMS Medical Evidence Form (CMS-2728), completed on all incident ESRD patients. LN-ESRD was defined by a primary attributed cause of ESRD of secondary glomerulonephritis due to SLE on the CMS-2728 (ICD-9 code = 710.0). We obtained transplant and donor characteristics from UNOS. Census 2000 data on characteristics of the residential neighborhood, as defined by patient 5-digit ZIP code tabulation area (ZCTA), were obtained from the Minnesota Population Center (12) and linked by patient ZIP code to the USRDS data. Of the 4786 U.S. LN-ESRD patients receiving a first transplant on or after 1/1/00, 43 were excluded due to missing race/ethnicity, leaving 4743 for descriptive analyses (99.1% of available cases), and an additional 463 were excluded from models due to missing covariates of interest, leaving 4280 (89.4% of available cases) in the final models.
Study Variables
Wait time to transplant
Our exposure was the wait time to transplant, defined as time on dialysis prior to receiving a first transplant (date of first kidney transplant – date of first ESRD service). Because of a priori assumptions about the non-linearity of the association of the exposure with graft failure (2–5), wait time to transplant was examined based on categories by proposed rheumatology and nephrology cutoffs (<3, 3–12, 12–24, 24–36, and ≥36 months) as well as by splines (see Statistical Analysis).
Time to graft failure
Our outcome was time from transplant to graft failure (return to dialysis, receipt of a second kidney transplant, or death), defined as: (date of graft failure or censoring) – (date of transplant). Patients who did not have a graft failure in the observed study period were censored at the last date of follow-up (9/30/11).
Other variables
Sociodemographics of interest included age, sex, race/ethnicity, and insurance prior to ESRD (from the CMS-2728). Due to the relative lack of information at the individual level on socioeconomic status (SES) and the potential for neighborhood effects independent of individual SES, we also examined the percentage of residents reporting black race, the percentage of households living below 100% of the federal poverty threshold, and the percentage of residents aged ≥25 without a high school degree or equivalent in the patient’s residential ZCTA. Access to pre-ESRD care was determined by whether patients saw a nephrologist prior to starting ESRD treatment, from the CMS-2728. Smoking, BMI, comorbid conditions, and serum albumin and hemoglobin at the start of ESRD were also obtained from the CMS-2728. Recipient blood group, recipient peak panel reactive antibody (PRA) status, donor type (living vs. deceased), donor age, number of human leukocyte antigen (HLA) mismatches between donor and recipient, graft cold ischemia time, and occurrence of delayed graft function (defined as dialysis treatment in the week following transplantation) were obtained from UNOS.
Statistical Analysis
Patient characteristics were summarized overall and by categories of time to transplant, and Kaplan-Meier curves of time to graft failure by time to transplant were constructed. Scatter plots of crude graft failure risk showed a potential non-linear association of time to transplant with graft failure, and statistically significant departures from linearity were seen (P<0.001, P=0.32, and P=0.005 for overall, black, and white patients, respectively). Thus, Cox proportional hazards models with time to transplant parameterized as a restricted cubic spline with five knots placed at Harrell’s percentiles (13) were used to graph continuous, potentially non-linear functions of hazard ratios (HRs) for graft failure, as well as estimate HRs (14) at the medians of the intervals of interest (<3, 3–12, 12–24, 24–36, and ≥36 months). Those factors we found to be associated with both time to transplant and time to graft failure and were not thought a priori to be mediators of the association (e.g., delayed graft function) were considered potential confounders. Potential effect modification by individual race and insurance and by neighborhood composition of race, poverty, and education was tested using pairwise z tests of log(HR) values. Those variables without significant missing data (e.g., peak PRA) and that resulted in a ≥10% change in the estimate of the association of wait time to transplant with time to graft failure—after backward elimination of all potential confounders that did not change the estimate by at least 10% when removed—were included in the full model. Multilevel models with clustering at the neighborhood level were not necessary because 93% of neighborhoods (ZCTAs) included in this analysis had only one (77%) or two (16%) cases. Stata v. 13 (StataCorp, College Station, TX) was used for all analyses.
We examined the robustness of our results in several sensitivity analyses. First, models additionally adjusting for peak PRA and for pre-ESRD care (available 2005+ only)—as well as albumin, additional transplant factors, and propensity for early transplantation—were used to examine the effect of these potentially important confounders on our results. Propensity for early kidney transplantation (within <3 months vs. ≥3 months) was calculated from logistic models with adjustment for the same predictors used in the full Cox models. Because graft failures within 30 days might represent technical failures of the transplant surgery, analyses excluding these observations were performed. Analyses of graft failures excluding death and of patient death were also performed for comparison. While not an a priori effect modification of interest, we ran stratified models to examine whether the observed effects differed by donor type. Because disease course, wait times, and outcomes may differ for children vs. adults, we adjusted for pediatric status in addition to age. Finally, results using simple categorization (without allowing for a non-linear, continuous association) were estimated and compared to the main results.
Results
Characteristics of the Study Population
There were 1239 graft failures among 4743 transplant recipients with LN-ESRD, contributing a total of 21,507 person-years (median follow-up, 4 years). In general, the percentage of recipients who experienced graft failure over study follow-up was higher among those who waited longer periods on dialysis (25% for 3–12 months and 27–30% for >12 months) compared to those who were transplanted <3 months after start of dialysis (16%; Table 1). The mean age of incident ESRD was 35 years; 81% were female, 41% were black, and 25% had Medicaid (Table 1). Patients with longer wait times to transplant were generally younger, more likely to be black, to have Medicaid coverage, and to live in areas with higher proportions black, poor, and uneducated residents. They were also less likely to have pre-ESRD care and have a living donor; and had greater peak PRA, lower albumin and hemoglobin levels, and greater numbers of HLA mismatches, relative to those who waited shorter periods for their transplants (Table 1). Overall, nonparametric tests for trend across categories gave similar P values to ANOVA and χ2 tests (data not shown). Patients excluded from the models below due to missing covariates were not different from the overall population, including by race (38.0% vs. 40.9% black; P=0.13), except that those excluded were more likely to experience graft failure (32.2% vs. 25.5%; P=0.002) and were less likely to have a living donor (37.1% vs. 45.4%; P=0.001) or have hypertension (67.6% vs. 76.2%; P<0.001).
Table 1.
Characteristics of U.S. patients with end-stage renal disease attributed to lupus nephritis, who received a transplant (1/1/00-9/30/11), overall and by categories of time to transplant.
| Time to transplant
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | Overall | 0–3 months | 3–12 months | 12–24 months | 24–36 months | >36 months | P* |
| N (%) | 4743 | --- | 569 (12.0%) | 655 (13.8%) | 801 (16.9%) | 643 (13.6%) | 2075 (43.8%) | --- |
| Outcome | ||||||||
| No. (%) graft failures in category | 4743 | 1239 (26.1%) | 91 (16.0%) | 163 (24.9%) | 232 (29.0%) | 195 (30.3%) | 558 (26.9%) | <0.001 |
| Individual patient factors | ||||||||
| Mean age (SD) | 4743 | 35.1 (12.5) | 41.7 (12.1) | 35.8 (12.6) | 34.0 (13.0) | 34.3 (12.9) | 33.6 (11.6) | <0.001 |
| Sex (%) | 4743 | 0.22 | ||||||
| Female | 81.2 | 83.8 | 79.5 | 80.0 | 82.9 | 80.9 | ||
| Male | 18.8 | 16.2 | 20.5 | 20.0 | 17.1 | 19.1 | ||
| Race/ethnicity (%) | 4743 | <0.001 | ||||||
| White | 32.1 | 56.9 | 45.8 | 32.8 | 29.9 | 21.4 | ||
| Black | 40.6 | 15.8 | 28.4 | 39.1 | 42.3 | 51.3 | ||
| Hispanic | 17.2 | 12.7 | 17.9 | 17.9 | 18.0 | 17.8 | ||
| Other | 10.1 | 14.6 | 7.9 | 10.2 | 9.8 | 9.5 | ||
| Insurance pre-ESRD (%) | 4719 | <0.001 | ||||||
| Private | 46.7 | 69.4 | 55.9 | 47.5 | 43.5 | 38.1 | ||
| Medicaid | 25.0 | 8.3 | 18.5 | 25.2 | 30.8 | 29.9 | ||
| Other** | 28.3 | 22.3 | 25.6 | 27.3 | 25.7 | 31.9 | ||
| Pre-ESRD care (%)*** | 1356 | <0.001 | ||||||
| Yes | 85.7 | 97.4 | 87.3 | 78.9 | 79.5 | 82.6 | ||
| No | 14.3 | 2.6 | 12.7 | 21.1 | 20.5 | 17.4 | ||
| Median days to waitlisting (IQR) **** | 4306 | 278 (79,620) | −160 (−407, −55) | −86 (−23,172) | 242 (114,363) | 343 (176,606) | 524 (240,1094) | <0.001 |
| Smoking (%) | 4743 | 0.14 | ||||||
| Yes | 2.5 | 1.4 | 2.1 | 3.0 | 1.7 | 2.9 | ||
| No | 97.5 | 98.6 | 97.9 | 97.0 | 98.3 | 97.1 | ||
| Mean (SD) BMI | 4572 | 25.5 (6.2) | 25.3 (5.8) | 25.2 (5.6) | 25.3 (6.2) | 25.3 (6.1) | 25.7 (6.4) | 0.21 |
| BMI ≥35 (%) | 4572 | 0.02 | ||||||
| Yes | 7.4 | 6.0 | 5.9 | 6.3 | 7.0 | 9.0 | ||
| No | 92.6 | 94.0 | 94.1 | 93.7 | 93.0 | 91.0 | ||
| No. of comorbidities (%) | 4743 | 0.08 | ||||||
| 0 | 21.0 | 21.1 | 20.6 | 19.9 | 20.4 | 21.8 | ||
| 1 | 62.2 | 65.2 | 65.2 | 61.3 | 60.3 | 61.3 | ||
| 2+ | 16.8 | 13.7 | 14.2 | 18.8 | 19.3 | 16.9 | ||
| Hypertension (%) | 4743 | 0.56 | ||||||
| Yes | 75.4 | 76.6 | 76.6 | 76.4 | 75.4 | 74.2 | ||
| No | 24.6 | 23.4 | 23.4 | 23.6 | 24.6 | 25.8 | ||
| CVD (%) | 4743 | 0.53 | ||||||
| Yes | 11.1 | 11.1 | 10.4 | 12.2 | 12.4 | 10.6 | ||
| No | 88.9 | 88.9 | 89.6 | 87.8 | 87.6 | 89.5 | ||
| Blood group (%) | 4444 | <0.001 | ||||||
| AB | 4.2 | 5.1 | 4.3 | 5.5 | 5.6 | 2.9 | ||
| A | 32.2 | 38.2 | 34.9 | 36.6 | 31.6 | 28.2 | ||
| B | 14.5 | 11.1 | 13.9 | 15.3 | 12.9 | 15.9 | ||
| O | 49.1 | 45.7 | 47.0 | 42.7 | 49.9 | 53.0 | ||
| Peak PRA (%) | 2781 | <0.001 | ||||||
| <20% | 54.6 | 70.0 | 68.0 | 61.0 | 56.8 | 44.1 | ||
| 20–80% | 28.2 | 18.7 | 22.1 | 25.3 | 26.3 | 33.9 | ||
| >80% | 17.2 | 11.3 | 9.9 | 13.6 | 16.9 | 22.0 | ||
| Mean serum albumin at ESRD start (SD) | 3732 | 3.1 (0.8) | 3.7 (0.5) | 3.2 (0.8) | 3.0 (0.8) | 2.9 (0.8) | 3.0 (0.8) | <0.001 |
| Mean serum hemoglobin at ESRD start (SD) | 4307 | 9.5 (1.9) | 10.7 (1.7) | 9.8 (1.9) | 9.5 (1.8) | 9.3 (1.9) | 9.2 (1.9) | <0.001 |
| Patient neighborhood factors | ||||||||
| Percentage black in zip code (%) | 4527 | <0.001 | ||||||
| Above median (8.0%) | 50.1 | 33.5 | 42.2 | 47.8 | 52.1 | 57.6 | ||
| At or below median | 49.9 | 66.5 | 57.8 | 52.2 | 47.9 | 42.4 | ||
| Percentage non-HS grads in zip code (%) | 4527 | <0.001 | ||||||
| Above median (16.9%) | 50.0 | 32.2 | 41.8 | 48.3 | 51.1 | 57.9 | ||
| At or below median | 50.0 | 67.8 | 58.2 | 51.7 | 48.9 | 42.1 | ||
| Percentage living in poverty in zip code (%) | 4527 | <0.001 | ||||||
| Above median (11.2%) | 50.1 | 36.4 | 40.7 | 47.7 | 51.7 | 57.4 | ||
| At or below median | 49.9 | 63.6 | 59.3 | 52.3 | 48.3 | 42.6 | ||
| Donor/transplant factors | ||||||||
| Type (%) | 4730 | <0.001 | ||||||
| Living | 44.6 | 79.3 | 74.7 | 61.6 | 40.8 | 20.4 | ||
| Deceased | 55.4 | 20.7 | 25.3 | 38.4 | 59.2 | 79.6 | ||
| Mean donor age (SD) | 4509 | 36.6 (14.5) | 39.4 (12.9) | 38.1 (12.3) | 35.9 (14.4) | 35.6 (14.7) | 36.0 (15.4) | <0.001 |
| No. HLA mismatches (%) | 4642 | <0.001 | ||||||
| 0 | 11.9 | 16.5 | 16.0 | 13.6 | 12.5 | 8.5 | ||
| 1–2 | 15.1 | 23.5 | 22.6 | 20.3 | 15.5 | 8.5 | ||
| 3–4 | 38.5 | 35.9 | 37.8 | 41.1 | 38.7 | 38.4 | ||
| 5–6 | 34.4 | 24.1 | 23.6 | 25.0 | 33.3 | 44.6 | ||
| Mycophenolate mofetil use | 4304 | 0.43 | ||||||
| No | 15.3 | 16.7 | 10.9 | 16.3 | 17.2 | 15.4 | ||
| Yes | 84.7 | 83.3 | 89.1 | 83.7 | 82.9 | 84.6 | ||
| Mean cold ischemia time, hours (SD)***** | 2344 | 18.2 (9.8) | 18.2 (9.2) | 17.7 (9.5) | 18.8 (9.7) | 17.6 (9.4) | 18.3 (10.0) | 0.53 |
| Delayed graft function (%) | 4670 | <0.001 | ||||||
| Yes | 12.0 | 1.4 | 4.8 | 8.1 | 11.8 | 18.7 | ||
| No | 88.0 | 98.6 | 95.2 | 91.9 | 88.2 | 81.3 | ||
SD, standard deviation; ESRD, end-stage renal disease; BMI, body mass index; CVD, cardiovascular disease (includes pericarditis); PRA, panel reactive antibody; HLA, human leukocyte antigens.
P by ANOVA (continuous variables) or chi-square (categorical variables).
Includes none, Medicare, VA, and other.
2005+ only.
Days from start of ESRD treatment to waitlisting; negative values indicate placement on the waitlist prior to start of ESRD treatment (pre-emptive waitlisting).
Among transplants with deceased donors only.
Association of Wait Time to Transplant with Graft Failure
Crude analyses
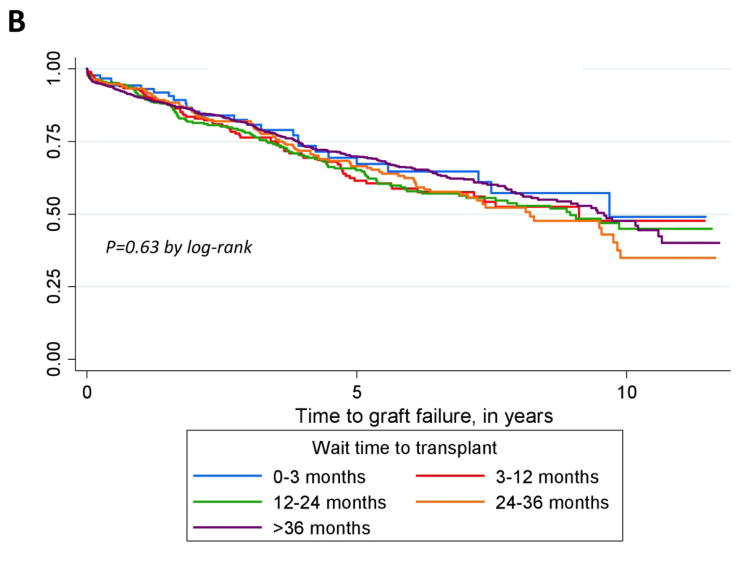
In Kaplan-Meier analyses by categorized time to transplant, LN-ESRD patients whose wait time to transplant was <3 months had longer times to graft failure than those whose wait times were ≥3 months (Figure 1A). Race-stratified analyses (Figure 1, B and C) suggested that this overall pattern held among whites (Figure 1C) but not among blacks (Figure 1B).
Figure 1.
Kaplan-Meier curves for time to graft failure among all (A), black (B), and white (C) U.S. patients with end-stage renal disease attributed to lupus nephritis, who received a transplant (1/1/00-9/30/11), by categories of time to transplant.
Effect modification
Interactions of wait time to transplant with black vs. white race in full models were statistically significant in the 24- to 36-month and >36-month intervals of wait time to transplant (P=0.029 and <0.001, respectively) but not in earlier intervals (P=0.15 and 0.10 in 3–12 and 12–24 vs. <3 months). However, there were no statistically significant interactions of wait time to transplant with Medicaid vs. private insurance or high vs. low neighborhood SES indicators, with adjustment. Thus, further analyses were shown overall and stratified by black vs. white race only.
Adjusted analyses
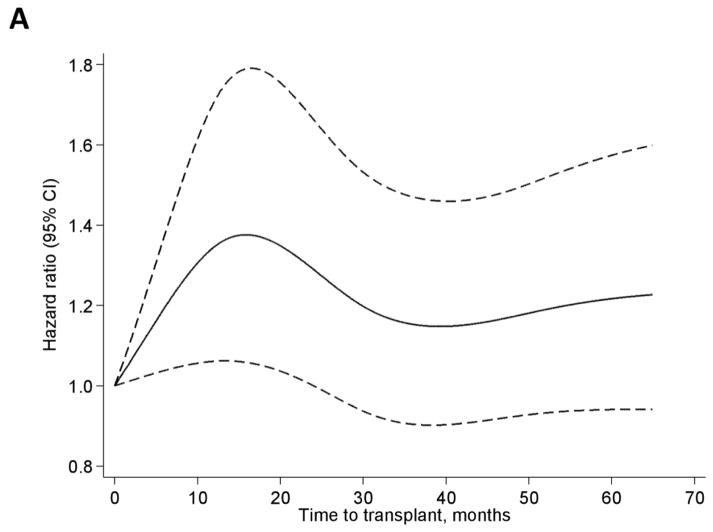
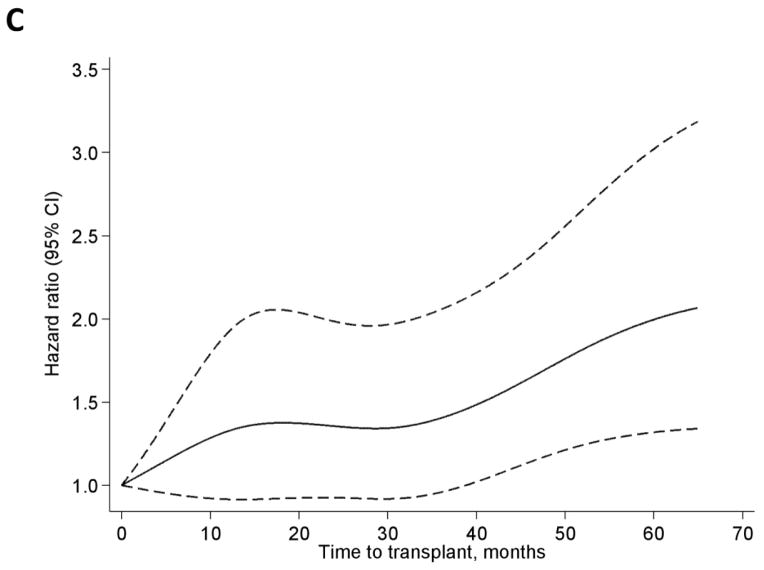
In the overall LN-ESRD population, wait times to transplant of 3–12 months and >12 months were associated with about 1.5- and 2-fold increased risk of graft failure, respectively, relative to <3 months of wait time, in crude analyses (Table 2). While these associations were attenuated with adjustment, particularly for age and race, even with full adjustment, wait times of 3–12 or 12–24 months were associated with 25% and 37% increased risk of graft failure, respectively, relative to wait times of <3 months. Similar associations and patterns were seen among whites, except that wait times >36 months were associated with nearly 2-fold risk of graft failure with full adjustment (Table 2). Among blacks, crude associations showed elevated risks that were not statistically significant among those with longer time to transplant; with adjustment, longer wait time was not associated with graft failure and even appeared (among those waiting >24 months) possibly protective against graft failure, relative to wait times <3 months (Table 2). Plots indicate a fairly steep increase in the adjusted HR of graft failure for wait time to transplant up to ~20 months in the overall population, with a subsequent slight decline and a slight increase after ~40 months (Figure 2A). Among blacks, the HR is maximized at ~12 months, with wide confidence intervals containing the null value at all time points (Figure 2B), whereas whites show a steadily increasing pattern (Figure 2C). It is worth noting that, with adjustment for age, insurance, hemoglobin, and donor type, blacks in this population remained at >40% greater risk of graft failure overall compared to whites (HR=1.41, 95% confidence interval, 1.21–1.63).
Table 2.
Crude and adjusted hazard ratios for graft failure among U.S. patients with end-stage renal disease attributed to lupus nephritis, who received a transplant (1/1/00-9/30/11), from restricted cubic splines of time to transplant.
| Time to transplant (months) | Median value in interval | Hazard ratio (95% CI) for graft failure at median value in interval*
|
|||
|---|---|---|---|---|---|
|
Adjusted
|
|||||
| Unadjusted | +Demographic | +Clinical | +Transplant | ||
| Overall | |||||
| <3 | 0 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 3–12 | 7.92 | 1.51 (1.27–1.80) | 1.32 (1.10–1.57) | 1.28 (1.07–1.53) | 1.25 (1.05–1.49) |
| 12–24 | 17.15 | 1.95 (1.51–2.51) | 1.54 (1.19–2.00) | 1.47 (1.13–1.92) | 1.37 (1.05–1.79) |
| 24–36 | 30.11 | 1.89 (1.50–2.37) | 1.46 (1.15–1.85) | 1.39 (1.10–1.77) | 1.20 (0.94–1.53) |
| >36 | 59.20 | 2.20 (1.76–2.76) | 1.59 (1.25–2.02) | 1.49 (1.17–1.90) | 1.21 (0.94–1.57) |
| Black | |||||
| <3 | 0 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 3–12 | 8.38 | 1.28 (0.96–1.72) | 1.14 (0.84–1.54) | 1.12 (0.83–1.51) | 1.07 (0.79–1.45) |
| 12–24 | 17.71 | 1.42 (0.91–2.22) | 1.16 (0.73–1.82) | 1.12 (0.71–1.77) | 1.01 (0.64–1.60) |
| 24–36 | 29.30 | 1.21 (0.81–1.79) | 0.97 (0.65–1.46) | 0.94 (0.63–1.42) | 0.78 (0.51–1.18) |
| >36 | 61.01 | 1.21 (0.82–1.78) | 0.98 (0.66–1.47) | 0.94 (0.63–1.41) | 0.74 (0.48–1.13) |
| White | |||||
| <3 | 0 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 3–12 | 7.52 | 1.32 (1.01–1.74) | 1.31 (1.00–1.72) | 1.24 (0.94–1.64) | 1.23 (0.93–1.63) |
| 12–24 | 17.41 | 1.57 (1.07–2.31) | 1.53 (1.04–2.27) | 1.42 (0.95–2.11) | 1.37 (0.92–2.06) |
| 24–36 | 29.86 | 1.59 (1.12–2.26) | 1.54 (1.08–2.21) | 1.45 (1.01–2.08) | 1.34 (0.92–1.97) |
| >36 | 54.99 | 2.52 (1.76–3.63) | 2.42 (1.66–3.51) | 2.16 (1.47–3.18) | 1.98 (1.31–2.99) |
CI, confidence interval. N=4280, 1750, and 1354 for overall, black, and white models, respectively. Demographic = age, race (overall model only; referent group=white), and insurance at start of end-stage renal disease (referent group=Medicaid); clinical = hemoglobin at start of end-stage renal disease; transplant = donor type (referent group=living donor).
Overall median values used for race-stratified analyses for comparability of hazard ratios.
Figure 2.
Hazard ratios by restricted cubic splines among all (A), black (B), and white (C) U.S. patients with end-stage renal disease attributed to lupus nephritis, who received a transplant (1/1/00-9/30/11). Adjusted for age, race (A only), insurance at start of ESRD, hemoglobin at start of ESRD, and donor type. Knots were placed at Harrell’s percentiles (corresponding to values of 0, 13.1, 30.6, 52.2, and 103.4 months).
Sensitivity analyses
With additional adjustment for PRA, we found that longer wait time to transplant was associated with higher risk of graft failure among whites but lower risk among blacks, although these associations were not statistically significant for either group, except for >36 vs. <3 months in whites (Table 3). Adjustment for albumin did not change the results (data not shown). Adjustment for pre-ESRD care (among those incident in 2005 or later) showed similar patterns of results to the primary analyses but with much less precision due to the reduced sample size, particularly among the groups with longer wait times. Adjustment for delayed graft function (a potential mediator), donor characteristics (age and race), and HLA mismatches did not change results, nor did adjustment for propensity to receive an early transplant (data not shown). Adjustment for proxies of secular trends in treatment, transplant year and treatment with mycophenolate mofetil (vs. azathioprine or other immunosuppressants), also did not change the results (data not shown). Excluding graft failures within 30 days and excluding deaths with functioning grafts from the graft failure definition (309/1239 graft failures) did not substantially change the results (Table 3). Risk of mortality after transplant did not differ by wait time to transplant, overall or stratified by race (data not shown). Analyses stratified by donor type showed that the effects seen in the primary analyses were stronger among those with living vs. deceased donors; additionally, the protective effects of longer wait time suggested among blacks in the primary analyses were statistically significant among those with deceased donors (Table 3). However, numbers of deceased donors in the referent groups were small (n=21 and 68 for blacks and whites, respectively), and these patients were older (48.6 and 50.4 years) and more likely to have private insurance (81.0% and 63.2%). Finally, indicators for pediatric status did not substantially change overall results, and associations from the primary analyses using restricted cubic splines of wait time to transplant were similar to those seen in analyses with simple categorization of wait time (data not shown).
Table 3.
Sensitivity analyses: adjusted hazard ratios among U.S. patients with end-stage renal disease attributed to lupus nephritis, who received a transplant (1/1/00-9/30/11), from restricted cubic splines of time to transplant.
| Time to transplant (months) | Hazard ratio (95% CI) for graft failure at median value in interval
|
|||||
|---|---|---|---|---|---|---|
| Adjusted for peak PRA (N=2442) | Adjusted for pre-ESRD care (2005+ only; N=1275) | Graft failures excluding deaths with functioning transplant* (N=4280) | Graft failures within 30 days excluded (N=4149) | Stratified by donor type:
|
||
| Living (N=1944) | Deceased (N=2336) | |||||
| Overall | ||||||
| <3 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 3–12 | 1.10 (0.89–1.35) | 1.56 (1.08–2.25) | 1.26 (1.02–1.55) | 1.25 (1.05–1.49) | 1.19 (0.94–1.50) | 1.12 (0.85–1.49) |
| 12–24 | 1.12 (0.82–1.53) | 1.79 (1.05–3.05) | 1.37 (1.05–1.88) | 1.37 (1.05–1.79) | 1.46 (1.04–2.04) | 1.05 (0.69–1.62) |
| 24–36 | 1.03 (0.77–1.37) | 1.25 (0.72–2.16) | 1.16 (0.87–1.55) | 1.20 (0.94–1.53) | 1.76 (1.28–2.42) | 0.76 (0.52–1.11) |
| >36 | 1.09 (0.81–1.47) | 1.51 (0.77–2.98) | 1.07 (0.79–1.45) | 1.21 (0.94–1.57) | 1.27 (0.87–1.86) | 0.86 (0.59–1.26) |
| Black | ||||||
| <3 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 3–12 | 0.93 (0.65–1.33) | 1.17 (0.68–2.00) | 1.07 (0.76–1.51) | 1.07 (0.79–1.45) | 1.09 (0.73–1.64) | 0.83 (0.52–1.33) |
| 12–24 | 0.83 (0.48–1.42) | 1.12 (0.51–2.43) | 1.02 (0.61–1.72) | 1.01 (0.64–1.60) | 1.14 (0.63–2.08) | 0.64 (0.31–1.34) |
| 24–36 | 0.69 (0.42–1.13) | 0.76 (0.35–1.64) | 0.79 (0.49–1.27) | 0.78 (0.51–1.18) | 1.06 (0.61–1.83) | 0.47 (0.24–0.91) |
| >36 | 0.68 (0.41–1.12) | 0.67 (0.27–1.68) | 0.67 (0.41–1.08) | 0.74 (0.48–1.13) | 0.70 (0.37–1.31) | 0.49 (0.26–0.95) |
| White | ||||||
| <3 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 3–12 | 1.15 (0.83–1.61) | 1.44 (0.72–2.85) | 1.20 (0.84–1.71) | 1.23 (0.93–1.63) | 0.99 (0.69–1.42) | 1.47 (0.94–2.30) |
| 12–24 | 1.23 (0.77–1.98) | 1.51 (0.58–3.96) | 1.26 (0.76–2.10) | 1.37 (0.92–2.06) | 1.26 (0.76–2.10) | 1.41 (0.74–2.72) |
| 24–36 | 1.21 (0.78–1.89) | 1.22 (0.38–3.97) | 1.12 (0.69–1.81) | 1.34 (0.92–1.97) | 2.29 (1.43–3.66) | 0.75 (0.41–1.36) |
| >36 | 1.97 (1.21–3.21) | 11.0 (2.61–46.1) | 1.83 (1.11–3.04) | 1.98 (1.31–2.99) | 1.88 (1.05–3.35) | 1.66 (0.90–3.04) |
CI, confidence interval; PRA, panel reactive antibody; ESRD, end-stage renal disease. Adjusted for age, race (overall model only; referent group=white), insurance at start of end-stage renal disease (referent group=Medicaid), hemoglobin at start of end-stage renal disease, and donor type (non-donor type-stratified models only; referent group=living donor). PRA: <20% (referent group), 20–80%, and ≥80%); pre-ESRD care: yes and no (=referent group).
Censoring 309/1239 graft failure events that were deaths with a functioning transplant.
Discussion
In this national study of kidney transplant recipients with ESRD secondary to LN, we found that longer wait times to transplant were not associated with lower risk of graft failure among these patients, as might be expected from current clinical recommendations (2–5). Rather, we found that longer time on dialysis was generally associated with increased risk of graft failure among LN-ESRD patients, relative to those patients who were transplanted in the first 3 months of ESRD treatment, although results were not always statistically significant. Our effect estimates were similar to those seen in the overall ESRD population, in whom wait times to transplantation of >6 months and >1 year, relative to 0–15 days, have been shown to be associated with approximately 25% and 40% increased graft failure risk (6). In our study, relative to waiting <3 months, waiting ≥3 years for kidney transplantation was associated with a 2-fold risk of graft failure among white LN-ESRD patients, whereas longer wait time was generally associated with similar risk of graft failure among black LN-ESRD patients. Even in the fully adjusted models, where there was a non-statistically significant suggestion of a protective effect among black LN-ESRD patients whose wait times were ≥2 years, we did not see increased risk of graft failure among those transplanted early. While the confounding effect of SLE activity at the start of ESRD cannot be fully accounted for with adjustment for markers such as albumin, hemoglobin, and peak PRA, nevertheless these results provide, to our knowledge, a first examination of the association between wait time to transplant and graft outcomes in a nationally representative population of U.S. LN-ESRD patients that can be used to generate hypotheses and guide future study of this issue.
Patients with LN-ESRD could, in many ways, be considered ideal kidney transplant candidates, due to their relative youth (median age, 38) (11), lower likelihood of malignancies or cardiovascular contraindications (15), close medical supervision and potentially better pre-ESRD management by multiple providers (including rheumatologists and nephrologists) (16), and demonstrated adherence to complex immunosuppression regimens (15, 16). These patients may also be more likely to identify living donors; we found that transplants from living donors were overrepresented in these recipients with LN-ESRD (45%, compared to 33% of all U.S. transplant recipients in 2011) (11).
There are also unique barriers to transplant among LN-ESRD patients, such as the potential for post-transplant recurrence of LN and subsequent development of glomerulonephritis in the graft, making SLE a potential contraindication to transplantation (17). However, in a recent national study of transplant recipients with SLE (n=6850) (18), only 2% were reported to have recurrent LN, and only 7% of all graft failures in this population were attributed to recurrent LN (18). Further, graft and patient survival are comparable among U.S. patients with ESRD due to LN vs. other causes (19, 20).
Despite the increasing evidence of likely equivalent transplant outcomes among LN-ESRD patients (18–20), the incidence of kidney transplantation is not increasing among LN-ESRD patients (21). Greater demand on the organ supply from the growing overall ESRD population as well as CMS policies that currently limit medication coverage among younger patients who qualify for Medicare based solely on ESRD status (22) may contribute to this observed discrepancy. However, lingering provider beliefs about the necessity of waiting periods to establish relative quiescence of SLE in the setting of ESRD prior to kidney transplantation (3, 5) may also play a role.
Our results suggest U.S. recommendations for transplantation in LN-ESRD (2–5) may not align with evidence from the target population. To our knowledge, no studies have reported the association of graft failure with duration of wait time to transplant in LN-ESRD patients in the United States, or in Canada or Europe, where renal transplantation guidelines similarly recommend waiting periods prior to transplantation for LN-ESRD patients (23, 24). Chung et al. (10) recently examined this issue in a single-center study (n=31) in Taiwan and found a slightly increased risk for graft dysfunction and equivalent risk for graft failure with longer wait times, although their results were not statistically significant.
Importantly for the U.S. population, we found a potential effect modification by race, in that longer wait times were associated with greater risk of graft failure among white but not black kidney transplant recipients with LN-ESRD and that there was a possible protective effect of wait times of ≥2 years among blacks. This observation could be due to unexplained differences in disease pathology and course between white and black LN-ESRD patients. We found that early transplant, possibly indicating SLE quiescence prior to the need for renal replacement therapy, was more common among whites than blacks.
Black ESRD patients treated with dialysis have long been known to have a survival advantage over their white counterparts (25, 26), although this pattern may be reversed in younger ESRD patients (27). Social differences associated with race that affect access to care could also play a role, although our failure to find evidence of effect modification by insurance status, poverty, or education does not support this explanation. Unavoidable bias inherent in the study design, namely index event bias—which occurs when examined risk factors (here, longer wait time to transplant) are not seen in the unselected (non-transplanted) population (28)—may also explain the results. It is also possible that the overwhelming effect of race on graft failure in the LN-ESRD population masks any effect of prolonged wait time in this subpopulation, although our estimates and estimates in another national U.S. study suggested only a 1.4-fold increased risk of graft failure for blacks vs. whites (9).
Confounding due to differences in unmeasured SLE activity (confounding by indication) may be the most serious threat to the internal validity of our findings. Although we tried to control for potential proxies (hemoglobin, albumin, and peak PRA) and for the propensity to be transplanted early, the USRDS does not have information on SLE-specific disease activity prior to transplantation and during the first year of dialysis, which could have been associated with decisions to delay transplantation for some patients and may have also influenced graft outcomes. However, in their Taiwanese population, Chung et al. (10) found that pre-transplant SLE activity was not associated with graft dysfunction or failure. Future studies in U.S. SLE cohorts or registries that collect information on SLE activity could potentially examine whether a similar lack of effect of SLE activity exists in the U.S. kidney transplant recipients with SLE.
Other residual confounders may have influenced our results. Receipt of a kidney graft has long been known to be differential by race in the overall ESRD population (29, 30). This could lead to important, unobserved differences in the white and black LN-ESRD kidney transplant recipient populations. However, we found that 41% of transplant recipients with LN-ESRD were black, compared to 45% of all LN-ESRD patients (11), suggesting receipt, if not timing, of transplant may not be differential by race among U.S. LN-ESRD patients. Unmeasured provider characteristics that are associated with wait times could also be associated with graft outcomes.
In addition to the limitations noted above, the potentially low sensitivity of attributed ESRD cause (31) could bias our results. Additionally, our individual socioeconomic status data were limited and some misclassification due to assigning neighborhood-level characteristics to individuals, particularly using ZCTAs rather than census tract or blocks (32), is likely. However, our study also has several strengths, including the capture of all U.S. patients who receive kidney transplants, limited loss to follow-up with no competing risks, and limited potential for selection bias due to excluded data.
In summary, we found that, among U.S. LN-ESRD patients receiving a kidney transplant, waiting 3 or 12 months on dialysis treatment was generally associated with equal or even greater risk of graft failure compared to being transplanted within 3 months, which is not expected given current clinical recommendations. As in the general ESRD population, waiting to transplant may not advantage LN-ESRD kidney transplant recipients in terms of graft outcomes. Even in the case of apparently equivalent graft outcomes among black LN-ESRD transplant recipients, regardless of waiting time, delays in transplantation may be not only unnecessary but also detrimental to other outcomes important to this young population, particularly quality of life, perceived health status, and employment (33). While these results should be considered hypothesis-generating due to the limitations of the data, future studies with SLE cohorts could determine whether longer wait times are associated with increased risk of graft failure, independent of SLE activity, strengthening the evidence for standardizing recommendations. Further, compared to the general ESRD population, LN-ESRD patients receive medical care by multiple providers, resulting in greater opportunities to intervene early to decrease wait time to transplant and, potentially, to improve transplant outcomes.
Significance and Innovations.
We found that longer wait times to transplant may not advantage U.S. kidney transplant recipients with ESRD secondary to systemic lupus erythematosus (SLE) and lupus nephritis, in terms of graft outcomes
While these results are hypothesis-generating, future cohort studies could determine whether longer wait times remain associated with increased risk of graft failure, independent of clinically recognized SLE activity that may delay transplantation
Patients with end-stage renal disease secondary to lupus nephritis receive medical care by multiple providers, including nephrologists and rheumatologists, resulting in greater opportunities to intervene early to decrease wait time to transplant and, potentially, improve transplant outcomes
Acknowledgments
Financial Support
L.C.P. was supported by the Laney Graduate School, Emory University. R.E.P. was supported in part by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH; ULl TR000454 and KL2TR000455). R.E.P. and S.O.P are both supported in part by 1R24MD008077-01 through the National Institute on Minority Health and Health Disparities. M.R.K. is supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the NIH (K01HD074726). S.S.L. and C.D. are supported in part by the NIH (R01AR065493).
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government. This work was presented in part at the World Transplant Congress, held July 26–31, 2014, in San Francisco, California. R.E.P. was supported in part by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH; ULl TR000454 and KL2TR000455). R.E.P. and S.O.P are both supported in part by 1R24MD008077-01 through the National Institute on Minority Health and Health Disparities. M.R.K. is supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the NIH (K01HD074726). S.S.L. and C.D. are supported in part by the NIH (R01AR065493).
Footnotes
Disclosure
S.O.P. is a minority shareholder in Fresenius College Park Dialysis, College Park, Georgia.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Renal transplantation in congenital and metabolic diseases. A report from the ASC/NIH renal transplant registry. J Am Med Assoc. 1975;232(2):148–53. doi: 10.1001/jama.1975.03250020022018. [DOI] [PubMed] [Google Scholar]
- 2.Imboden JB, Hellmann DB, Stone JH, editors. Current diagnosis and treatment: rheumatology. New York, NY: McGraw-Hill; 2013. [Google Scholar]
- 3.Mojcik CF, Klippel JH. End-stage renal disease and systemic lupus erythematosus. Am J Med. 1996;101(1):100–7. doi: 10.1016/s0002-9343(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 4.Balow JE. Renal manifestations of systemic lupus erythematosus. In: Greenberg A, editor. Primer on kidney diseases. Philadelphia: Saunders Elsevier; 2009. pp. 208–213. [Google Scholar]
- 5.Moroni G, Tantardini F, Ponticelli C. Renal replacement therapy in lupus nephritis. J Nephrol. 2003;16(6):787–91. [PubMed] [Google Scholar]
- 6.Goldfarb-Rumyantzev A, Hurdle JF, Scandling J, Wang Z, Baird B, Barenbaum L, et al. Duration of end-stage renal disease and kidney transplant outcome. Nephrol Dial Transplant. 2005;20(1):167–75. doi: 10.1093/ndt/gfh541. [DOI] [PubMed] [Google Scholar]
- 7.Press R, Carrasquillo O, Nickolas T, Radhakrishnan J, Shea S, Barr RG. Race/ethnicity, poverty status, and renal transplant outcomes. Transplantation. 2005;80(7):917–24. doi: 10.1097/01.tp.0000173379.53347.31. [DOI] [PubMed] [Google Scholar]
- 8.Stephens MR, Evans M, Ilham MA, Marsden A, Asderakis A. The influence of socioeconomic deprivation on outcomes following renal transplantation in the United kingdom. Am J Transplant. 2010;10(7):1605–12. doi: 10.1111/j.1600-6143.2010.03041.x. [DOI] [PubMed] [Google Scholar]
- 9.Nee R, Jindal RM, Little D, Ramsey-Goldman R, Agodoa L, Hurst FP, et al. Racial differences and income disparities are associated with poor outcomes in kidney transplant recipients with lupus nephritis. Transplantation. 2013;95(12):1471–8. doi: 10.1097/TP.0b013e318292520e. [DOI] [PubMed] [Google Scholar]
- 10.Chung MC, Yu TM, Shu KH, Lan JL, Chen DY, Ho HC, et al. Influence of pretransplantation dialysis time and lupus activity on outcome of kidney transplantation in systemic lupus erythematosus. Transplant Proc. 2014;46(2):336–8. doi: 10.1016/j.transproceed.2013.11.085. [DOI] [PubMed] [Google Scholar]
- 11.United States Renal Data System. USRDS. 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. Available at: www.usrds.org. [Google Scholar]
- 12.Minnesota Population Center. National Historical Geographic Information System: Version 2.0. Minneapolis, MN: University of Minnesota; 2011. Available at: www.nhgis.org. [Google Scholar]
- 13.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic regression, and survival analyses. New York: Springer; 2001. [Google Scholar]
- 14.Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011;11(1):1–29. [Google Scholar]
- 15.Batabyal P, Chapman JR, Wong G, Craig JC, Tong A. Clinical practice guidelines on wait-listing for kidney transplantation: consistent and equitable? Transplantation. 2012;94(7):703–13. doi: 10.1097/TP.0b013e3182637078. [DOI] [PubMed] [Google Scholar]
- 16.Danovitch G, editor. Handbook of Kidney Transplantation. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010. [Google Scholar]
- 17.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, et al. Racial disparities in access to renal transplantation--clinically appropriate or due to underuse or overuse? N Engl J Med. 2000;343(21):1537–44. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contreras G, Mattiazzi A, Guerra G, Ortega LM, Tozman EC, Li H, et al. Recurrence of lupus nephritis after kidney transplantation. J Am Soc Nephrol. 2010;21(7):1200–7. doi: 10.1681/ASN.2009101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward MM. Outcomes of renal transplantation among patients with end-stage renal disease caused by lupus nephritis. Kidney Int. 2000;57(5):2136–43. doi: 10.1046/j.1523-1755.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 20.Bunnapradist S, Chung P, Peng A, Hong A, Chung P, Lee B, et al. Outcomes of renal transplantation for recipients with lupus nephritis: analysis of the Organ Procurement and Transplantation Network database. Transplantation. 2006;82(5):612–8. doi: 10.1097/01.tp.0000235740.56573.c6. [DOI] [PubMed] [Google Scholar]
- 21.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63(6):1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grubbs V, Plantinga LC, Vittinghoff E, O’Hare AM, Dudley RA. Medicare immunosuppressant coverage and access to kidney transplantation: a retrospective national cohort study. BMC Health Serv Res. 2012;12:254. doi: 10.1186/1472-6963-12-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, et al. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. Can Med Assoc J. 2005;173(10):1181–4. doi: 10.1503/cmaj.051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalble T, Alcaraz K, Budde K, Humke U, Karam G, Lucan M, et al. Guidelines on renal transplantation. Arnhem: European Association of Urology; 2009. Available at: www.uroweb.org/fileadmin/tx_eauguidelines/2009/Full/Renal_Transplant.pdf. [Google Scholar]
- 25.Ojo AO, Port FK, Wolfe RA, Mauger EA, Williams L, Berling DP. Comparative mortality risks of chronic dialysis and cadaveric transplantation in black end-stage renal disease patients. Am J Kidney Dis. 1994;24(1):59–64. doi: 10.1016/s0272-6386(12)80160-0. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 27.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, et al. Association of race and age with survival among patients undergoing dialysis. J Am Med Assoc. 2011;306(6):620–6. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smits LJ, van Kuijk SM, Leffers P, Peeters LL, Prins MH, Sep SJ. Index event bias-a numerical example. J Clin Epidemiol. 2013;66(2):192–6. doi: 10.1016/j.jclinepi.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med Clin N Am. 2005;89(5):1003–31. ix. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Navaneethan SD, Singh S. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplantation. 2006;20(6):769–75. doi: 10.1111/j.1399-0012.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 31.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, et al. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol. 2010;5(11):2046–52. doi: 10.2215/CJN.03550410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–82. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 33.Kutner NG, Brogan D, Kutner MH. End-stage renal disease treatment modality and patients’ quality of life. Longitudinal assessment. Am J Nephrol. 1986;6(5):396–402. doi: 10.1159/000167200. [DOI] [PubMed] [Google Scholar]