Abstract
Opiates are indispensable for the treatment of moderate to severe pain. The gastrointestinal tract is one of the major victims of the undesired effects of opiates, because the enteric nervous system expresses all major subtypes of opioid receptors. As a result, propulsive motility and secretory processes in the gut are inhibited by opioid analgesics, and the ensuing constipation is one of the most frequent and troublesome adverse reactions. Many treatments involving laxatives, prokinetic drugs and opioid-sparing regimens have been explored to circumvent opioid-induced bowel dysfunction, but the outcome has in general been unsatisfactory. Specific antagonism of peripheral opioid receptors offers a more rational approach to the management of the adverse actions of opioid analgesics in the gut. This goal is currently addressed by the use of opioid receptor antagonists with limited absorption such as oral naloxone and by the development of peripherally restricted opioid receptor antagonists such as methylnaltrexone and alvimopan. These investigational drugs hold considerable promise in preventing constipation due to opiate treatment, whereas the analgesic action of opiates remains unabated. Postoperative ileus associated with opioid-induced postsurgical pain control is likewise ameliorated by the compounds. With this proof of concept, several phase III studies are under way to define optimal dosage, dosing regimen as well as long-term efficacy and safety of methylnaltrexone and alvimopan. In addition, there is preliminary evidence that these peripherally restricted opioid receptor antagonists may act as prokinetic drugs in their own right.
Keywords: Alvimopan, methylnaltrexone, naloxone, opioid peptides, enteric nervous system, opioid-induced bowel dysfunction, constipation, peripherally restricted opioid receptor antagonists, prokinetic effects
Introduction
Opium derived from the unripe seed capsules of Papaver somniferum has been used since ancient times to treat diarrhoea. We now know that the biological effects of opium are due not only to morphine and codeine but also other drugs such as papaverine, all of which affect the function of the gastrointestinal (GI) tract. Despite many attempts to develop other strong pain therapeutics, opioid analgesics have remained the mainstay of therapy in many patients with moderate to severe pain. However, the use of opioid analgesics is associated with a number of adverse effects among which those on the GI tract are most troublesome in terms of frequency and severity. The constipation associated with opioid medication can be disabling to a degree that opioid treatment needs to be reduced or even abandoned. The traditional approach to ameliorate opioid-induced constipation is laxative co-medication which, however, can be both ineffective and distasteful to the patient (1,2,3).
The spectrum of adverse opioid actions on the gut reflects the ability of these analgesic drugs to directly interact with pathways of the enteric nervous system that regulate GI motility and secretion (4,5,6,7,8). In addition, there is evidence that some GI effects of opioid receptor agonists can be mediated by opioid receptors in the brain (9). However, experimental and clinical studies with opioid receptor antagonists that are unable to enter the brain have shown that the adverse GI effect profile of opioid receptor agonists is essentially peripheral in origin (9). Consequently, peripherally restricted opioid receptor antagonists (PRORAs) such as N-methylnaltrexone and alvimopan represent a significant advance in improving opiate therapy of pain (2,7). The current review starts by providing a brief overview of the neurobiological mechanisms whereby opioids cause constipation. After describing the clinical features of opioid-induced bowel dysfunction (OBD) the article goes on to discuss the emerging strategies to avoid OBD and the expanding range of indications in which PRORAs are likely to be of therapeutic benefit.
The opioid system in the gastrointestinal tract
Independently of their plant, mammalian or synthetic origin, opioids are neuroactive substances, the actions of which are mediated by the principal μ-, κ- and δ-opioid receptors. Many neuroactive drugs act on the gut because the alimentary canal is equipped with the largest collection of neurons outside the brain, known as the enteric nervous system (ENS). The enteric neurons originate from the myenteric and submucosal plexuses, supply all layers of the alimentary canal and thus are in a position to regulate virtually each aspect of digestion (10,11,12). Many of the transmitters and neuropeptides occurring in the brain are also expressed by the ENS, and the same is true for transmitter and neuropeptide receptors. Thus, enteric neurons synthesize and release not only acetylcholine, substance P, nitric oxide, adenosine triphosphate, vasoactive intestinal polypeptide and 5-hydroxytryptamine but also opioid peptides as their transmitters.
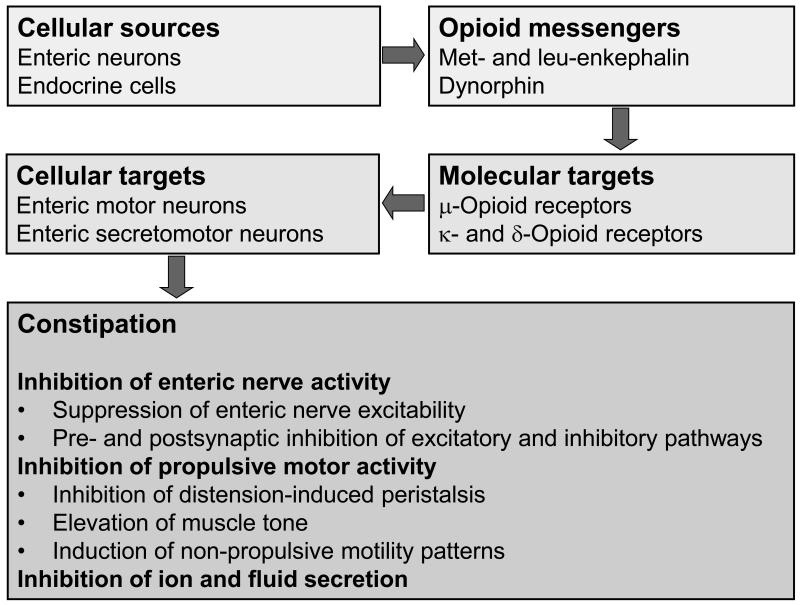
The presence of an elaborate opioid system (Figure 1) in the gut explains why exogenous opioid analgesics inhibit GI function. Met-enkephalin, leu-enkephalin, β-endorphin and dynorphin are among the endogenous opioid peptides occurring in the GI tract where they have been localized to both neurons and endocrine cells of the mucosa (2,4,5,8,13). Further analysis has revealed that opioid peptides are present in distinct classes of enteric neurons, notably in myenteric neurons projecting to the circular muscle and in neurons of descending enteric pathways (11,12,13). Opioid receptors of the μ-, κ- and δ-subtype have been localized to the GI tract of rodents and humans, but their relative distribution varies with GI layer, GI region and species (2,8,13). In the human gut, μ-opioid receptors are present on myenteric and submucosal neurons and on immune cells in the lamina propria (13).
Figure 1.
Schematic overview of the gastrointestinal opioid system relevant to constipation.
Opioid physiology and pharmacology in the gastrointestinal tract
When released from enteric neurons, opioid peptides modify GI function by interaction with opioid receptors present on the enteric circuitry controlling motility and secretion. The inhibitory effect of opioid receptor agonists on peristalsis in the guinea-pig small intestine is thought to arise primarily from interruption of transmission within enteric nerve pathways governing muscle activity (7,8,14). Transmission is blocked both via presynaptic and postsynaptic sites of action on enteric neurons, whereby the release and action of transmitters are attenuated (5,14). It is important to realize that opioid receptor agonists can interrupt both excitatory and inhibitory neural inputs to GI muscle (14). Suppression of excitatory neural inputs causes inhibition of the release of excitatory transmitters such as acetylcholine and blockade of distension-induced peristaltic contractions, whereas blockade of inhibitory neural inputs results in depression of nitric oxide release from inhibitory motor neurons, disinhibition of GI muscle activity, elevation of resting muscle tone and non-propulsive motility patterns (5,7,8,14,15).
Depending on whether interruption of excitatory or inhibitory neural pathways is prevailing, muscle relaxation or spasm will ensue in response to opiate administration. In addition, opioids may directly activate the interstitial cell–muscle network. As a result, μ-opioid receptor agonists inhibit gastric emptying, increase pyloric muscle tone, induce pyloric and duodenojejunal phasic pressure activity, disturb the migrating myoelectrical complex, delay transit through the small and large intestine, and elevate the resting anal sphincter pressure (2,8,14). The halt in propulsive motility combines with inhibition of GI ion and fluid transport. Through prolonged contact of the intestinal contents with the mucosa and interruption of prosecretory enteric reflexes, opioids attenuate the secretion of electrolytes and water and facilitate the net absorption of fluid (2,5,8,16,17). As a result, the patient experiences constipation and abdominal discomfort, two major aspects of OBD.
When pharmacological measures to control OBD are considered, the question arises as to which opioid receptor subtypes are involved. Opioid receptors belong to the family of metabotropic membrane receptors that couple via the Gi/Go subtypes of G-proteins to cellular transduction processes. Once activated by agonists, μ-opioid receptors undergo endocytosis in a concentration-dependent manner (13,14). The cellular effects of myenteric μ-opioid receptor activation are brought about by a multiplicity of signalling pathways including activation of potassium channels, membrane hyperpolarization, inhibition of calcium channels and reduced production of cyclic adenosine monophosphate (14). Studies with isolated tissues from the human intestine show that δ-, κ- and μ-opioid receptors contribute to opiate-induced inhibition of muscle activity (2,8). Propulsive motility in the rat intestine is blocked by δ- and μ-, but not κ-, opioid receptor agonists (5), whereas peristalsis in the guinea-pig intestine is suppressed by activation of κ- and μ-, but not δ-, opioid receptors (18), much as opiate-induced inhibition of cholinergic transmission in the guinea-pig gut is mediated by μ- and κ-opioid receptors.
Although the available evidence indicates that OBD is mediated by opioid receptors in the gut (2,8), there are experimental data to show that opioids acting within the brain can also influence GI function. Thus, intradural injection of opioid analgesics delays intestinal transit at doses that are considerably lower than equieffective intravenous doses (9). However, opiate-induced blockade of gut motility correlates better with opiate concentrations in the gut than with opiate concentrations in the brain (19). In addition, the N-methyl quaternary analogues of naloxone and naltrexone, which do not cross the blood-brain barrier, are able to fully antagonize the effects of morphine in the canine and rat intestine (2,9). It follows that the adverse influence of opiates on GI function results primarily from interaction with opioid receptors in the gut, an inference that is backed by the experimental and clinical effects of PRORAs.
Opioid-induced bowel dysfunction
A delay in GI transit and constipation are the most common and often disabling side effects of opioid analgesics. However, constipation is just one symptom of OBD, whose manifestations comprise incomplete evacuation, abdominal distension, bloating, abdominal discomfort and increased gastro-oesophageal reflux. In addition, OBD may lead to secondary complications such as pseudo-obstruction of the bowel, anorexia, nausea, vomiting and interference with oral drug administration and absorption (2,8). The symptoms associated with OBD can profoundly impair the quality of life and in some patients can be so severe that they prefer to discontinue analgesic therapy rather than experience the discomfort arising from OBD (2). Unlike other adverse effects of chronic opioid therapy such as sedation, nausea and vomiting which often resolve with continued use, OBD generally persists throughout treatment (2). However, tolerance to the effects of morphine on enteric neurons has been found to occur under experimental conditions (14).
Specific management of opioid-induced bowel dysfunction
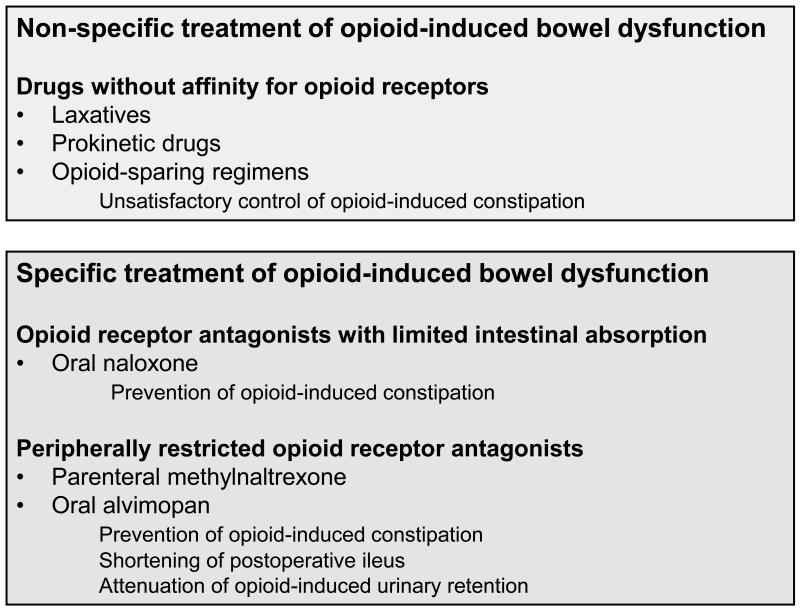
The pharmacological management of OBD involves two approaches (Figure 2): non-specific treatment with laxatives and prokinetic drugs and specific treatment with opioid receptor antagonists (1,2,20). Since the non-specific regimens often do not provide satisfactory relief from the GI manifestations of opioid-induced analgesia, various opportunities in the specific treatment of OBD have been explored. The primary objective of these approaches is to prevent GI symptoms rather than treat established motor stasis due to opioid use (2). For this reason, many opioid-sparing regimens such as non-steroidal anti-inflammatory drugs have been tested to circumvent the adverse GI sequelae of opioid use. However, these regimens lack efficacy in severe pain states and themselves have adverse effects on the gut. Transdermal administration of opiates such as fentanyl has also been reported to cause less constipation and to result in a better quality of life rating than oral administration of morphine (2,21). However, none of these attempts is able to solve the problem of OBD in a satisfactory manner.
Figure 2.
Non-specific and specific treatment of opioid-induced bowel dysfunction.
Since opioid-induced analgesia is primarily mediated by μ-opioid receptors in the central nervous system, the rational approach to prevent OBD would be to combine opioid analgesics with opioid receptor antagonists that cannot penetrate the blood-brain barrier. As a result, the adverse effects of opioid analgesics on the GI system would be suppressed whereas their central analgesic action would be preserved. This approach has been validated by the use of opioid receptor antagonists with limited systemic absorption and by the development of PRORAs such a N-methylnaltrexone and alvimopan (2,8,22,23). The latter two compounds are currently in clinical trials for the management of OBD and postoperative ileus and may also turn out to become prokinetic drugs to relieve intestinal stasis unrelated to opiate use.
Opioid receptor antagonists with limited systemic absorption: oral naloxone
The first attempt to selectively target opioid receptors in the periphery was made with naloxone and related tertiary opioid receptor antagonists such as nalmefene (2). Naloxone is a pan-opioid receptor antagonist (24) whose systemic bioavailability following oral administration is as low as 2 % because of extensive first-pass metabolism. Consequently, oral naloxone has been found to reduce constipation but not antinociception caused by morphine in rats (25). These observations have been confirmed in clinical studies in which oral naloxone turned out to improve OBD without necessarily compromising opiate-induced analgesia (1,26,27). It needs to be realized, however, that naloxone can easily cross the blood-brain barrier and hence, despite its low oral bioavailability, can reverse analgesia if given at sufficient doses (2). Thus, the therapeutic range of naloxone is rather narrow because of the need to titrate peripherally versus centrally active doses (26). Despite this limitation, a combination of oral oxycodone and naloxone at the weight ratio of 2:1 has been licensed in Germany, given that a phase III trial had shown that the combination has a low potential to induce OBD whereas the analgesic effect is preserved (28).
One of the major metabolites of naloxone is naloxone-3-glucuronide which following oral administration to rodents is absorbed to a negligible degree but can counteract opiate-induced inhibition of GI motility (29,30). Experimental observations similar to those with naloxone glucuronide have been made with nalmefene glucuronide, a metabolite of the μ-opioid receptor antagonist nalmefene (29). A pilot study in humans, however, indicates that oral nalmefene glucuronide is not sufficiently selective for the gut in order to be clinically useful (31).
Peripherally restricted opioid receptor antagonists
Quaternary analogues of opioid receptor antagonists such as naloxone and naltrexone display a pharmacokinetic profile of limited absorption from the gut and inability to enter the brain (2,22). Thus, intraluminal administration of N-methylnaloxone to an isolated vascularly perfused segment of the rat colon prevents intravascularly administered morphine from depressing motility, although the compound is not absorbed from the lumen to a degree that it becomes measurable in the vasculature (30). Similarly, N-methylnaloxone and N-methylnaltrexone attenuate morphine-evoked electrical activity in the canine duodenum at doses that are devoid of effects on the central nervous system (32). N-methylnaltrexone and another highly polar opioid receptor antagonist, alvimopan, also fail to enter the brain in humans and have a promising potential to relieve OBD in a well-tolerated manner without compromising central analgesia. It is worth noting, however, that N-methylnaltrexone and alvimopan differ in their opioid receptor subtype selectivity and intrinsic activity on the isolated guinea-pig ileum (33). While N-methylnaltrexone reduces electrically induced contractions and spontaneous activity of the muscle, alvimopan increases these parameters. The action of alvimopan appears to involve both μ- and κ-opioid receptors (33).
Methylnaltrexone
Attaching a methyl group to the amine configuration in naltrexone results in N-methylnaltrexone (in brief methylnaltrexone), a drug that has greater polarity and lower lipid solubility than its parent compound. As a result, methylnaltrexone exhibits low oral bioavailability due to limited absorption and does not cross the blood-brain barrier (8,22,34,35). Consequently, this μ-opioid receptor-preferring antagonist (IC50 at human μ-opioid receptors = 70 nM) offers the potential to prevent or reverse the undesired side effects of opioids in the gut without compromising analgesia or precipitating opioid withdrawal symptoms that are predominantly mediated by opioid receptors in the brain (22,34). This claim has been supported by several studies which show that, following subcutaneous or intravenous administration of methylnaltrexone together with a centrally active opiate, the adverse effect of the opiate on GI function is prevented without attenuation of analgesia in dogs and humans, whereas in rats analgesia is appreciably compromised (22,34,35). This species-dependence of the peripheral selectivity of methylnaltrexone arises from its demethylation to naltrexone which readily penetrates the blood-brain barrier: demethylation occurs in mice and rats but is negligible in dogs and humans (36).
A number of phase I and II studies has established the pharmacodynamic, pharmacokinetic, therapeutic and safety profile of methylnaltrexone (22,34,35,37). The drug has been formulated as a solution for intravenous or subcutaneous administration and as capsules/tablets for oral administration. Both the parenteral and oral formulations as well as single and repeated dosage regimens have been found efficacious in preventing the morphine-induced prolongation of gastric emptying and orocaecal transit time without significantly attenuating morphine-induced analgesia (22,34,35). In this context it is worth noting that methylnaltrexone is also able to ameliorate opioid-induced urinary retention (38).
Phase II studies have shown that methylnaltrexone is capable of relieving constipation in methadone-maintained, opioid-dependent volunteers. In a double-blind, randomized and placebo-controlled trial it has been found that intravenous methylnaltrexone shortens the orocaecal transit time and causes laxation, but does not elicit opioid withdrawal (39). The utility of methylnaltrexone to selectively counteract opiate-induced stasis in the GI tract has also been proven in phase II and III studies of patients with advanced illness requiring high doses of opiates for pain control (34,35).
Postoperative ileus could be another indication for methylnaltrexone. This condition is thought to involve activation of opioid mechanisms in the gut and exacerbated by the use of opioid analgesics for the control of postoperative pain (40,41). A phase II trial has shown that patients with postoperative ileus following open segmental colonic resection benefit from treatment with methylnaltrexone, since upper and lower bowel function recover approximately 1 day earlier than in placebo-treated patients, whereas no difference in opioid use or mean pain scores was observed (34). Several phase III studies are currently under way to establish the efficacy and safety of methylnaltrexone in postoperative ileus (35).
At therapeutic doses (0.3 - 0.45 mg/kg intravenously and up to 19.2 mg/kg per os) methylnaltrexone is well tolerated, an outcome that is also true when methylnaltrexone is repeatedly administered at 0.3 mg/kg intravenously every 6 h (37). Thus far, only two types of adverse reactions to methylnaltrexone have been reported. One of them relates to the vascular system, given that transient orthostatic hypotension can occur at supra-therapeutic doses (8,22,34,35). This reaction may be related to facial flushing and mild light-headedness, symptoms that have occasionally been reported (35). The other type of adverse effect comprises gut-related reactions such as abdominal cramps, soft stools and diarrhoea (8,22,34,35,37).
Alvimopan
Alvimopan is a μ-opioid receptor-preferring antagonist with a peripherally restricted site of action and a potency (IC50 at human μ-opioid receptors = 0.77 nM) considerably higher than that of methynaltrexone. Given its polar structure, alvimopan exhibits both low systemic absorption (oral bioavailability of 0.03 % in dogs and 6 % in humans) and a limited ability to enter the brain (22,42,43,44). Since it is rapidly degraded after intravenous injection, alvimopan is formulated for oral intake, in which case it potently blocks μ-opioid receptors in the gut with a prolonged duration of action. Several preclinical and phase I studies have established the pharmacokinetics, safety, efficacy and selectivity of alvimopan in its antagonism of peripheral opioid receptors (2,8,22,42,43,44). In these studies, alvimopan was found to prevent opioid receptor agonists from delaying GI transit in healthy subjects without antagonizing central opioid effects such as analgesia and pupillary constriction (43,44,45).
In subsequent phase II trials the utility of alvimopan in preventing or treating OBD was explored (8,22,44,46,47). In patients on chronic opioid therapy for non-malignant pain or opioid addiction, alvimopan (0.5 or 1 mg once daily for 21 days) was found to ameliorate constipation without attenuating opioid analgesia (46). These results were confirmed in a phase III trial involving more than 500 subjects taking opioids for non-cancer pain (47). Alvimopan (0.5 or 1 mg twice daily for 6 weeks) was able to increase spontaneous bowel movements during the initial 3 weeks of treatment and to improve other symptoms of OBD (straining, stool consistency, incomplete evacuation, abdominal bloating and discomfort and decreased appetite) over the whole treatment period, while analgesia was not compromised (47).
Since opioid mechanisms and OBD are thought to contribute to postoperative ileus (2,40,41), several phase II and III studies have addressed the ability of alvimopan to improve postoperative bowel function (2,8,22,44,48,49). The results of these studies indicate that alvimopan can shorten postoperative ileus, although with somewhat varying results. This may in part be due to the rather wide range of doses (1 – 12 mg) tested as well as to differences in pharmacokinetics, given that the rate of alvimopan absorption is slowed in surgical patients, relative to healthy controls (50). The patients enrolled in the studies underwent radical abdominal hysterectomy or bowel resection, and in most studies the initial dose of alvimopan was given 2 h before surgery, and the drug subsequently dosed twice daily. Postoperatively, patient-controlled analgesia with intravenous opioids was instituted. Alvimopan (12 mg) was found to shorten both the time to first bowel movement and the duration of hospitalisation on average by 18 h (49).
Studies addressing the acute safety of alvimopan in patients with OBD have shown that the drug is well tolerated, the adverse effects being primarily bowel-related and including nausea, vomiting and abdominal discomfort (43). When alvimopan was tested for 3 – 6 weeks, the most prevalent adverse reactions comprised abdominal pain, nausea and diarrhoea and occurred predominantly during the initial period of treatment (46,47). It is at present too early to judge the long-term safety of alvimopan, given that a phase III trial was halted in 2007 after a numerical imbalance in the number of ischaemic cardiovascular events and neoplasm cases had been observed in patients on alvimopan, relative to placebo (3).
Opioid receptor antagonists as potential prokinetics
From the effects of opioid receptor antagonists on GI function it would appear that endogenous opioid peptides play a role in the fine tuning of digestion. For example, distension-evoked peristalsis can be facilitated by naloxone in various preparations of the guinea-pig, rabbit, cat and rat isolated small intestine (5,7,18). In the guinea-pig small intestine, the effect of naloxone is mimicked by selective antagonists at μ- and κ-opioid receptors, but not by antagonism at δ-opioid receptors (18). It follows that endogenous opioid peptides released in the course of propulsive motility participate in the neural control of peristalsis as they dampen peristaltic performance via activation of μ- and κ-opioid receptors (8,18). Naloxone has been found to accelerate transit in the colon but not small intestine of healthy human volunteers, this effect being shared by the μ-opioid receptor-preferring antagonists methylnaltrexone and alvimopan (37,45). Thus, PRORAs have the potential to act as prokinetics in their own right and to alleviate intestinal motor stasis unrelated to opiate use, such as chronic idiopathic constipation and intestinal pseudo-obstruction (6,8,34,43).
There is emerging evidence that GI pathophysiology leading to GI motor inhibition is associated with upregulation and/or overactivity of the opioid system in the alimentary canal. For instance, experimental inflammation enhances the potency of μ-opioid receptor agonists to inhibit GI transit and increases the expression of μ-opioid receptors in the intestine of mice (51,52). Abdominal surgery leads to an increase in the circulating levels of endomorphin in humans (53) and causes internalization of μ-opioid receptors in the myenteric plexus of the guinea-pig intestine (13). These observations reflect a role of endogenous opioids in the pathophysiology of postoperative motor disturbances. Consistent with this concept is a limited number of small studies showing that naloxone can reverse idiopathic chronic constipation and have beneficial activity in patients with intestinal pseudo-obstruction and constipation-predominant irritable bowel syndrome (8,22). PRORAs may hence be able to normalize pathological inhibition of gut function that arises from an upregulation and/or overactivity of the opioid system in the GI tract (6,8).
Conclusions
The GI tract is one of the major targets of the undesired effects of opiates, because the ENS expresses all major subtypes of opioid receptors which when activated dampen GI function. This may be physiologically meaningful under conditions where the endogenous opioid system is activated, but represents a nuisance when exogenous opiates are administered for therapeutic purposes. The development of opioid receptor antagonists with restricted access to the central nervous system has opened up a new avenue to selectively control the adverse actions of opioid analgesics in the gut. This concept has been validated by the clinical efficacy of oral naloxone, parenteral methylnaltrexone and oral alvimopan, which are able to prevent the undesired opioid effects on GI function while the desired action of opiates on central pain control is preserved. With this proof of concept, further studies defining optimal dosage, dosing regimen as well as long-term efficacy and safety are needed (8,23). These studies will also reveal whether PRORAs could act as prokinetic drugs in their own right.
Acknowledgements
Work in the authors’ laboratory is supported by FWF - The Austrian Scientific Research Funds, the Austrian Federal Ministry of Science and Research, and the Zukunftsfonds Steiermark. The author thanks Ulrike Holzer-Petsche for critically reading the manuscript.
References
- 1).Meissner W, Schmidt U, Hartmann M, Kath R, Reinhart K. Oral naloxone reverses opioid-associated constipation. Pain. 2000;84:105–109. doi: 10.1016/S0304-3959(99)00185-2. [DOI] [PubMed] [Google Scholar]
- 2).Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 3).Herbert MK, Holzer P. Standardized concept for the treatment of gastrointestinal dysmotility in critically ill patients--current status and future options. Clin Nutr. 2008;27:25–41. doi: 10.1016/j.clnu.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4).Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988;40:121–162. [PubMed] [Google Scholar]
- 5).De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103–115. doi: 10.1016/0163-7258(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 6).Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett. 2004;361:192–195. doi: 10.1016/j.neulet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 7).Sanger GJ, Tuladhar BR. The role of endogenous opiates in the control of gastrointestinal motility: predictions from in vitro modelling. Neurogastroenterol Motil. 2004;16(Suppl 2):38–45. doi: 10.1111/j.1743-3150.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 8).Holzer P. Treatment of opioid-induced gut dysfunction. Expert Opin Investig Drugs. 2007;16:181–194. doi: 10.1517/13543784.16.2.181. [DOI] [PubMed] [Google Scholar]
- 9).Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol. 1985;25:249–273. doi: 10.1146/annurev.pa.25.040185.001341. [DOI] [PubMed] [Google Scholar]
- 10).Holzer P, Schicho R, Holzer-Petsche U, Lippe IT. The gut as a neurological organ. Wien Klin Wochenschr. 2001;113:647–660. [PubMed] [Google Scholar]
- 11).Brookes SJH. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12).Furness JB. The Enteric Nervous System. Blackwell; Oxford: 2006. [Google Scholar]
- 13).Sternini C, Patierno S, Selmer IS, Kirchgessner A. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 2):3–16. doi: 10.1111/j.1743-3150.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 14).Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 15).Lenard L, Halmai V, Barthó L. Morphine contracts the guinea pig ileal circular muscle by interfering with a nitric oxide mediated tonic inhibition. Digestion. 1999;60:562–566. doi: 10.1159/000007707. [DOI] [PubMed] [Google Scholar]
- 16).Beubler E, Lembeck F. Inhibition of stimulated fluid secretion in the rat small and large intestine by opiate agonists. Naunyn-Schmiedeberg’s Arch Pharmacol. 1979;306:113–118. doi: 10.1007/BF00498980. [DOI] [PubMed] [Google Scholar]
- 17).Turnberg LA. Antisecretory activity of opiates in vitro and in vivo in man. Scand J Gastroenterol. 1983;84:79–83. [PubMed] [Google Scholar]
- 18).Shahbazian A, Heinemann A, Schmidhammer H, Beubler E, Holzer-Petsche U, Holzer P. Involvement of μ- and κ-, but not δ-, opioid receptors in the peristaltic motor depression caused by endogenous and exogenous opioids in the guinea-pig intestine. Br J Pharmacol. 2002;135:741–750. doi: 10.1038/sj.bjp.0704527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Bianchi G, Ferretti P, Recchia M, Rocchetti M, Tavani A, Manara L. Morphine tissue levels and reduction of gastrointestinal transit in rats. Correlation supports primary action site in the gut. Gastroenterology. 1983;85:852–858. [PubMed] [Google Scholar]
- 20).Herndon CM, Jackson KC, Hallin PA. Management of opioid-induced gastrointestinal effects in patients receiving palliative care. Pharmacotherapy. 2002;22:240–250. doi: 10.1592/phco.22.3.240.33552. [DOI] [PubMed] [Google Scholar]
- 21).Allan L, Hays H, Jensen NH, De Waroux BI, Bolt M, Donald R, Kalso E. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. Br Med J. 2001;322:1154–1158. doi: 10.1136/bmj.322.7295.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).DeHaven-Hudkins DL, DeHaven RN, Little PJ, Techner LM. The involvement of the μ-opioid receptor in gastrointestinal pathophysiology: therapeutic opportunities for antagonism at this receptor. Pharmacol Ther. 2008;117:162–187. doi: 10.1016/j.pharmthera.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 23).Becker G, Galandi D, Blum HE. Peripherally acting opioid antagonists in the treatment of opiate-related constipation: a systematic review. J Pain Symptom Manage. 2007;34:547–565. doi: 10.1016/j.jpainsymman.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 24).Greenwood-Van Meerveld B, Gardner CJ, Little PJ, Hicks GA, DeHaven-Hudkins DL. Preclinical studies of opioids and opioid antagonists on gastrointestinal function. Neurogastroenterol Motil. 2004;16(Suppl 2):46–53. doi: 10.1111/j.1743-3150.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 25).Jurna I, Kaiser R, Kretz O, Baldauf J. Oral naloxone reduces constipation but not antinociception from oral morphine in the rat. Neurosci Lett. 1992;142:62–64. doi: 10.1016/0304-3940(92)90620-m. [DOI] [PubMed] [Google Scholar]
- 26).Sykes NP. An investigation of the ability of oral naloxone to correct opioid-related constipation in patients with advanced cancer. Palliat Med. 1996;10:135–144. doi: 10.1177/026921639601000208. [DOI] [PubMed] [Google Scholar]
- 27).Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manage. 2002;23:48–53. doi: 10.1016/s0885-3924(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 28).Müller-Lissner S, Leyendecker P, Ruckes MC, Fleischer W, Reimer K. Oral prolonged release (pr) oxycodone/naloxone combination reduces opioid-induced bowel dysfunction (OIBD) Eur J Pain. 2007;11(Suppl 1):S82. doi: 10.1016/j.ejpain.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 29).Simpkins JW, Smulkowski M, Dixon R, Tuttle R. Evidence for the delivery of narcotic antagonists to the colon as their glucuronide conjugates. J Pharmacol Exp Ther. 1988;244:195–205. [PubMed] [Google Scholar]
- 30).Reber P, Brenneisen R, Flogerzi B, Batista C, Netzer P, Scheurer U. Effect of naloxone-3-glucuronide and N-methylnaloxone on the motility of the isolated rat colon after morphine. Dig Dis Sci. 2007;52:502–507. doi: 10.1007/s10620-006-9563-9. [DOI] [PubMed] [Google Scholar]
- 31).Cheskin LJ, Chami TN, Johnson RE, Jaffe JH. Assessment of nalmefene glucuronide as a selective gut opioid antagonist. Drug Alcohol Depend. 1995;39:151–154. doi: 10.1016/0376-8716(95)01153-p. [DOI] [PubMed] [Google Scholar]
- 32).Russell J, Bass P, Goldberg LI, Schuster CR, Merz H. Antagonism of gut, but not central effects of morphine with quaternary narcotic antagonists. Eur J Pharmacol. 1982;78:255–261. doi: 10.1016/0014-2999(82)90026-7. [DOI] [PubMed] [Google Scholar]
- 33).Beattie DT, Cheruvu M, Mai N, O’Keefe M, Johnson-Rabidoux S, Peterson C, Kaufman E, Vickery R. The in vitro pharmacology of the peripherally restricted opioid receptor antagonists, alvimopan, ADL 08-0011 and methylnaltrexone. Naunyn-Schmiedeberg’s Arch Pharmacol. 2007;375:205–220. doi: 10.1007/s00210-007-0146-x. [DOI] [PubMed] [Google Scholar]
- 34).Yuan CS, Israel RJ. Methylnaltrexone, a novel peripheral opioid receptor antagonist for the treatment of opioid side effects. Expert Opin Investig Drugs. 2006;15:541–552. doi: 10.1517/13543784.15.5.541. [DOI] [PubMed] [Google Scholar]
- 35).Reichle FM, Conzen PF. Methylnaltrexone, a new peripheral μ-receptor antagonist for the prevention and treatment of opioid-induced extracerebral side effects. Curr Opin Investig Drugs. 2008;9:90–100. [PubMed] [Google Scholar]
- 36).Kotake AN, Kuwahara SK, Burton E, McCoy CE, Goldberg LI. Variations in demethylation of N-methylnaltrexone in mice, rats, dogs, and humans. Xenobiotica. 1989;19:1247–1254. doi: 10.3109/00498258909043176. [DOI] [PubMed] [Google Scholar]
- 37).Yuan CS, Doshan H, Charney MR, O’Connor M, Karrison T, Maleckar SA, Israel RJ, Moss J. Tolerability, gut effects, and pharmacokinetics of methylnaltrexone following repeated intravenous administration in humans. J Clin Pharmacol. 2005;45:538–546. doi: 10.1177/0091270004273491. [DOI] [PubMed] [Google Scholar]
- 38).Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther. 2007 doi: 10.1038/sj.clpt.6100164. doi: 10.1038/sj.cipt.6100164. [DOI] [PubMed] [Google Scholar]
- 39).Yuan CS, Foss JF, O’Connor M, Osinski J, Karrison T, Moss J, Roizen MF. Methylnaltrexone for reversal of constipation due to chronic methadone use: A randomized, controlled trial. J Am Med Assoc. 2000;283:367–372. doi: 10.1001/jama.283.3.367. [DOI] [PubMed] [Google Scholar]
- 40).Schmidt J, Stoffels B, Nazir A, Dehaven-Hudkins DL, Bauer AJ. Alvimopan and COX-2 inhibition reverse opioid and inflammatory components of postoperative ileus. Neurogastroenterol Motil. 2008 doi: 10.1111/j.1365-2982.2007.01078.x. in press. [DOI] [PubMed] [Google Scholar]
- 41).Fukuda H, Suenaga K, Tsuchida D, Mantyh CR, Pappas TN, Hicks GA, DeHaven-Hudkins DL, Takahashi T. The selective μ opioid receptor antagonist, alvimopan, improves delayed GI transit of postoperative ileus in rats. Brain Res. 2006;1102:63–70. doi: 10.1016/j.brainres.2006.02.092. [DOI] [PubMed] [Google Scholar]
- 42).Schmidt WK. Alvimopan (ADL 8-2698) is a novel peripheral opioid antagonist. Am J Surg. 2001;182(Suppl):27S–38S. doi: 10.1016/s0002-9610(01)00784-x. [DOI] [PubMed] [Google Scholar]
- 43).Camilleri M. Alvimopan, a selective peripherally acting μ-opioid antagonist. Neurogastroenterol Motil. 2004;17:157–165. doi: 10.1111/j.1365-2982.2005.00640.x. [DOI] [PubMed] [Google Scholar]
- 44).Neary P, Delaney CP. Alvimopan. Expert Opin Investig Drugs. 2005;14:479–488. doi: 10.1517/13543784.14.4.479. [DOI] [PubMed] [Google Scholar]
- 45).Gonenne J, Camilleri M, Ferber I, Burton D, Baxter K, Keyashian K, Foss J, Wallin B, Du W, Zinsmeister AR. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3:784–791. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]
- 46).Paulson DM, Kennedy DT, Donovick RA, Carpenter RL, Cherubini M, Techner L, Du W, Ma Y, Schmidt WK, Wallin B, Jackson D. Alvimopan: an oral, peripherally acting, μ-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction - a 21-day treatment-randomized clinical trial. J Pain. 2005;6:184–192. doi: 10.1016/j.jpain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 47).Webster L, Jansen JP, Peppin J, Lasko B, Irving G, Morlion B, Snidow J, Pierce A, Mortensen E, Kleoudis C, Carter E. Alvimopan, a peripherally acting μ-opioid receptor (PAM-OR) antagonist for the treatment of opioid-induced bowel dysfunction: Results from a randomized, double-blind, placebo-controlled, dose-finding study in subjects taking opioids for chronic non-cancer pain. Pain. 2008 doi: 10.1016/j.pain.2007.11.008. in press. [DOI] [PubMed] [Google Scholar]
- 48).Tan EK, Cornish J, Darzi AW, Tekkis PP. Meta-analysis: alvimopan vs. placebo in the treatment of post-operative ileus. Aliment Pharmacol Ther. 2007;25:47–57. doi: 10.1111/j.1365-2036.2006.03150.x. [DOI] [PubMed] [Google Scholar]
- 49).Delaney CP, Wolff BG, Viscusi ER, Senagore AJ, Fort JG, Du W, Techner L, Wallin B. Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase III studies. Ann Surg. 2007;245:355–363. doi: 10.1097/01.sla.0000232538.72458.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Foss JF, Fisher DM, Schmith VD. Pharmacokinetics of alvimopan and its metabolite in healthy volunteers and patients in postoperative ileus trials. Clin Pharmacol Ther. 2007 doi: 10.1038/sj.clpt.6100292. doi: 10.1038/sj.clpt.6100292. [DOI] [PubMed] [Google Scholar]
- 51).Puig MM, Pol O. Peripheral effects of opioids in a model of chronic intestinal inflammation in mice. J Pharmacol Exp Ther. 1998;287:1068–1075. [PubMed] [Google Scholar]
- 52).Pol O, Alameda F, Puig MM. Inflammation enhances μ-opioid receptor transcription and expression in mice intestine. Mol Pharmacol. 2001;60:894–899. doi: 10.1124/mol.60.5.894. [DOI] [PubMed] [Google Scholar]
- 53).Yoshida S, Ohta J, Yamasaki K, Kamei H, Harada Y, Yahara T, Kaibara A, Ozaki K, Tajiri T, Shirouzu K. Effect of surgical stress on endogenous morphine and cytokine levels in the plasma after laparoscopoic or open cholecystectomy. Surg Endosc. 2000;14:137–140. doi: 10.1007/s004649900085. [DOI] [PubMed] [Google Scholar]