Abstract
Neuropeptide-Y (NPY) is involved in the regulation of ingestive behaviour and energy homeostasis. Since deletion of the NPY Y2 and Y4 receptor gene increases and decreases food intake, respectively, we examined whether water intake during the light and dark phase is altered in Y2 and Y4 receptor knockout mice. The water consumption of mice staying in their home cages was measured by weighing the water bottles at the beginning and end of the light phase during 4 consecutive days. Control, Y2 and Y4 receptor knockout mice did not differ in their water intake during the light phase. However, during the dark phase Y2 and Y4 receptor knockout mice drank significantly more (46-63 %, P<0.05) water than the control mice. The total daily water intake over 24 h was also enhanced. The enhanced water intake during the dark phase was not altered by the β-adrenoceptor antagonist propranolol or the angiotensin AT1 receptor antagonist telmisartan (each injected intraperitoneally at 10 mg/kg). These data indicate that NPY acting via Y2 and Y4 receptors plays a distinctive role in the regulation of nocturnal water consumption. While β-adrenoceptors and angiotensin AT1 receptors do not seem to be involved, water intake in Y2 and Y4 receptor knockout mice may be enhanced because presynaptic autoinhibition of NPY release and inhibition of orexin neurons in the central nervous system are prevented.
Keywords: Neuropeptide-Y, Y2 receptor gene knockout, Y4 receptor gene knockout, propranolol, telmisartan, nocturnal water consumption, water homeostasis, Y2 autoreceptors
Introduction
Neuropeptide-Y (NPY) is involved in the regulation of food intake and energy homeostasis. For instance, deletion of the NPY Y2 and Y4 receptor gene increases and decreases food intake, respectively [14,25,30,31,33]. In contrast, the drinking of water provided ad libitum has not yet been systematically investigated in NPY receptor knockout mice. Thiele et al. [33] have reported that in an ethanol/water preference test Y2 receptor knockout (Y2−/−) mice drink more water, but less ethanol, than wild-type controls. When one of us (S.D.) noted that a colony of Y4 receptor knockout (Y4−/−) mice drank more water than the respective controls, we decided to carry out an explorative study of the spontaneous drinking behaviour in both Y2−/− and Y4−/− mice. Three particular aims were pursued. Firstly, we recorded the water intake during the light and dark phase and estimated the total daily intake of water in Y2−/− and Y4−/− mice relative to their controls. Secondly, we investigated whether the increase in dark phase water intake observed in Y2−/− and Y4−/− mice was inhibited by the β-adrenoceptor antagonist propranolol. This possibility was envisaged because β-adrenoceptor agonists stimulate water intake [20,23] and NPY is associated with noradrenergic neurons of the central and sympathetic nervous system [16,22]. Thirdly, we examined whether the enhanced water intake in Y2−/− and Y4−/−mice is normalized by the angiotensin AT1 receptor antagonist telmisartan, given that angiotensin II is a central messenger that stimulates drinking [3,12].
Materials and Methods
Experimental animals
This study was approved by an ethical committee at the Federal Ministry of Education, Science and Culture of the Republic of Austria and conducted according to the Directive of the European Communities Council of 24 November 1986 (86/609/EEC). The experiments were carried out with female germline Y2−/− and Y4−/− mice and non-induced conditional Y2 and Y4 receptor knockout (FY2 and FY4) mice (Department of Pharmacology, Medical University of Innsbruck, Austria) weighing 16-24 g. The generation of Y2−/−, Y4−/−, FY2 and FY4 mice and the demonstration of the absence or presence of Y2 and Y4 receptors have been described previously [30,31]. Germline Y2−/− mice were generated from the same founders on the same mixed C57BL/6–129SvJ background as the conditional FY2 and FY4 knockout mice. Non-induced conditional FY2 and FY4 knockout mice were used as controls in all experiments and termed control mice throughout the paper.
Experimental protocols
The mice were housed in groups of 3-4 per cage under controlled temperature (21 °C) and a 12 h light/dark cycle (lights on at 6:00 AM, lights off at 6:00 PM). Tap water and standard laboratory food were provided ad libitum throughout the study. Since only a limited number of female control, Y2−/− and Y4−/− mice was available for the study, four experiments were carried out with most mice at 1 week intervals, if not stated otherwise. In experiment 1 the water intake during the light phase of 4 consecutive days and the 3 intervening periods of dark phase was estimated. To this end, the weight of the water bottles of each cage was determined at 7:45 AM and 4:30 PM. The water bottles were cleaned and refilled every second day. Experiment 2 was carried out to test whether intraperitoneal (IP) injection of vehicle (physiological saline) would alter drinking during the dark phase. To this end the water intake during the dark phase of 3 consecutive days was estimated. On the second day, physiological saline (0.15 M NaCl, 2 ml/kg) was injected IP at 5:30 PM and 11:30 PM. Experiments 3 and 4 were identical with the second experiment, except that instead of vehicle two doses of propranolol (each dose at 10 mg/kg; experiment 3) and telmisartan (each dose at 10 mg/kg; experiment 4) were injected IP at 5:30 PM and 11:30 PM. An interval of 2 weeks was allowed between the third and fourth experiment to ensure complete washout of propranolol. The experiment involving telmisartan was repeated with naïve Y2−/−, Y4−/− and control mice in order to prove that previous treatment with vehicle and propranolol did not modify the effect of telmisartan (experiment 5). These additional mice were injected either with telmisartan (10 mg/kg IP at 5:30 PM and 11:30 PM) or its vehicle.
The experimental design entailed that the water intake per cage (i.e., per 3-4 mice) was determined. Since, in addition, the weight of the mice was recorded at the beginning of each experiment, the water intake during the light and dark phase was expressed relative to body weight. The total intake of water per day was calculated by summing up the water intake of one light phase and the following dark phase.
Drugs
(±)-Propranolol hydrochloride (Sigma, Vienna, Austria) was dissolved in saline at a concentration of 5 mg/ml and injected IP at a volume of 2 ml/kg. Telmisartan (gift of Boehringer Ingelheim, Biberach, Germany) was dissolved in 0.15 M NaOH, the pH of this solution being adjusted with 0.15 M HCl to 9.5 [12]. The injection solution contained 5 mg/ml telmisartan and was administered IP at a volume of 2 ml/kg. The vehicle solution was prepared in an analogous manner.
Statistics
Statistical evaluation of the results was performed on Statistica (StatSoft Inc., Tulsa, OK, USA) with two-way analysis of variance (ANOVA) for repeated measures (to identify differences between genotypes over time) or three-way ANOVA for repeated measures (to identify differences between genotypes and treatments over time). Since no significant effect of time and treatment and no significant interaction between time, genotype and treatment were found, the data were reevaluated with one-way ANOVA and significant differences between the genotypes identified with the Holm-Sidak method (SigmaStat, SPSS Inc., Chicago, IL, USA). All data are presented as means ± s.e. mean, n referring to the number of cages in the respective group. Probability values of P < 0.05 were regarded as significant.
Results
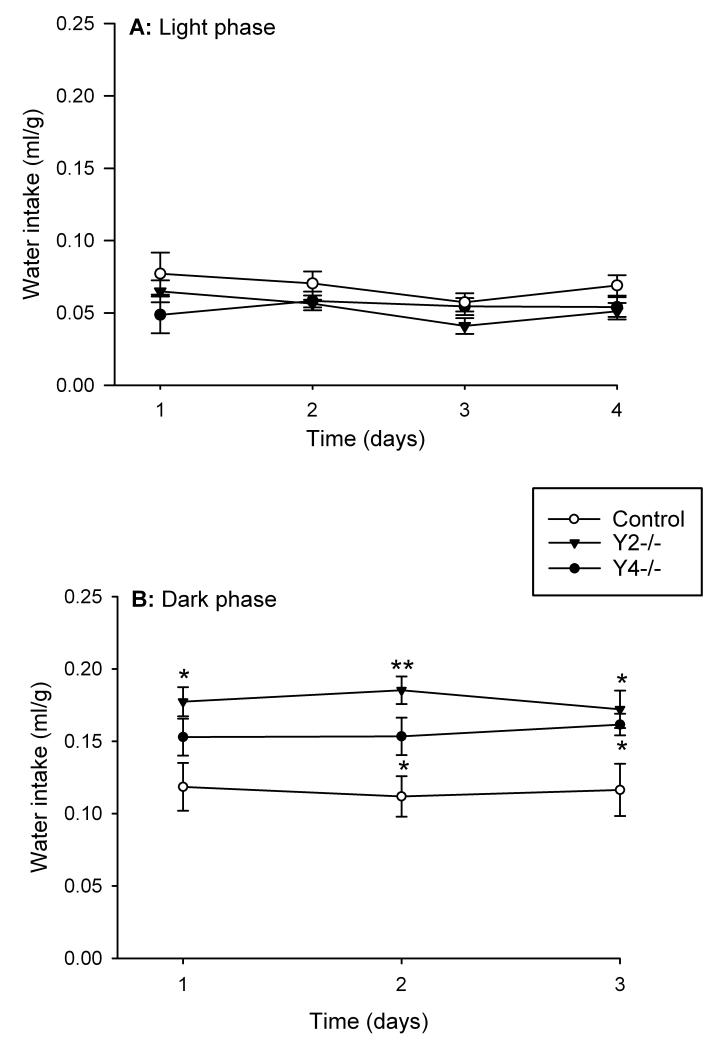
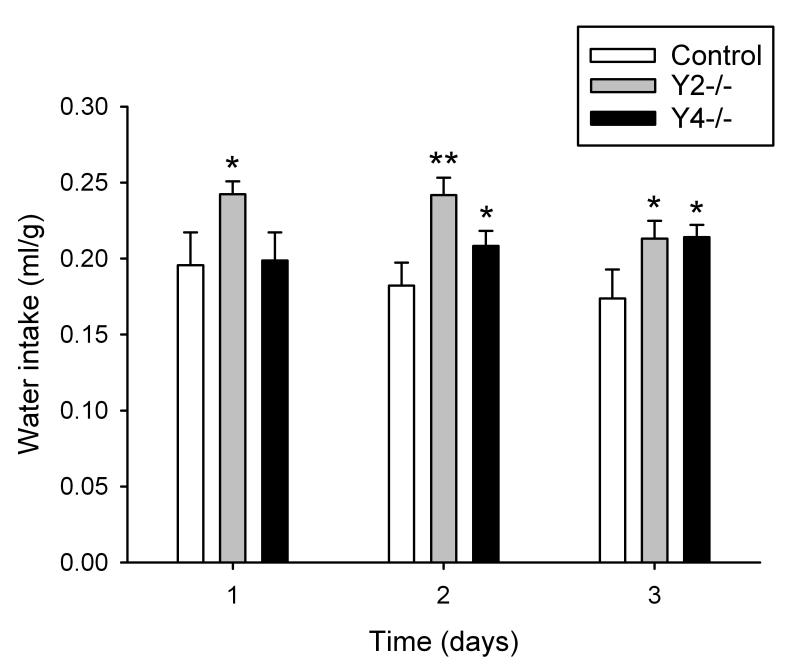
The water intake of control (FY2 and FY4) mice during the light phase was in the range of 0.057-0.077 ml/g (Figure 1A) while that during the dark phase was in the range of 0.112-0.119 ml/g (Figure 1B) and significantly higher (P<0.05) than during the light phase. Y2−/− and Y4−/− mice did not significantly differ from control mice in their water intake during the light phase (Figure 1A). During the dark phase, however, Y2−/− mice drank significantly more water than the control mice as recorded over 3 consecutive days (Figure 1B). The total daily (24 h) intake of water was also significantly increased in Y2−/− mice during this observation period (Figure 2). Y4−/− mice likewise drank more water during the dark phase than control mice, but this difference was statistically significant only on the second and third day of the 3 day recording period (Figure 1B). A similar observation was made for the total daily (24 h) intake of water which in Y4−/− mice was elevated only on the second and third day of the 3 day recording period (Figure 2).
Figure 1.
Water intake in control, Y2−/− and Y4−/− mice during the light phase (A) of 4 consecutive days and the 3 intervening periods of dark phase (B). The water intake is expressed as ml per g body weight. Means ± s.e. mean, n=6. * P<0.05, ** P<0.01 versus control.
Figure 2.
Total daily (24 h) water intake in control, Y2−/− and Y4−/− mice during 3 consecutive days. The water intake is expressed as ml per g body weight. Means ± s.e. mean, n=6. * P<0.05, ** P<0.01 versus control.
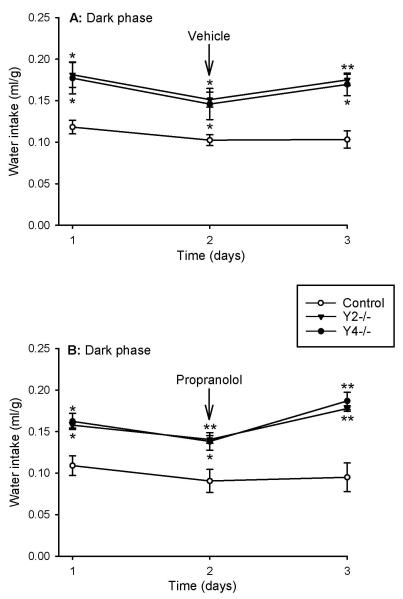
IP injection of physiological saline (the vehicle for propranolol) at 5:30 PM and 11:30 PM had no significant influence on the water intake during the dark phase, although the injection tended to reduce the drinking of water (Figure 3A). Importantly, the difference in the water intake during the dark phase between Y2−/− and Y4−/− mice, on the one hand, and control mice, on the other hand, was maintained after IP injection of vehicle (Figure 3A). The same observation was made after IP injection of propranolol (10 mg/kg at 5:30 PM and 11:30 PM) which did not prevent Y2−/− and Y4−/− mice from drinking significantly more water during the dark phase than the control mice (Figure 3B).
Figure 3.
Effect of vehicle (A) and propranolol (B) on water intake in control, Y2−/− and Y4−/− mice during the dark phase of 3 consecutive days. Vehicle (2 ml/kg) or propranolol (10 mg/kg) was injected intraperitoneally at 5:30 PM and 11:30 PM of the second day. The water intake is expressed as ml per g body weight. Means ± s.e. mean, n=4. * P<0.05, ** P<0.01 versus control.
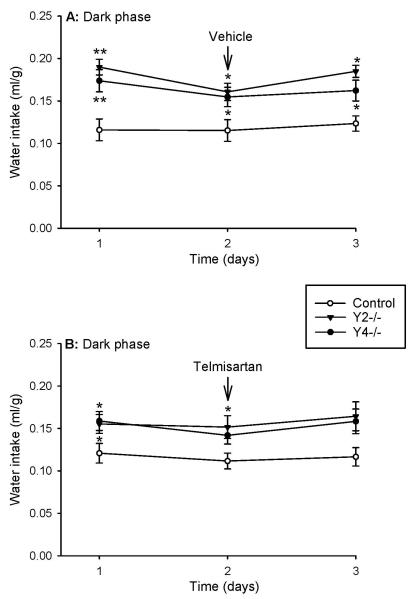
Since the results of experiments 4 and 5 involving telmisartan were indistinguishable, they were combined and are presented as one experiment. The vehicle for telmisartan (injected IP at 5:30 PM and 11:30 PM) did not influence the water intake during the dark phase (Figure 4A). As after injection of vehicle, analysis of the water intake following injection of telmisartan (10 mg/kg injected IP at 5:30 PM and 11:30 PM) failed to reveal any significant effect of treatment over time (Figure 4B). For this reason it cannot be deduced that the lack of a significant difference in the nocturnal water intake between control mice and Y2−/− mice (day 3) as well as Y4−/− mice (days 2 and 3), as shown in Figure 4B, reflects an effect of telmisartan.
Figure 4.
Effect of vehicle (A) and telmisartan (B) on water intake in control, Y2−/− and Y4−/− mice during the dark phase of 3 consecutive days. Vehicle (2 ml/kg) or telmisartan (10 mg/kg) was injected intraperitoneally at 5:30 PM and 11:30 PM of the second day. The water intake is expressed as ml per g body weight. Means ± s.e. mean, n=7-9. * P<0.05, ** P<0.01 versus control.
Discussion
The results of the present study demonstrate that Y2−/− and Y4−/− mice consume significantly more water during the dark phase than control mice, whereas the water intake during the light phase is unchanged. Our finding obtained with Y2−/− mice is consistent with the report of Thiele et al. [33] that, in an ethanol/water preference test, Y2−/− mice drink more water, but less ethanol, than wild-type controls during a 24 h observation period. The present data significantly extend this observation and reveal an implication of both Y2 and Y4 receptors in the circadian control of spontaneous water intake. The enhancement of nocturnal water consumption by Y2 and Y4 receptor gene knockout is reminiscent of the ability of Y1 and Y5 receptor blockade to inhibit feeding preferentially during the dark phase [7,15]. Being night-active animals, mice normally have a higher food and water intake during the dark phase than during the light phase. Although in Y2−/− mice water intake is enhanced in parallel with an increase in food intake [25,30,33], it is thought that the orexigenic effect of NPY takes place independently of its effect on drinking behaviour [24,28,32,33]. Such a relationship can be ruled out in Y4−/− mice in which water intake is enhanced despite reduced food consumption [14,31].
Hints at the mechanisms whereby Y2 and Y4 receptor gene knockout boosts nocturnal drinking may be deduced from the ability of intracerebroventricular and intrahypothalamic administration of NPY to augment spontaneous water intake in rabbits, rats and mice, an action that involves Y4 receptors [4,28,29,32,34]. As discussed by Thiele et al. [33], the increased water consumption seen in Y2−/− mice could be related to the function of Y2 receptors as presynaptic autoreceptors that inhibit NPY release [2]. If so, knockout of the Y2 receptor would remove any feedback inhibition of the release of NPY and, consequently, enforce postsynaptic transmission via NPY. This argument is supported by the finding that Y2 autoreceptor stimulation inhibits the release of NPY from neurons in the rat hypothalamus [17]. Furthermore, Y2 receptors are expressed in several brain regions relevant to the regulation of drinking behaviour [10], including area postrema, nucleus of the solitary tract, paraventricular nucleus of the hypothalamus, preoptic nucleus, bed nucleus of stria terminalis and amygdala [9,13,26,27].
Whether the rise in nocturnal water intake observed in Y4−/− mice can be explained in an analogous manner awaits to be determined. It is worth noting that Y4 receptors are expressed by orexin-containing neurons in the lateral hypothalamus [4], and that intracerebroventricularly administered orexin is known to increase water intake [18]. There is electrophysiological evidence that NPY inhibits orexin neurons in the murine hypothalamus by multiple pre- and postsynaptic mechanisms [11]. The postsynaptic receptors depressing the activity of orexin neurons are of the Y1 type, while the receptors mediating presynaptic inhibition have tentatively been classified as Y2/Y5 receptors [11] but remain to be fully characterized. It is thus conceivable that Y2 and, speculatively, Y4 receptor gene knockout removes an inhibitory drive on hypothalamic orexin neurons, which in turn results in stimulation of water consumption.
In explaining as to how Y2 and Y4 receptor gene knockout enhances water intake during the dark phase, several other factors have to be taken into account. In particular, changes in the intestinal ion and water transport, alterations of renal function and modifications in various messenger systems relevant to water homeostasis need to be considered. NPY is known to inhibit intestinal ion and water secretion, an action that in the mouse and human colon involves Y1, Y2 and Y4 receptors [5,6]. In the kidney, NPY is known to induce vasoconstriction via Y1 receptor activation and to elicit diuresis via stimulation of Y2 and Y5 receptors [1]. While investigation of intestinal and renal factors was beyond the scope of this explorative study, we addressed the possibility that β-adrenoceptors and angiotensin AT1 receptors, two interrelated systems known to stimulate water intake, may contribute to the increase in nocturnal water intake seen in Y2−/− and Y4−/− mice.
The involvement of β-adrenoceptors was considered because NPY is associated with noradrenergic neurons in the central and sympathetic nervous system [16,22]. β-Adrenoceptor agonists stimulate water intake through activation of the renin-angiotensin system [20,23). In addition, β-adrenoceptors play a role in the dipsogenic effect of angiotensin II in the preoptic area and hypothalamus [3,8]. However, our data obtained with propranolol rule out any implication of β-adrenoceptors in the increased nocturnal consumption of water exhibited by Y2−/− and Y4−/− mice. We think this conclusion to be valid because (±)-propranolol injected systemically at doses in the range of 10 mg/kg reaches and blocks both peripheral and central β-adrenoceptors of mice without causing sedation [21,36].
Although angiotensin II is a potent dipsogenic messenger in the brain [3,12], we do not think that this peptide contributes to the elevated nocturnal water consumption seen in Y2−/− and Y4−/−mice. This conjecture is deduced from the failure of the angiotensin AT1 receptor antagonist telmisartan to normalize the increase in nocturnal water intake caused by Y2 and Y4 receptor knockout. The validity of our conclusion is based on the reported ability of telmisartan (10 mg/kg administered systemically) to abolish the dipsogenic response to intracerebroventricular injection of angiotensin II, an effect that is sustained for 24 h [12]. The lack of involvement of angiotensin II in the enhanced drinking behaviour shown by Y2−/− and Y4−/− mice is in keeping with the finding that NPY and angiotensin II activate different neurons in the paraventricular nucleus of the hypothalamus [19]. This instance suggests that NPY and angiotensin II regulate disparate mechanisms of water homeostasis. A downstream mediator of angiotensin II is vasopressin which affects drinking indirectly through promotion of water reabsorption in the kidney, and it awaits to be determined whether this pathway contributes to the alterations observed here.
Because of its exploratory nature, the current study has some limitations which need to be addressed in interpreting the implications of the findings. The present experiments were initiated following the discovery of a differential water consumption in a colony of female control, Y2−/− and Y4−/− mice that were obtained to study gastric nociception [35]. Thus, the experimental design was confined by the number of female animals available for the study, which explains the sequential rather than parallel study design. Although the data collected in a sequential protocol might be complicated by alterations of water intake over time, we are confident that such temporal changes did not occur because the differences in the nocturnal water intake between control, Y2−/− and Y4−/−mice were consistently seen in the sequential study runs (compare Figures 1B, 3A and 4A). We are likewise certain that telmisartan, relative to vehicle, failed to abrogate the increase in nocturnal water intake seen in Y2−/− and Y4−/− mice. This inference is based on the data of two studies which were combined because the number of cages used in each study alone was too small to allow for a statistical analysis by two-way ANOVA. In neither of the two studies had telmisartan any effect different from that of the vehicle. We did not measure food intake in control, Y2−/− and Y4−/−mice, because these parameter has already been examined [14,25,30,31,33]. A follow-up study, however, is warranted to simultaneously monitor the spontaneous food and water consumption in individual mice so that any impact of Y2 and Y4 receptor function on the relationship between water and food homeostasis can be analyzed. Given that functional changes produced by germline gene knockout may be masked by compensatory changes and that the metabolic set-points are subtly different in germline versus conditional F2Y mutants [30], it will also be important to confirm the present observations by appropriate NPY receptor antagonists as they become available. Unfortunately, the Y2 receptor antagonist BIIE0246 does not cross the blood-brain barrier (personal communication by H.N. Doods, Boehringer Ingelheim) at effective and selective doses as confirmed in a recent study [35].
In summary, our study has shown that Y2 and Y4 receptor gene knockout leads to enhanced consumption of water specifically during the dark phase. Although we cannot rule out that the knockout approach causes compensatory changes in multiple messenger systems relevant to drinking, our data reveal that endogenous NPY acting via Y2 and Y4 receptors plays a distinctive role in nocturnal water homeostasis. We hypothesize that Y2−/− and Y4−/− mice drink more water than control mice because they lack presynaptic autoinhibition of NPY release and NPY-mediated inhibition of orexin neurons in the central nervous system, whereas β-adrenoceptors and angiotensin AT1 receptors are not involved.
Acknowledgements
This work was supported by the Jubilee Funds of the Austrian National Bank (grant 9858), the Austrian Scientific Research Funds (FWF grant L25-B05) and the Zukunftsfonds Steiermark (grant 262). The authors thank Dr. Wolfgang Wienen of Boehringer Ingelheim (Biberach, Germany) for the kind gift of telmisartan.
References
- [1].Bischoff A, Michel MC. Renal effects of neuropeptide Y. Pflügers Arch. 1998;435:443–453. doi: 10.1007/s004240050538. [DOI] [PubMed] [Google Scholar]
- [2].Broberger C, Landry M, Wong H, Walsh JN, Hökfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- [3].Camargo LA, Saad WA, Camargo GP. Effects of subtypes alpha- and beta-adrenoceptors of the lateral hypothalamus on the water and sodium intake induced by angiotensin II injected into the subfornical organ. Brain Res. 2000;881:176–181. doi: 10.1016/s0006-8993(00)02840-7. [DOI] [PubMed] [Google Scholar]
- [4].Campbell RE, Smith MS, Allen SE, Grayson BE, Ffrench-Mullen JM, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J Neurosci. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cox HM, Pollock EL, Tough IR, Herzog H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides. 2001;22:445–452. doi: 10.1016/s0196-9781(01)00355-2. [DOI] [PubMed] [Google Scholar]
- [6].Cox HM, Tough IR. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br J Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Criscione L, Rigollier P, Batzl-Hartmann C, Rueger H, Stricker-Krongrad A, Wyss P, Brunner L, Whitebread S, Yamaguchi Y, Gerald C, Heurich RO, Walker MW, Chiesi M, Schilling W, Hofbauer KG, Levens N. Food intake in free-feeding and energy-deprived lean rats is mediated by the neuropeptide Y5 receptor. J Clin Invest. 1998;102:2136–2145. doi: 10.1172/JCI4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].da Silva RK, Menani JV, Saad WA, Renzi A, Silveira JE, Luiz AC, Camargo LA. Role of the alpha 1-, and alpha 2- and beta-adrenoceptors of the median preoptic area on the water intake, renal excretion, and arterial pressure induced by ANG II. Brain Res. 1996;717:38–43. doi: 10.1016/0006-8993(95)01553-1. [DOI] [PubMed] [Google Scholar]
- [9].Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- [10].Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- [11].Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, Culman J, Unger T. AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther. 2001;298:62–70. [PubMed] [Google Scholar]
- [13].Gustafson EL, Smith KE, Durkin MM, Walker MW, Gerald C, Weinshank R, Branchek TA. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol Brain Res. 1997;46:223–235. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- [14].Herzog H. Transgenic and knockout models in NPY research. In: Michel C, editor. Neuropeptide Y and Related Peptides. Handbook of Experimental Pharmacology. Vol. 162. Springer; Berlin: 2004. pp. 447–478. [Google Scholar]
- [15].Kanatani A, Hata M, Mashiko S, Ishihara A, Okamoto O, Haga Y, Ohe T, Kanno T, Murai N, Ishii Y, Fukuroda T, Fukami T, Ihara M. A typical Y1 receptor regulates feeding behaviors: effects of a potent and selective Y1 antagonist, J-115814. Mol Pharmacol. 2001;59:501–505. doi: 10.1124/mol.59.3.501. [DOI] [PubMed] [Google Scholar]
- [16].Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- [17].King PJ, Williams G, Doods H, Widdowson PS. Effect of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246, on neuropeptide Y release. Eur J Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- [18].Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T. Orexins/hypocretins regulate drinking behaviour. Brain Res. 1999;842:256–261. doi: 10.1016/s0006-8993(99)01884-3. [DOI] [PubMed] [Google Scholar]
- [19].Lambert PD, Phillips PJ, Wilding JP, Bloom SR, Herbert J. c-Fos expression in the paraventricular nucleus of the hypothalamus following intracerebroventricular infusions of neuropeptide Y. Brain Res. 1995;670:59–65. doi: 10.1016/0006-8993(94)01224-6. [DOI] [PubMed] [Google Scholar]
- [20].Lehr D, Mallow J, Krukowski M. Copious drinking and simultaneous inhibition of urine flow elicited by beta-adrenergic stimulation and contrary effect of alpha-adrenergic stimulation. J Pharmacol Exp Ther. 1967;158:150–163. [PubMed] [Google Scholar]
- [21].Levy A, Ngai SH, Finck AD, Kawashima K, Spector S. Disposition of propranolol isomers in mice. Eur J Pharmacol. 1976;40:93–100. doi: 10.1016/0014-2999(76)90358-7. [DOI] [PubMed] [Google Scholar]
- [22].Malmström RE. Pharmacology of neuropeptide Y receptor antagonists. Focus on cardiovascular functions. Eur J Pharmacol. 2002;447:11–30. doi: 10.1016/s0014-2999(02)01889-7. [DOI] [PubMed] [Google Scholar]
- [23].Meyer DK, Rauscher W, Peskar B, Hertting G. The mechanism of the drinking response to some hypotensive drugs: activation of the renin-angiotensin system by direct or reflex-mediated stimulation of beta-receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 1973;276:13–24. doi: 10.1007/BF00500774. [DOI] [PubMed] [Google Scholar]
- [24].Morley JE, Flood JF. The effect of neuropeptide Y on drinking in mice. Brain Res. 1989;494:129–137. doi: 10.1016/0006-8993(89)90151-0. [DOI] [PubMed] [Google Scholar]
- [25].Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, Ernfors P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- [26].Naveilhan P, Neveu I, Arenas E, Ernfors P. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87:289–302. doi: 10.1016/s0306-4522(98)00141-9. [DOI] [PubMed] [Google Scholar]
- [27].Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- [28].Pau MY, Pau KY, Spies HG. Characterization of central actions of neuropeptide Y on food and water intake in rabbits. Physiol Behav. 1988;44:797–802. doi: 10.1016/0031-9384(88)90065-0. [DOI] [PubMed] [Google Scholar]
- [29].Raposinho PD, Pedrazzini T, White RB, Palmiter RD, Aubert ML. Chronic neuropeptide Y infusion into the lateral ventricle induces sustained feeding and obesity in mice lacking either Npy1r or Npy5r expression. Endocrinology. 2004;145:304–310. doi: 10.1210/en.2003-0914. [DOI] [PubMed] [Google Scholar]
- [30].Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Fürtinger S, Jenkins A, Cox HM, Sperk G, Hökfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA. 2002;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- [33].Thiele TE, Naveilhan P, Ernfors P. Assessment of ethanol consumption and water drinking by NPY Y2 receptor knockout mice. Peptides. 2004;24:975–983. doi: 10.1016/j.peptides.2004.03.009. [DOI] [PubMed] [Google Scholar]
- [34].van Dijk G, Strubbe JH. Time-dependent effects of neuropeptide Y infusion in the paraventricular hypothalamus on ingestive and associated behaviors in rats. Physiol Behav. 2003;79:575–580. doi: 10.1016/s0031-9384(03)00125-2. [DOI] [PubMed] [Google Scholar]
- [35].Wultsch T, Painsipp E, Thoeringer CK, Herzog H, Sperk G, Holzer P. Endogenous neuropeptide Y depresses the afferent signaling of gastric acid challenge to the mouse brainstem via neuropeptide Y type Y2 and Y4 receptors. Neuroscience. 2005 doi: 10.1016/j.neuroscience.2005.08.038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yoshimura H, Kihara Y, Ogawa N. Psychotropic effects of adrenergic beta-blockers on agonistic behavior between resident and intruder mice. Psychopharmacology (Berlin) 1987;91:445–450. doi: 10.1007/BF00216009. [DOI] [PubMed] [Google Scholar]