Summary
The maintenance of gastrointestinal mucosal integrity depends on the rapid alarm of protective mechanisms in the face of pending injury. Two populations of extrinsic primary afferent neurons, vagal and spinal, subserve this goal through different mechanisms. These sensory neurons react to gastrointestinal insults by triggering protective autonomic reflexes including the so-called cholinergic anti-inflammatory reflex. Spinal afferents, in addition, can initiate protective tissue reactions at the site of assault through release of calcitonin gene-related peptide from their peripheral endings. The protective responses triggered by sensory neurons comprise alterations in gastrointestinal blood flow, secretion and motility as well as modifications of immune function. This article focusses on significant advances that during the past couple of years have been made in identifying molecular nocisensors on afferent neurons and in dissecting the signalling mechanisms whereby afferent neurons govern inflammatory processes in the gut.
Keywords: Acid-sensing ion channel-3 (ASIC3), calcitonin gene-related peptide (CGRP), cholinergic anti-inflammatory reflex, gastroduodenal bicarbonate secretion, gastrointestinal blood flow, gastrointestinal immune system, primary afferent neurons, transient receptor potential vanilloid 1 (TRPV1) ion channel
Introduction
The physiological role of the gastrointestinal (GI) tract is not only to take up and digest food and absorb nutrients and water, but also to sort out and eliminate harmful and useless material. These seemingly conflicting tasks require a molecular analysis of the luminal contents and the functional status of the GI tract, so that the appropriate effector programmes can be selected [1]. To this end, the digestive system is endowed with an elaborate network of surveillance systems among which sensory neurons play a particular role. Thus, the gut is supplied by intrinsic sensory neurons of the enteric nerve plexuses as well as extrinsic spinal and vagal afferent neurons which are in close contact with two important non-neural surveillance systems in the mucosa: endocrine and immune cells [1]. With these connections and their sensory modalities, GI sensory neurons are able to recognize subtle changes in the chemical and physical environment within the lumen, interstitial space, vasculature and muscle of the gut.
Sensory neurons subserve homeostasis and protection from adverse conditions through several mechanisms. These include (i) sensation of pain, alterations in (ii) emotion, affect and cognition, induction of (iii) autonomic reflexes and (iv) neuroendocrine responses, and initiation of (v) protective tissue reactions at the site of assault (Figure 1). Within the gut, the protective mechanisms triggered by sensory neurons comprise alterations in blood flow, secretion and motility and modifications of immune function. Work in the past decade has identified a phenomenal variety of molecular sensors that are expressed by primary afferent neurons and enable them to carry out their surveillance tasks [2]. This gain of knowledge has considerably advanced the understanding of both the physiology and pharmacology of afferent neurons in maintaining homeostasis of the GI mucosa in the face of challenge and injury. The current article highlights some of the most important advances that have been made in this field during the past two years.
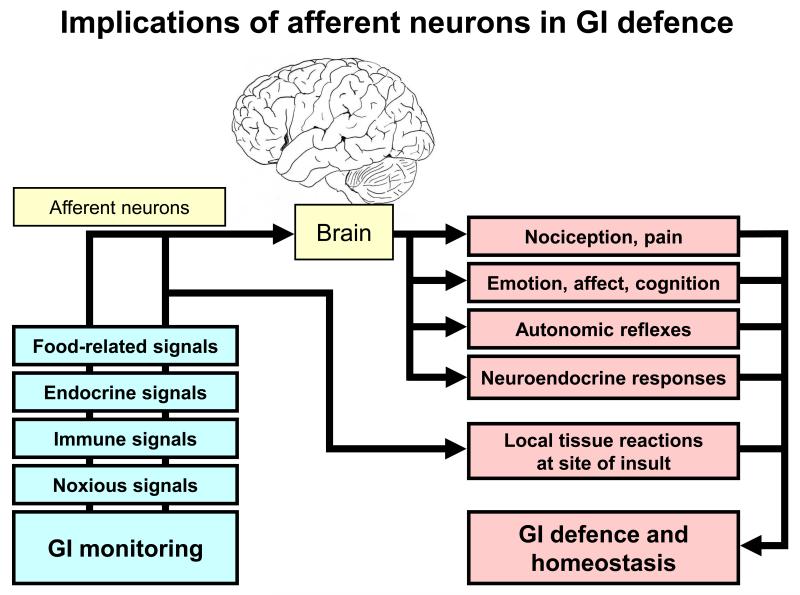
Figure 1.
Implications of afferent neurons in gastrointestinal (GI) defence. The graph shows that primary afferent neurons monitor the chemical and physical environment within the GI tract as they are able to respond to a variety of signal modalities. Via brain-mediated reactions and reflexes and through local neuropeptide release at the site of insult they contribute to GI defence and homeostasis.
Coordinated protection of the oesophago-gastro-duodenal region
Despite its essential role in digestion, gastric acid is a constant threat to the integrity of the mucosa in the stomach and the adjacent oesophageal and duodenal regions. Effective protection from the autoaggressive potential of acid is provided by mucosal defence mechanisms and appropriate compartmentalisation of the oesophago-gastro-duodenal region (Figure 2). The latter strategy is to prevent the escape of injurious concentrations of acid from the stomach, the mucosa of which is most resistant to intrusion by H+. Both the lower oesophageal sphincter (LOS) and the pyloric sphincter are under the control of neural reflexes involving acid-sensitive neurons. The tone of these sphincters is adjusted such that the levels of acid present in the oesophagus, stomach and duodenum are balanced with the mucosal defence mechanisms in these compartments [3].
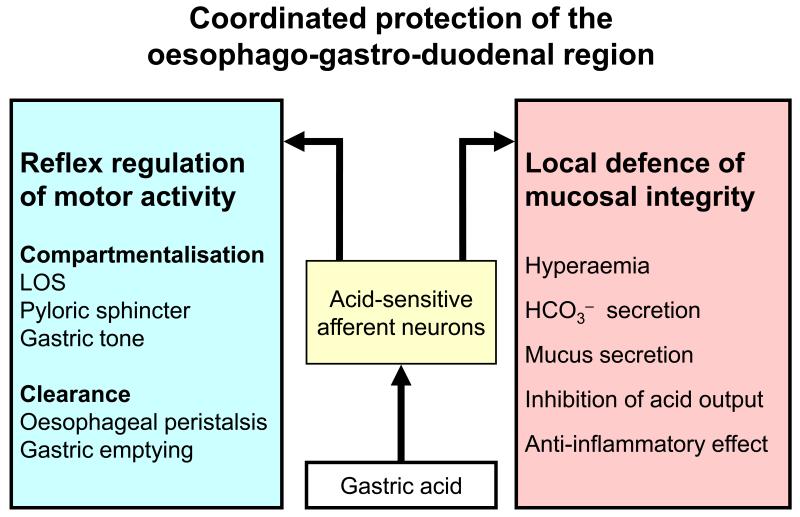
Figure 2.
Coordinated protection of the oesophago-gastro-duodenal region. The graph shows that acid-sensitive afferent neurons protect the foregut from gastric acid by reflex regulation of motor activity in the lower oesophageal sphincter (LOS), stomach and pyloric sphincter and by governing local tissue reactions supporting the defence of the mucosa.
The pyloric sphincter controls gastric emptying and ensures that the acidified gastric contents are delivered to the duodenum at a rate that enables this most proximal region of the small intestine to cope with the imposed acid load [3]. The LOS, in turn, prevents gastric acid from refluxing into the oesophagus and causing damage to the oesophageal mucosa. Transient lower oesophageal sphincter relaxations (TLOSRs), triggered by gastric distension, are thought to be a major cause for gastro-oesophageal reflux disease (GORD). Gastro-oesophageal vagal afferents express gamma-aminobutyric acid (GABA) receptors of the GABAB type, activation of which reduces the mechanosensitivity of gastro-oesophageal vagal afferents involved in the reflex regulation of LOS tone [4]. The GABAB receptor agonist baclofen is very active in inhibiting TLOSRs and has been shown to ameliorate GORD in adults as well as children [5]. Stimulation of afferent neurons in the oesophagus by local administration of capsaicin improves motor performance of the oesophageal body in GORD patients with ineffective motility [6]. Thus, afferent neurons seem to contribute to the protection of the oesophageal mucosa from gastric contents in a dual manner: by regulating the competence of the LOS and by facilitating clearance of the oesophagus from refluxing acid (Figure 2).
Spinal afferent nerve fibres as local emergency system in the gastrointestinal mucosa
There is ample evidence that afferent neurons originating from the dorsal root ganglia participate in the local regulation of GI circulation, secretion, motility, mucosal homeostasis and mucosal repair [1,7,8,9,10]. These tasks are accomplished by an efferent-like mode of operation: Calcitonin gene-related peptide (CGRP) is released from the peripheral fibres of sensory neurons and, in turn, modifies the activity of several GI effector systems. It has not yet been ascertained whether the efferent-like mode of action operates in parallel with the afferent mode of action of sensory neurons or whether different populations of sensory neurons subserve either an afferent or efferent-like function [11].
Although the local protective role of spinal afferent neurons has been demonstrated to take place in all regions of the GI tract from the oesophagus to the colon [7], most studies of this unique defensive action have been conducted in the gastroduodenal region. Here, the efferent-like mode of operation is best portrayed by the protective response of the gastric and duodenal mucosa to acid backdiffusion from the lumen into the mucosa [7,9]. If the mucosal barrier is disturbed or disrupted in the presence of luminal acid, the surge of acid intruding the lamina propria stimulates spinal afferents which via local CGRP release and nitric oxide (NO) formation cause prompt hyperaemia in the gastroduodenal mucosa, facilitate other mechanisms of defence such as bicarbonate (HCO3−) secretion and inhibit gastric acid secretion [1,7,8,9,12,13].
Further study of the efferent-like defensive action of sensory neurons has shown that the CGRP/NO messenger system stimulates mucin synthesis in the gastric corpus mucosa [14] and reduces myoelectrical activity in gastric smooth muscle [15]. Importantly, stimulation of sensory nerve fibres also exerts an anti-inflammatory action (Figure 3) which is brought about by a CGRP-induced increase in prostacyclin (PGI2) formation and a decrease in tumour necrosis factor-alpha release and tissue accumulation of neutrophils [16]. Through this action, sensory nerve stimulation reduces stress-related gastric mucosal damage and ischaemia reperfusion-induced liver injury [16,17,18,19]. The protective role of sensory neurons is gender-dependent, given that oestrogen enhances the expression of CGRP in dorsal root ganglion cells and the availability of releasable peptide in the stomach, whereas ovariectomy reduces the expression of CGRP and makes the mucosa more vulnerable to stress-induced injury [17,19].
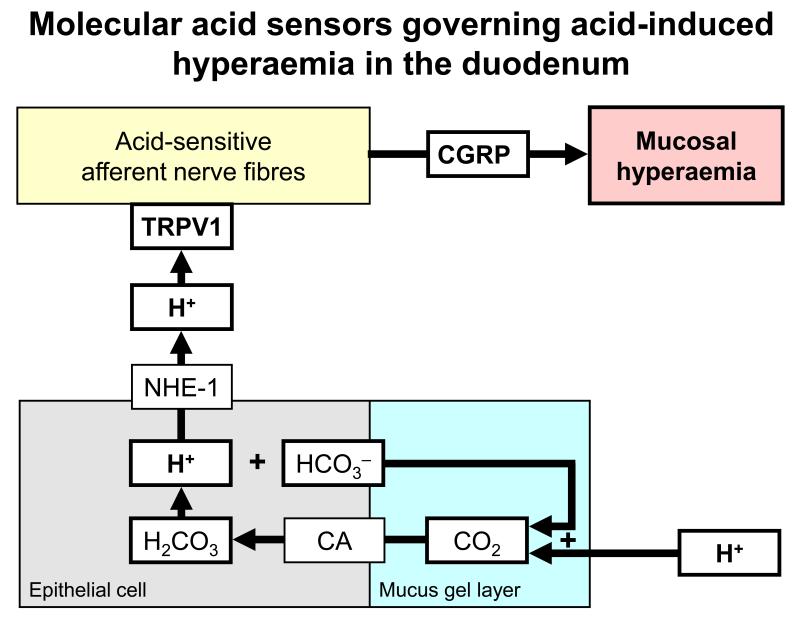
Figure 3.
Molecular acid sensors governing acid-induced hyperaemia in the duodenum [24]. The graph shows that luminal acid diffusing into the mucus gel layer of the duodenal mucosa interacts with HCO3− to form CO2. This molecule easily traverses the apical plasma membrane of epithelial cells where it is hydrated to carbonic acid by carbonic anhydrase (CA). Carbonic acid dissociates into HCO3− and H+ which exits the cells via the basolateral sodium-proton exchanger-1 (NHE-1) and lowers interstitial pH. Subepithelial acidosis activates TRPV1-bearing sensory nerve fibres that release the vasodilator peptide CGRP.
It is intriguing to note that some gastroprotective agents such as the histamine H2 receptor antagonist lafutidine are able to stimulate sensory neurons, which may contribute to their gastroprotective effect [20,21]. The molecular mode of action whereby lafutidine excites sensory neurons, causes CGRP release and attenuates ethanol- and stress-induced gastric injury is different from that of capsaicin [20,21] but has not yet been elucidated. Sensory neuron dysfunction, to the contrary, impairs GI mucosal protection [7]. For example, the dwindling capacity of the gastric mucosa to defend itself against injury in ageing rats is associated with a reduced number of mucosal CGRP-containing nerve fibres and with a decreased ability of CGRP to facilitate gastric mucin synthesis [14]. Chronic gastritis appears to be associated with an enhanced expression of CGRP in the human stomach [22] and, in patients infected with Helicobacter pylori, symptoms of functional dyspepsia have been correlated with enhanced expression of CGRP and substance P in the antral mucosa [23]. The pathogenic relevance of these findings to the local inflammatory process awaits to be determined.
Molecular acid sensors of afferent neurons involved in the protection of the gastroduodenal mucosa
Most sensory neurons respond to extracellular acidosis. There is emerging evidence that the acid-sensitive ion channel TRPV1 (transient receptor potential vanilloid-1) plays a role in signalling for duodenal hyperaemia in the face of luminal acidification [24,25]. TRPV1 is expressed by many afferent neurons innervating the rodent and human GI tract [26,27,28,29,30,31,32]. Since TRPV1 is located on sensory nerve terminals in the lamina propria behind the epithelium, the mucosal acid signal must be transduced across the epithelium (Figure 3). This transepithelial signalling pathway involves CO2 which is formed when excess luminal H+ combines with HCO3− secreted into the mucosal gel layer [24,25]. Easily traversing the apical plasma membrane of epithelial cells, CO2 is hydrated by carbonic anhydrase to carbonic acid which dissociates into HCO3− and H+. H+, in turn, exits via the basolateral sodium-proton exchanger-1 and lowers interstitial pH, which activates TRPV1-bearing sensory nerve fibres that release the vasodilator peptide CGRP [24,25].
The implication of TRPV1 in neural acid sensing has previously been envisaged from the effect of capsaicin, a ligand known to stimulate TRPV1 [26]. Thus, the capsaicin-evoked gastric hyperaemia [33] gastric mucosal protection [34,35] and gastroduodenal bicarbonate secretion [12,36] are antagonized by the TRPV1 blocker capsazepine. Unlike the acid-induced hyperaemia in the duodenum [24], the acid-evoked secretion of bicarbonate [12] and hyperaemia in the rat stomach [33] remain unaltered by capsazepine. This finding does not totally rule out any implication of TRPV1 because capsazepine is a class B blocker of TRPV1, inhibiting channel activation by capsaicin more potently than that by acid. It is, however, conceivable that there are regional differences in the receptor mechanisms whereby acid challenge activates sensory neurons, a conjecture that is in keeping with the multiplicity of acid-sensing ion channels expressed by sensory neurons [37].
Acid-sensing ion channels (ASICs) comprising ASIC1, ASIC2 and ASIC3 represent another class of molecular acid sensors present on primary afferent neurons in the GI tract [27,38]. While ASIC3 participates in the inflammation-induced hypersensitivity of vagal afferents to gastric acid [39] and spinal afferents to colorectal distension [40], it has not yet been examined whether sensory neuron-mediated GI mucosal protection involves ASIC3.
Mucosal factors stimulating afferent neurons involved in gastric mucosal protection
Bradykinin, ghrelin and melatonin have been identified as factors that facilitate GI mucosal protection through sensory neuron-dependent mechanisms. The effect of bradykinin to stimulate gastroduodenal HCO3− secretion through an action involving sensory neurons is mediated by bradykinin B2 receptors and prostaglandin E2 [12,13]. Ghrelin is produced by endocrine cells of the gastric mucosa and known to excite vagal afferents which express ghrelin receptors [41]. The ability of this peptide to attenuate ischaemia reperfusion-induced injury in the gastric mucosa involves activation of sensory neurons and formation of NO [42]. Melatonin, which likewise is a GI hormone, attenuates stress-induced gastric lesions through stimulation of melatonin MT2 receptors and CGRP-releasing sensory neurons [43].
Pro-inflammatory effects mediated by sensory neurons in the gut
Despite the evidence that primary afferent neurons releasing CGRP contribute to GI mucosal defence, this functional implication must not be generalized because there is evidence that under certain conditions sensory neurons exacerbate inflammatory tissue reactions. For instance, gastritis induced by iodoacetamide or diquat is significantly reduced by capsaicin-induced ablation of sensory neurons [44] and colitis evoked by dextrane sulfate sodium is attenuated by TRPV1 blockers [45]. Likewise, ileitis induced by Clostridium difficile toxin A or the endocannabinoids anandamide and 2-arachidonoyl glycerol is ameliorated by capsazepine [46], and pancreatic islet inflammation in an experimental model of type-1 diabetes is inhibited by ablation of TRPV1-expressing sensory neurons [47]. In contrast, the effect of dinitrobenzene sulfonic acid to induce colitis and disturb colonic smooth muscle activity is increased in TRPV1 knockout mice [48].
The proinflammatory role of TRPV1-bearing sensory neurons in the ileitis evoked by Clostridium difficile toxin A is thought to arise from the formation of endocannabinoids which stimulate TRPV1 and thereby cause the release of substance P from sensory nerve fibres [46]. Substance P, in turn, activates enteric neurons and immune cells, which ultimately results in hypersecretion, inflammation and mucosal damage [46]. Whether hydrogen sulfide contributes to these processes awaits to be determined. Formed by cystathionine gamma-lyase and cystathionine beta-synthase in enteric neurons, hydrogen sulfide enhances intestinal chloride secretion via an action involving TRPV1 and capsaicin-sensitive afferent neurons [49].
The vagal anti-inflammatory reflex
Cytokine-responsive vagal afferent neurons participate in the communication between the peripheral immune system and the brain [50,51]. This function is supported by a particular proximity of vagal afferent nerve fibres to immunologically relevant structures such as hepatic Kupffer cells (macrophage-like cells), paraganglia and connective tissue containing macrophages and dendritic cells [50,51]. Bacterial lipopolysaccharide (endotoxin) is able to cause release of interleukin-1beta from these cells, the cytokine in turn leading to excitation of vagal afferents [50,51].
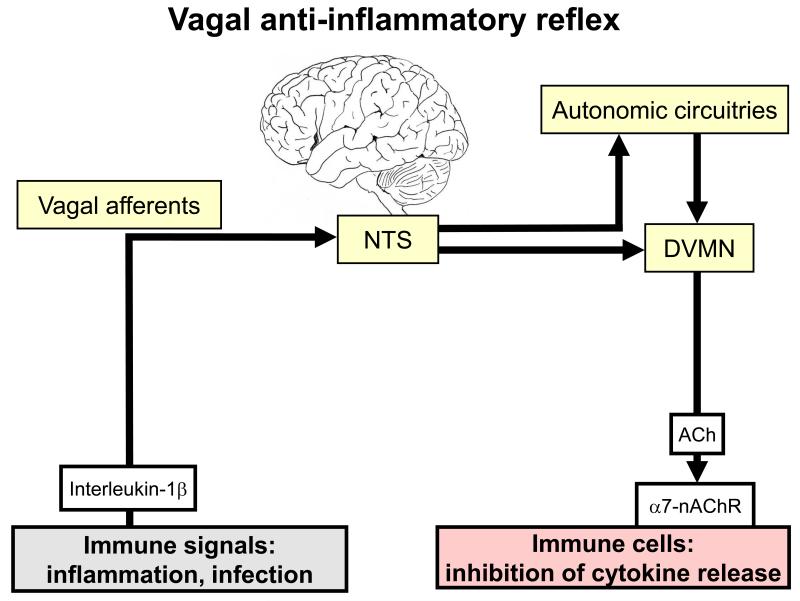
Through these properties, vagal afferents are thought to mediate a vago-vagal anti-inflammatory reflex (Figure 4). Peripheral immune and inflammatory signals trigger an input to the brain both via vagal afferents and circumventricular organs that are devoid of a blood-brain barrier. These signals are processed by the brainstem and central autonomic circuitries to provide an output via cholinergic vagal efferents [52]. Acetylcholine, released from efferent axons in the periphery, activates alpha7 subunit-containing nicotinic receptors on tissue macrophages and other immune cells (Figure 4), which results in inhibition of pro-inflammatory cytokine release and suppression of inflammation [52,53]. The specific involvement of alpha7 subunit-containing nicotinic receptors makes it conceivable that alpha7 subunit-selective agonists such as GTS-21 represent a new type of anti-inflammatory agent that is devoid of an action on autonomic ganglia in which transmission is mediated primarily by alpha3/beta4 subunit-containing nicotinic receptors [54].
Figure 4.
Vagal anti-inflammatory reflex [52]. The graph shows a vago-vagal reflex, the afferent arm of which is activated by pro-inflammatory cytokines such as interleukin-1beta. Following processing in the nucleus tractus solitarii (NTS) and in autonomic circuitries of the brain, efferent output is generated from the dorsal vagal motor nucleus (DVMN). Acetylcholine (ACh) released from vagal efferents activates alpha7 subunit-containing nicotinic acetylcholine receptors (alpha7-nAChR) on macrophages and other immune cells, which results in inhibition of pro-inflammatory cytokine release.
There is emerging evidence that the vagovagal anti-inflammatory reflex has an important role in controlling inflammation within the gut. Thus, dextrane sulfate sodium-induced colitis is exaggerated by vagotomy and hexamethonium and attenuated by nicotine [55]. The anti-inflammatory action of the vagus nerve involves a macrophage-dependent mechanism, because vagotomy fails to exacerbate colitis in mice that are deficient of macrophage colony-stimulating factor [55]. The extent of inflammation following abdominal surgery is a factor relevant to the severity and duration of postoperative ileus. Stimulation of nicotinic acetylcholine receptors has been reported to inhibit macrophage activation, ameliorate surgery-induced inflammation and reduce postoperative ileus through downstream activation of the Jak2-STAT3 signalling pathway [56].
Conclusions
The role of primary afferent neurons in monitoring actual or potential threats to the GI mucosa is of physiological relevance to body homeostasis. Long thought to subserve primarily nociception, sensory neurons are now recognized to enforce GI mucosal defence through several mechanisms (Figure 1), among which autonomic reflexes and the initiation of protective tissue reactions at the site of insult play a particular role. Significant progress has been made in the past years to identify the molecular sensors that enable afferent neurons to recognize potential threats. These nocisensors include TRPV1 and ASIC3 and receptors for local tissue mediators (e.g., bradykinin, ghrelin, melatonin, endocannabinoids, hydrogen sulfide) that are released upon challenge of the GI tract.
Autonomic reflexes that govern motor and immune functions of the GI tract are fine-tuned by the multiple sensory capacities of their afferent arm. For instance, the passage of gastric juice across the LOS and pyloric sphincter is controlled by chemo- and mechanosensitive afferent neurons that may be targeted in the design of novel GORD therapeutics. A particular aspect of GI homeostasis is highlighted by the discovery of vago-vagal anti-inflammatory reflexes that have a bearing on GI immune function. Since the anti-inflammatory reflex output to GI immune cells is mediated by alpha7 subunit-containing nicotinic receptors, it appears possible to develop alpha7 subunit-selective agonists as a new type of anti-inflammatory agent.
Acknowledgements
Work in the author’s laboratory was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Scientific Research Funds (FWF grant L25-B05).
References
- 1.Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci. 2006;125:70–75. doi: 10.1016/j.autneu.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holzer P. Treating visceral pain via molecular targets on afferent neurons: current and future. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain: Theory and Practice. Informa Healthcare; 2007. pp. 245–269. [Google Scholar]
- 3.**; Holzer P. Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am J Physiol. 2007;292:G699–G705. doi: 10.1152/ajpgi.00517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Short review focussing on neural and non-neural mechanisms of acid sensing throughout the gastrointestinal tract, with emphasis on the roles played by acid-sensitive ion channels such as TRPV1 and ASIC3.
- 4.*; Page AJ, O’Donnell TA, Blackshaw LA. Inhibition of mechanosensitivity in visceral primary afferents by GABAB receptors involves calcium and potassium channels. Neuroscience. 2006;137:627–636. doi: 10.1016/j.neuroscience.2005.09.016. [DOI] [PubMed] [Google Scholar]; Experimental study of the cellular mechanisms whereby the GABAB receptor agonist baclofen reduces mechanosensitivity of afferent neurons supplying the gastrointestinal tract.
- 5.Omari TI, Benninga MA, Sansom L, Butler RN, Dent J, Davidson GP. Effect of baclofen on esophagogastric motility and gastroesophageal reflux in children with gastroesophageal reflux disease: a randomized controlled trial. J Pediatr. 2006;149:468–474. doi: 10.1016/j.jpeds.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Grossi L, Cappello G, Marzio L. Effect of an acute intraluminal administration of capsaicin on oesophageal motor pattern in GORD patients with ineffective oesophageal motility. Neurogastroenterol Motil. 2006;18:632–636. doi: 10.1111/j.1365-2982.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 7.Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- 8.Szolcsanyi J, Bartho L. Capsaicin-sensitive afferents and their role in gastroprotection: an update. J Physiol (Paris) 2001;95:181–188. doi: 10.1016/s0928-4257(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 9.Akiba Y, Nakamura M, Nagata H, Kaunitz JD, Ishii H. Acid-sensing pathways in rat gastrointestinal mucosa. J Gastroenterol. 2002;37(Suppl 14):133–138. doi: 10.1007/BF03326432. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista S. Role of sensory neurons in restitution and healing of gastric ulcers. Curr Pharm Des. 2006;12:2977–2984. doi: 10.2174/138161206777947632. [DOI] [PubMed] [Google Scholar]
- 11.Holzer P, Maggi CA. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience. 1998;86:389–398. doi: 10.1016/s0306-4522(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 12.*; Aihara E, Hayashi M, Sasaki Y, Kobata A, Takeuchi K. Mechanisms underlying capsaicin-stimulated secretion in the stomach: comparison with mucosal acidification. J Pharmacol Exp Ther. 2005;315:423–432. doi: 10.1124/jpet.105.087619. [DOI] [PubMed] [Google Scholar]; Although both acid and capsaicin activate a common pathway that leads to bicarbonate secretion in the murine stomach, they do so via different sensors and prostaglandin mechanisms.
- 13.Aihara E, Sasaki Y, Ise F, Kita K, Nomura Y, Takeuchi K. Distinct mechanisms of acid-induced HCO3- secretion in normal and slightly permeable stomachs. Am J Physiol. 2006;291:G464–G471. doi: 10.1152/ajpgi.00048.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa T, Kusakabe T, Gono Y, Shikama N, Hiruma H, Kawakami T, Ishihara K. Nitric oxide synthase activity in rat gastric mucosa contributes to mucin synthesis elicited by calcitonin gene-related peptide. Biomed Res. 2006;27:117–124. doi: 10.2220/biomedres.27.117. [DOI] [PubMed] [Google Scholar]
- 15.Mizuguchi S, Ohno T, Hattori Y, Kamata K, Arai K, Saeki T, Saigenji K, Hayashi I, Kuribayashi Y, Majima M. Calcitonin gene-related peptide released by capsaicin suppresses myoelectrical activity of gastric smooth muscle. J Gastroenterol Hepatol. 2005;20:611–618. doi: 10.1111/j.1440-1746.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- 16.Okajima K, Harada N. Regulation of inflammatory responses by sensory neurons: molecular mechanism(s) and possible therapeutic applications. Curr Med Chem. 2006;13:2241–2251. doi: 10.2174/092986706777935131. [DOI] [PubMed] [Google Scholar]
- 17.*; Shimozawa N, Okajima K, Harada N, Arai M, Ishida Y, Shimada S, Kurihara H, Nakagata N. Contribution of sensory neurons to sex difference in the development of stress-induced gastric mucosal injury in mice. Gastroenterology. 2006;131:1826–1834. doi: 10.1053/j.gastro.2006.09.005. [DOI] [PubMed] [Google Scholar]; Oestrogen increases CGRP levels in dorsal root ganglia and reduces the vulnerability of the gastric mucosa to stress-induced injury.
- 18.Tan R, Bulbul M, Ongut G, Tosun O, Izgut-Uysal VN. Prostaglandins, capsaicin-sensitive sensory nerves and neutrophil infiltration, but not nitric oxide, contribute to cold restraint stress-induced gastric adaptation in rats. Clin Exp Pharmacol Physiol. 2006;33:946–951. doi: 10.1111/j.1440-1681.2006.04469.x. [DOI] [PubMed] [Google Scholar]
- 19.Shimozawa N, Okajima K, Harada N. Estrogen and isoflavone attenuate stress-induced gastric mucosal injury by inhibiting decreases in gastric tissue levels of CGRP in ovariectomized rats. Am J Physiol. 2007;292:G615–G619. doi: 10.1152/ajpgi.00117.2006. [DOI] [PubMed] [Google Scholar]
- 20.Fukushima K, Aoi Y, Kato S, Takeuchi K. Gastro-protective action of lafutidine mediated by capsaicin-sensitive afferent neurons without interaction with TRPV1 and involvement of endogenous prostaglandins. World J Gastroenterol. 2006;12:3031–3037. doi: 10.3748/wjg.v12.i19.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada N, Okajima K. Inhibition of neutrophil activation by lafutidine, an H 2-receptor antagonist, through enhancement of sensory neuron activation contributes to the reduction of stress-induced gastric mucosal injury in rats. Dig Dis Sci. 2007;52:469–477. doi: 10.1007/s10620-006-9620-4. [DOI] [PubMed] [Google Scholar]
- 22.Domotor A, Kereskay L, Szekeres G, Hunyady B, Szolcsanyi J, Mozsik G. Participation of capsaicin-sensitive afferent nerves in the gastric mucosa of patients with Helicobacter pylori-positive or-negative chronic gastritis. Dig Dis Sci. 2007;52:411–417. doi: 10.1007/s10620-006-9180-7. [DOI] [PubMed] [Google Scholar]
- 23.Mönnikes H, van der Voort IR, Wollenberg B, Heymann-Mönnikes I, Tebbe JJ, Alt W, Arnold R, Klapp BF, Wiedenmann B, McGregor GP. Gastric perception thresholds are low and sensory neuropeptide levels high in helicobacter pylori-positive functional dyspepsia. Digestion. 2005;71:111–123. doi: 10.1159/000084625. [DOI] [PubMed] [Google Scholar]
- 24.**; Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, Swenson ER, Kaunitz JD. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology. 2006;131:142–152. doi: 10.1053/j.gastro.2006.04.018. [DOI] [PubMed] [Google Scholar]; The hyperaemic response of the duodenal mucosa to luminal acidification depends on a cascade of events involving formation of CO2 in the mucus gel layer, carbonic anhydrase in the epithelial cells, the basolateral sodium-proton exchanger-1 and TRPV1 on sensory neurons releasing CGRP.
- 25.Montrose MH, Akiba Y, Takeuchi K, Kaunitz JD. Gastroduodenal mucosal defense. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract, Fourth Edition. Academic Press; 2006. pp. 1259–1291. [Google Scholar]
- 26.Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 28.Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol (London) 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faussone-Pellegrini MS, Taddei A, Bizzoco E, Lazzeri M, Vannucchi MG, Bechi P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem Cell Biol. 2005;124:61–68. doi: 10.1007/s00418-005-0025-9. [DOI] [PubMed] [Google Scholar]
- 30.Horie S, Michael GJ, Priestley JV. Co-localization of TRPV1-expressing nerve fibers with calcitonin-gene-related peptide and substance P in fundus of rat stomach. Inflammopharmacology. 2005;13:127–137. doi: 10.1163/156856005774423854. [DOI] [PubMed] [Google Scholar]
- 31.Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- 33.Tashima K, Nakashima M, Kagawa S, Kato S, Takeuchi K. Gastric hyperemic response induced by acid back-diffusion in rat stomachs following barrier disruption - relation to vanilloid type-1 receptors. Med Sci Monit. 2002;8:BR157–BR163. [PubMed] [Google Scholar]
- 34.Kato S, Aihara E, Nakamura A, Xin H, Matsui H, Kohama K, Takeuchi K. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem Pharmacol. 2003;66:1115–1121. doi: 10.1016/s0006-2952(03)00461-1. [DOI] [PubMed] [Google Scholar]
- 35.Horie S, Yamamoto H, Michael GJ, Uchida M, Belai A, Watanabe K, Priestley JV, Murayama T. Protective role of vanilloid receptor type 1 in HCl-induced gastric mucosal lesions in rats. Scand J Gastroenterol. 2004;39:303–312. doi: 10.1080/00365520310008647. [DOI] [PubMed] [Google Scholar]
- 36.Kagawa S, Aoi M, Kubo Y, Kotani T, Takeuchi K. Stimulation by capsaicin of duodenal HCO3− secretion via afferent neurons and vanilloid receptors in rats: comparison with acid-induced HCO3− response. Dig Dis Sci. 2003;48:1850–1856. doi: 10.1023/a:1025480003388. [DOI] [PubMed] [Google Scholar]
- 37.Holzer P. Acid-sensitive ion channels in gastrointestinal function. Curr Opin Pharmacol. 2003;3:618–625. doi: 10.1016/j.coph.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wultsch T, Painsipp E, Shahbazian A, Mitrovic M, Edelsbrunner M, Lazdunski M, Waldmann R, Holzer P. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach – brainstem axis. Pain. 2007 doi: 10.1016/j.pain.2007.04.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RC, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol. 2006;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- 42.*; Konturek PC, Brzozowski T, Walter B, Burnat G, Hess T, Hahn EG, Konturek SJ. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. Eur J Pharmacol. 2006;536:171–181. doi: 10.1016/j.ejphar.2006.02.032. [DOI] [PubMed] [Google Scholar]; The gastric orexigenic hormone ghrelin protects the gastric mucosa from ischaemia-reperfusion-induced injury and promotes healing of gastric lesions through stimulation of sensory neurons.
- 43.Brzozowski T, Konturek PC, Zwirska-Korczala K, Konturek SJ, Brzozowska I, Drozdowicz D, Sliwowski Z, Pawlik M, Pawlik WW, Hahn EG. Importance of the pineal gland, endogenous prostaglandins and sensory nerves in the gastroprotective actions of central and peripheral melatonin against stress-induced damage. J Pineal Res. 2005;39:375–385. doi: 10.1111/j.1600-079X.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 44.Larauche M, Anton PM, Peiro G, Eutamene H, Bueno L, Fioramonti J. Role of capsaicin-sensitive afferent nerves in different models of gastric inflammation in rats. Auton Neurosci. 2004;110:89–97. doi: 10.1016/j.autneu.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811–818. doi: 10.1111/j.1365-2982.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 46.McVey DC, Schmid PC, Schmid HH, Vigna SR. Endocannabinoids induce ileitis in rats via the capsaicin receptor (VR1) J Pharmacol Exp Ther. 2003;304:713–722. doi: 10.1124/jpet.102.043893. [DOI] [PubMed] [Google Scholar]
- 47.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 48.Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med. 2006;84:142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 49.Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 50.Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci Basic Clin. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 51.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 52.**; Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]; Concise review of the vagal cholinergic anti-inflammatory pathway that leads to suppression of cytokine release from immune cells and inhibition of inflammation.
- 53.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 54.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007 doi: 10.1097/01.CCM.0000259381.56526.96. in press. [DOI] [PubMed] [Google Scholar]
- 55.*; Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]; The vagus nerve plays an anti-inflammatory role in dextrane sulfate sodium-evoked colitis. The anti-inflammatory effect involves nicotinic acetylcholine receptors and a macrophage-dependent mechanism.
- 56.*; de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]; Experimentally induced postoperative inflammation and gastric ileus are attenuated by stimulation of the vagus nerve. This anti-inflammatory effect depends on activation of the Jak2-STAT3 signalling pathway in macrophages.