Abstract
Gastric distension causes cardiovascular reactions and enhances gastric compliance. Here we investigated how these responses are related to each other, whether they change upon repeated distension and which neural mechanisms are involved. Mean arterial blood pressure (MAP) in phenobarbital-anaesthetized rats was recorded from a carotid artery and gastric compliance determined with an electronic barostat. Runs of intermittent gastric distension were generated by stepwise increments (5 mm Hg) of intragastric pressure. While gastric compliance peaked at intragastric pressures of 20 mm Hg, the change in MAP (predominantly hypotension) was largest at intragastric pressures beyond 30 mm Hg. Repeated distension enhanced the MAP response to intragastric pressures beyond 35 mm Hg, whereas gastric compliance was facilitated primarily at intragastric pressures below 20 mm Hg. This facilitation of gastric compliance depended on the magnitude of the preceding distension. The MAP response to distension was enhanced by nitric oxide synthase inhibition, inhibited by subdiaphragmatic vagotomy but hardly affected by coeliac ganglionectomy. The facilitation of gastric compliance was changed by vagotomy in a complex manner but left unaltered by the other interventions. These findings show that isobaric gastric distension elicits both MAP and gastric compliance responses whose characteristics, mechanisms and sensitization properties differ profoundly.
Keywords: Cardiovascular reaction to gastric distension, gastric accommodation, electronic barostat, facilitation of gastric compliance on repeated gastric distension, extrinsic innervation of the stomach
INTRODUCTION
The primary motor function of the stomach is to receive, store and prepare food for digestion (1). This task is made possible by the accommodation reflex which, through active relaxation of the gastric fundus, allows for a volume increase without a rise in intragastric (IG) pressure and thus enables the stomach to incorporate large volumes during food intake (2,3). Gastric accommodation involves vago-vagal reflex pathways that activate inhibitory motor neurons of the enteric nervous system in the gastric wall (2,4). In addition, intrinsic neural reflex pathways participate in gastric accommodation to distension (4). Disturbances of these regulatory systems are thought to underlie functional disorders such as functional dyspepsia, in which relaxation of the gastric fundus in response to food intake is often impaired (3,5,6,7,8).
Apart from regulating gastric motility, distension also gives rise to autonomic reflexes and sensation (9). If IG pressure exceeds physiological levels, gastric relaxation is defective or afferent nerves have become hypersensitive, gastric distension elicits sensory discomfort and pain (3,5,6,7,8). In experimental animals visceral pain is assessed by pseudoaffective reflexes such as changes in blood pressure or visceromotor responses such as contractions of abdominal, hind limb and neck muscles (10,11,12,13,14,15,16,17). Importantly, the sensory gain of distension receptors in the human stomach is influenced by the tone of the gastric wall (18,19). Therefore, the overall aim of the present study was to record gastric compliance during isobaric distension of the stomach in anaesthetized rats, to determine the concomitant blood pressure response and to address some of the mechanisms governing these reactions.
The first specific aim was to characterize the relationship between gastric compliance, estimated with an electronic barostat, and the cardiovascular response to isobaric distension of the rat stomach over a range of physiological and supraphysiological IG pressures. These experiments revealed that repeated application of intermittent distension facilitated gastric compliance to a significant extent. Therefore, the second aim was to examine if this facilitation depends on the magnitude of the preceding distension and/or the interval between repeated distension protocols. Because hydrochloric acid (HCl) has been found to enhance gastric compliance and mechanosensation in humans (20), the third aim was to test whether acute exposure of the rat stomach to HCl has an influence on the gastric distension-evoked compliance and blood pressure reactions.
Nitric oxide (NO) is a transmitter of the inhibitory motor neurons mediating gastric relaxation (21,22,23), and in vivo studies have shown that gastric accommodation is significantly inhibited by NO synthase inhibitors (2,24). The fourth aim was, therefore, to examine whether NO participates in the gastric compliance and blood pressure response to gastric distension. This possibility was tested with NG-nitro-L-arginine methylester (L-NAME), an inhibitor of NO synthase. Since the stomach is innervated by vagal and spinal afferents, parasympathetic and sympathetic efferents as well as enteric neurons, the fifth and last aim was to explore some of the neural pathways underlying the blood pressure and gastric compliance response to gastric distension and the facilitation of compliance on repeated distension. This issue was addressed by acute bilateral subdiaphragmatic vagotomy and acute extirpation of the coeliac ganglion.
METHODS
Animal preparation and experimental procedures
This study was approved by an ethical committee of the Austrian Federal Ministry of Education, Science and Culture. Female Sprague-Dawley rats weighing 180 - 220 g were fasted for 20 h but had free access to water. After the induction of surgical anaesthesia with phenobarbital (220 mg/kg intraperitoneally) the rats were placed on a thermostated table, to maintain their rectal temperature at 37 degrees Celsius, and fitted with a tracheal cannula to facilitate spontaneous breathing. A cannula in the left jugular vein was used for continuous infusion of saline (0.9 % NaCl; 1.5 ml/h per rat), to avoid dehydration, and for intravenous administration of drugs. The mean arterial blood pressure (MAP) was measured via a pressure transducer attached to a cannula inserted into a common carotid artery. Signals were digitized and recorded on a personal computer for further analysis. In addition, the rats were laparotomized to ascertain the appropriate position of the IG bag in the fundus and to allow for IG administration of HCl via a transpyloric cannula.
IG pressure and volume were measured with an electronic barostat (Distender Series II Dual Drive Barostat for Rats; G&J Electronics, Willowdale, Ontario, Canada) connected to a non-compliant polyethylene bag (diameter: 2.5 cm, length: 5 cm, approximate capacity: 20 ml). The bag was attached to the distal end of a polyethylene tube (inner diameter: 1 mm) with a silk ligature that was sealed with a double-component glue. In keeping with recommendations as to how compliance of a spheric hollow organ is best measured (25,26,27), the bag was oversized with respect to the volume of the stomach so that the pressure-volume relationship of the gastric wall could be appropriately tested. Before being placed in the gastric fundus, the bag was finely folded and passed through the oesophagus into the stomach. The bag was then inflated with 6 ml air to ensure complete unfolding.
For IG administration of HCl, a cannula (inner diameter: 2.4 mm) was inserted through an incision in the proximal duodenum, passed through the pylorus and positioned in the stomach distally to the IG bag. The cannula was held in place by a ligature around the proximal duodenum. For administration and withdrawal of fluid the catheter was fitted with a three-way valve. One of the two exits was connected to a syringe for fluid administration, while the other exit was used to drain the gastric fluid (28). When the stomachs were treated with IG saline or HCl (2 ml), the fluid was slowly injected over a period of 10 s. After a 15 min exposure period, the stomach was allowed to drain before an intermittent distension protocol was run.
In one group of rats the subdiaphragmatic vagus nerves were cut, while in another group the coeliac ganglion with the adjacent superior mesenteric ganglion was removed (28). These nerve transections were performed immediately after laparotomy while control rats were sham-operated.
Experimental protocols
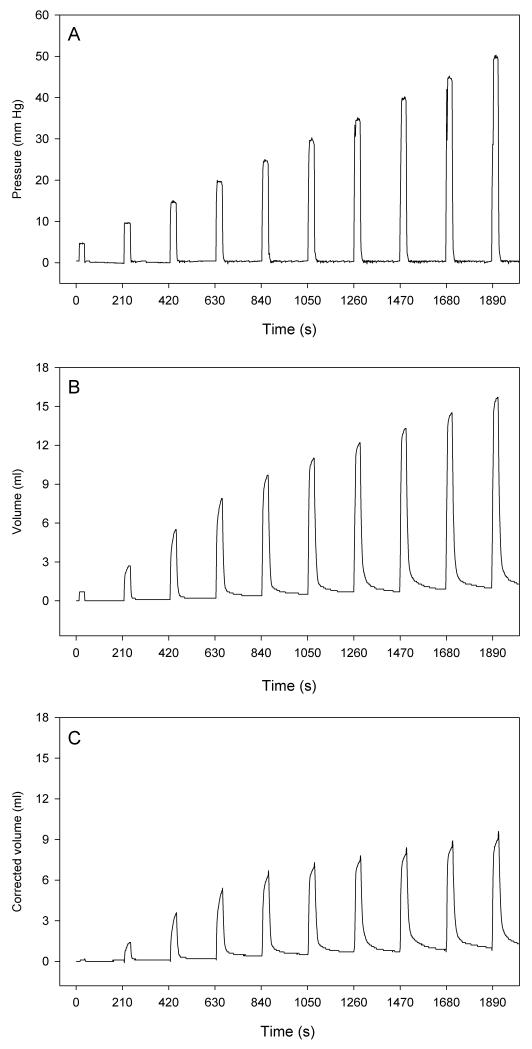
Following completion of surgery, the animals were allowed a period of 30 min to recover. Thereafter the first distension protocol was run. To this end, the barostat was operated via a host computer and the software Protocol Plus™ (G&J Electronics) which enabled pre-programmed distension sequences to be automatically delivered by the barostat. The method of ascending limits (AML) was chosen to generate a sequence of increasing step distensions (26) as shown in Figure 1. Specifically, the stomach was subjected to intermittent isobaric distensions with stepwise increments of IG pressure by 5 mm Hg, ranging from a minimal distending pressure of 5 mm Hg to a maximal pressure of 50 mm Hg. The distension steps lasted 30 s and were separated by periods of 3 min, during which the bag was deflated so that IG pressure returned to 0 mm Hg (Figure 1). Each rat was subjected to 2 or 3 AML runs (duration: 32 min), with 20 min rest periods between the runs, if not stated otherwise. The pressure and volume readings were saved by the barostat software which automatically calculated the corrected volume at each distension pressure according to Boyle’s law (26,27) and displayed it on the screen (Figure 1).
Figure 1.
Illustration of the intermittent gastric distension protocol (method of ascending limits) as delivered by the software Protocol Plus™. The intragastric pressure is increased by steps of 5 mm Hg, ranging from a minimal distending pressure of 5 mm Hg to a maximal pressure of 50 mm Hg. The distension steps last 30 s and are separated by periods of 3 min, during which the bag is deflated so that intragastric pressure returns to 0 mm Hg. The graph shows the recorded intragastric pressure (A), the recorded gastric volume (B) and the corrected gastric volume (C).
Seven different studies were carried out. In study 1 the effect of 3 consecutive AML runs (5 to 50 mm Hg) on gastric volume, gastric compliance and MAP was established. Since the rise of gastric compliance during the second AML run (run 2) was considerably larger than during the first run (run 1), this facilitation of gastric compliance was in the focus of the following experiments. Study 2 was performed to examine the facilitation of gastric compliance during 2 consecutive AML runs from 5 to 20 mm Hg and to compare it with the facilitation seen during 2 consecutive AML runs from 5 to 50 mm Hg. In study 3 we investigated whether the facilitation of gastric compliance varied if the rest period between 2 consecutive AML runs (5 to 20 mm Hg) was extended from 20 to 60 min. Study 4 tested whether the effect of 2 consecutive AML runs (5 to 50 mm Hg) on MAP and gastric compliance was altered by IG treatment with HCl. For this purpose, the gastric lumen was exposed to saline (2 ml) or HCl (0.2 M) for a 15 min period immediately before application of each AML run.
Study 5 examined whether the effect of 2 consecutive AML runs (run 1 from 5 to 20 mm Hg, run 2 from 5 to 50 mm Hg) on MAP and gastric compliance was altered by intravenous pretreatment with L-NAME. To this end, L-NAME (10 mg/kg; Bachem, Bubendorf, Switzerland) or its vehicle (saline, 1 ml/kg) was injected 25 min before application of run 1. Studies 6 and 7 were performed with rats subjected to sham operation, bilateral subdiaphragmatic vagotomy or coeliac ganglionectomy, respectively. In these animals the effect of 2 consecutive AML runs (run 1 from 5 to 25 mm Hg, run 2 from 5 to 50 mm Hg) on MAP and gastric compliance was investigated.
Evaluation of data and statistics
The alterations of MAP that occurred during intermittent gastric distension were often biphasic, involving both depressor and pressor reactions. For this reason, gastric distension-evoked MAP responses were expressed as total changes in MAP, incorporating both decreases and increases of MAP, if present. Gastric compliance was calculated as corrected gastric volume divided by IG pressure (ml/mm Hg). The facilitation of gastric compliance during 2 consecutive AML runs was calculated for each distension step and expressed as difference in the gastric compliance between each step in run 1 and run 2.
All data are presented as means ± SEM. Each experimental group comprised at least 5 animals. When the effect of treatments was tested, the design of the study was such that two rats were investigated in parallel, e.g., a rat subjected to the treatment under study and an appropriate control rat. Student’s two sample t test or one-way analysis of variance (ANOVA) followed by Tukey’s test was used to evaluate data obtained from two and more parallel experimental groups, respectively. Multiple readings within the same experimental group were analyzed with ANOVA for repeated measures followed by Tukey’s test. Probability values of P < 0.05 were regarded as significant.
RESULTS
Effect of three consecutive AML runs on MAP, gastric volume and gastric compliance
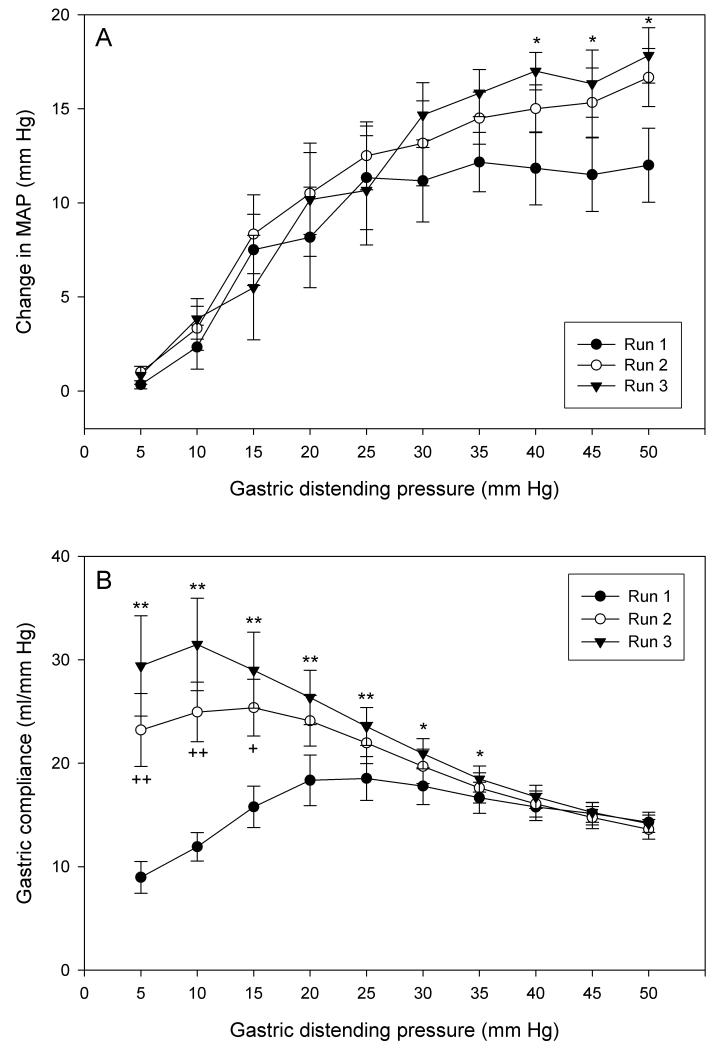
The effect of 3 consecutive AML runs (runs 1-3, 5 to 50 mm Hg, rest periods of 20 min between runs) on MAP and gastric compliance was examined. Intermittent gastric distension elicited brief changes in MAP (predominantly depressor effects), the magnitude of which was related to the IG pressure applied. The threshold for this cardiovascular effect was about 10 mm Hg (Figure 2A). The IG pressure - MAP change relationship recorded during AML runs 1 and 2 did not significantly differ from each other, although the MAP reaction in run 2 tended to be bigger than in run 1. During run 3, the MAP reaction to IG pressures higher than 35 mm Hg was significantly larger than during run 1 (Figure 2A).
Figure 2.
(A) Change in MAP and (B) gastric compliance during three runs of intermittent gastric distension from 5 to 50 mm Hg applied at inter-run intervals of 20 min. The values are means ± SEM; n = 6. * P < 0.05, ** P < 0.01 (run 3 vs. run 1); + P < 0.05, ++ P < 0.01 (run 2 vs. run 1).
Intermittent gastric distension was associated with increases in gastric volume, the magnitude of which depended on the IG pressure applied. Gastric compliance, expressed as corrected gastric volume divided by IG pressure, also rose. Unlike gastric volume, which continued to rise as long as the IG pressure was increased, gastric compliance recorded during run 1 reached a maximum at an IG pressure of 20 mm Hg. At higher IG pressure levels gastric compliance became smaller, which indicates that the capacity of the stomach to relax in response to distension was exhausted (Figure 2B). This IG pressure - gastric compliance relationship was significantly altered by repeated distension such that the maximum of the gastric compliance curve was successively enhanced and shifted towards lower IG pressure values (Figure 2B). In other terms, the gastric compliance response to IG pressures between 5 and 25 mm Hg underwent sensitization upon repeated distension. Resting periods of 20 min between the AML runs ensured that the baseline pressure and baseline volume returned to 0 before a new AML run was started. In all further experiments, this sensitization was portrayed by calculating the difference in gastric compliance between each step in run 1 and run 2, which revealed that sensitization was most pronounced at an IG pressure of 5 mm Hg and became smaller with higher IG pressures (Figure 3B).
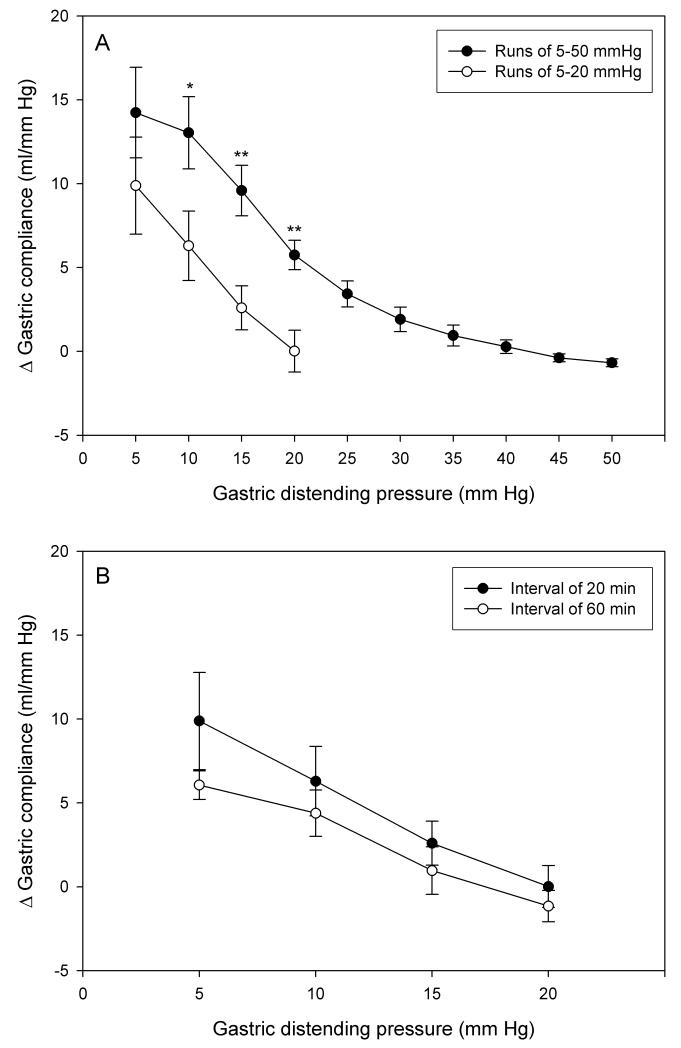
Figure 3.
Facilitation of gastric compliance on repeated gastric distension as expressed by the difference (D) in the gastric compliance at each step of distension runs 1 and 2. (A) Facilitation of gastric compliance induced by repeated distension runs from 5 to 20 mm Hg and 5 to 50 mm Hg applied at inter-run intervals of 20 min. (B) Facilitation of gastric compliance induced by repeated distension runs from 5 to 20 mm Hg applied at inter-run intervals of 20 min and 60 min. The values are means ± SEM; n = 6. * P < 0.05, ** P < 0.01 (vs. run from 5 to 20 mm Hg).
Facilitation of gastric compliance during two consecutive AML runs from 5 to 20 mm Hg and from 5 to 50 mm Hg
In order to examine whether the facilitation of gastric compliance upon repeated distension depended on the range of IG pressures applied during run 1, one group of rats was exposed to two consecutive AML runs from 5 to 20 mm Hg, while another group was submitted to two AML runs from 5 to 50 mm Hg. As can be seen in Figure 3A, the IG pressure - gastric compliance relationship differed significantly when distension run 1 comprised a range of 5 to 50 mm Hg as compared to a range of 5 to 20 mm Hg only. When the stomach was intermittently exposed to IG pressures up to 50 mm Hg during run 1, the facilitation of compliance was significantly larger than when the stomach was distended by pressures up to 20 mm Hg only. However, the facilitation of gastric compliance was still evident upon repeated distension in the range of 5 to 20 mm Hg.
Facilitation of gastric compliance during two consecutive AML runs with resting periods of 20 and 60 min in between
The facilitation of gastric compliance induced by two consecutive AML runs (each from 5 to 20 mm Hg) was not significantly altered if runs 1 and 2 were spaced apart 20 or 60 min, although with a resting period of 60 min the facilitation tended to become smaller (Figure 3B).
Effect of luminal acid exposure on MAP, gastric compliance and facilitation of gastric compliance
The effect of IG pretreatment with HCl on the MAP and gastric compliance responses during two consecutive AML runs (5 to 50 mm Hg) was examined. For this purpose, the gastric lumen was exposed to saline (2 ml) or HCl (0.2 M, 2 ml) for a 15 min period immediately before each AML run. The MAP responses recorded during run 1 and run 2 did not differ when the stomach had been pre-exposed to saline or HCl (n = 6). Likewise, the gastric compliance during run 1 and run 2 and the facilitation of gastric compliance following repeated runs of intermittent distension were not significantly altered by exposure to HCl (n = 6).
Effect of L-NAME on MAP, gastric compliance and facilitation of gastric compliance
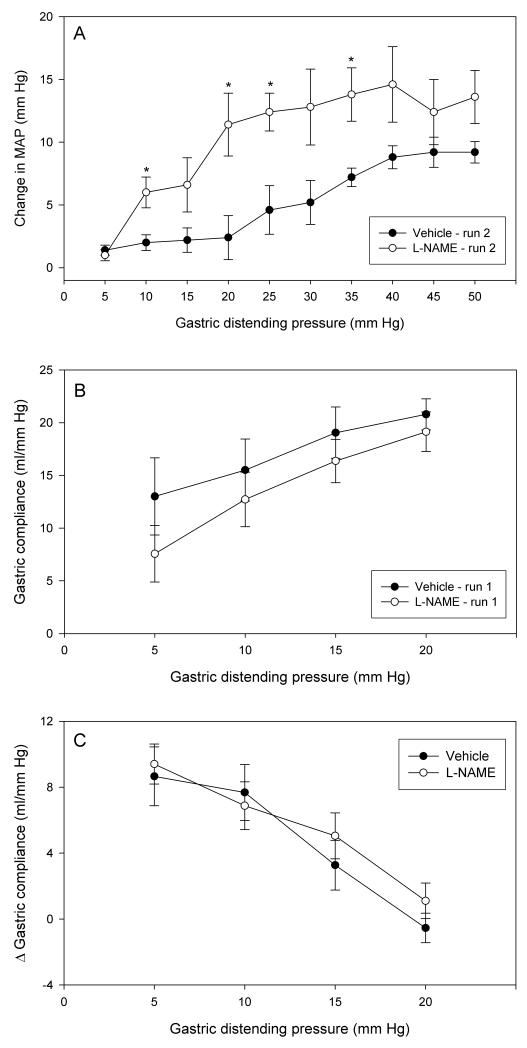
Within 15 min of its intravenous injection L-NAME (10 mg/kg) increased MAP from 74 ± 4.6 mm Hg to a maximum of 115 ± 4.8 mm Hg (n = 5) which was maintained throughout the experiment, whereas the vehicle (saline, 1 ml/kg) had no effect. Twenty-five min after injection of L-NAME, the first AML protocol was run (run 1 from 5 to 20 mm Hg), followed 20 min later by run 2 (5 to 50 mm Hg). Relative to saline, L-NAME enhanced the MAP reaction to intermittent distension both in run 1 (data not shown) and in run 2 (Figure 4A). The facilitation of the MAP response was most conspicuous at intermediate IG pressures ranging from 20 to 40 mm Hg. In contrast to the MAP reaction, gastric compliance in run 1 (Figure 4B) and run 2 (not shown) as well as the facilitation of gastric compliance following repeated runs of intermittent distension (Figure 4C) were not significantly modified by L-NAME. It may be noted, though, that in rats pretreated with L-NAME gastric compliance recorded during run 1 (Figure 4B) and run 2 (not shown) tended to be reduced compared to vehicle-pretreated rats.
Figure 4.
Effect of intravenous pretreatment with L-NAME on MAP and compliance during repeated gastric distension. (A) Change in MAP during the second run of intermittent gastric distension from 5 to 50 mm Hg. (B) Gastric compliance during the first run of intermittent gastric distension from 5 to 20 mm Hg. (C) Facilitation of gastric compliance on repeated gastric distension as expressed by the difference (D) in the gastric compliance at each step of distension runs 1 and 2 applied at an inter-run interval of 20 min. The animals were pretreated with vehicle (0.15 M NaCl, 1 ml/kg) or L-NAME (10 mg/kg) 25 min before application of run 1. The values are means ± SEM; n = 5. * P < 0.05 (vs. vehicle).
Effect of vagotomy on MAP, gastric compliance and facilitation of gastric compliance
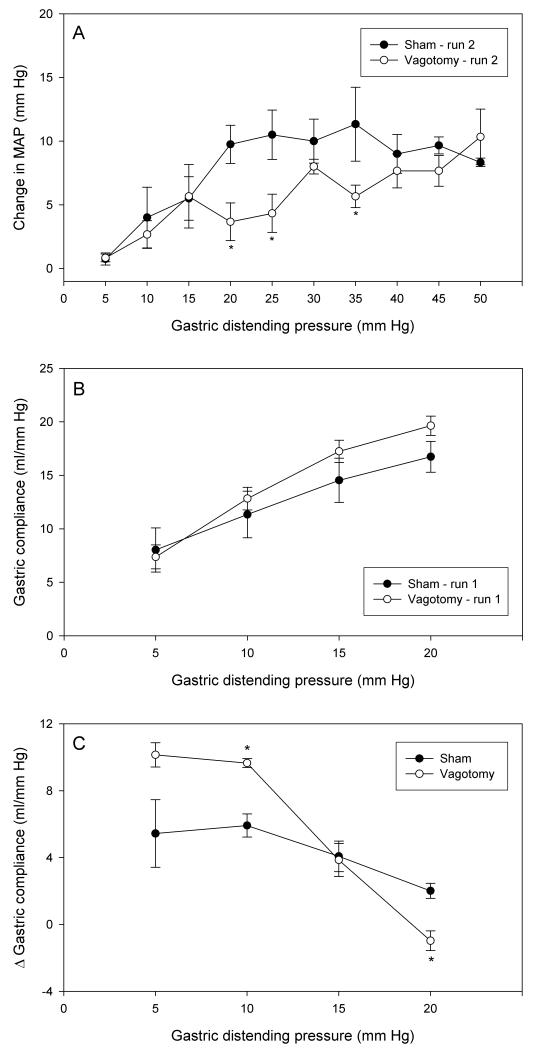
Acute bilateral subdiaphragmatic vagotomy was performed during instrumentation of the animals for the experiments and its influence on the parameters under study tested in 2 consecutive AML runs (run 1 from 5 to 20 mm Hg, run 2 from 5 to 50 mm Hg). Relative to sham operation, vagotomy attenuated the MAP reaction to intermittent distension both in run 1 (data not shown) and in run 2 (Figure 5A). The inhibition of the MAP response was most prominent at intermediate IG pressures ranging from 20 to 35 mm Hg. In contrast, gastric compliance tended to be enhanced by vagotomy both during run 1 (Figure 5B) and run 2 (not shown). This rise of compliance reached statistical significance only at an IG pressure of 10 mm Hg during run 2 (not shown). The facilitation of gastric compliance following repeated AML runs was modified by vagotomy in a complex manner (Figure 5C). While at low IG pressures of 5 to 10 mm Hg the facilitation was significantly enhanced, it was abolished at an IG pressure of 20 mm Hg (Figure 5C).
Figure 5.
Effect of bilateral subdiaphragmatic vagotomy, relative to sham operation, on MAP and compliance during repeated gastric distension. (A) Change in MAP during the second run of intermittent gastric distension from 5 to 50 mm Hg. (B) Gastric compliance during the first run of intermittent gastric distension from 5 to 20 mm Hg. (C) Facilitation of gastric compliance on repeated gastric distension as expressed by the difference (D) in the gastric compliance at each step of distension runs 1 and 2 applied at an inter-run interval of 20 min. Vagotomy and sham operation were performed during setup of the animals for the experiments. The values are means ± SEM; n = 5. * P < 0.05 (vs. vehicle).
Effect of coeliac ganglionectomy on MAP, gastric compliance and facilitation of gastric compliance
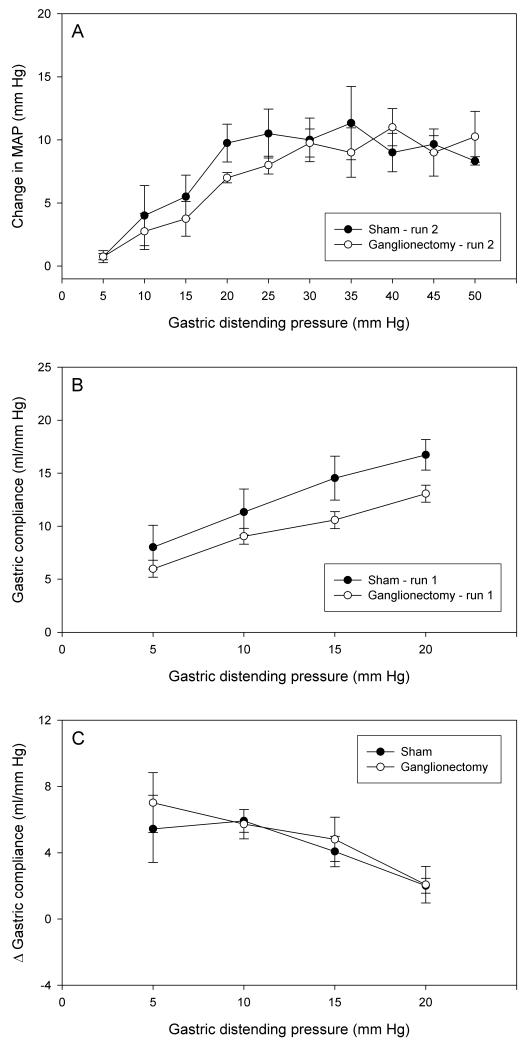
The experimental protocol used to evaluate the effect of coeliac ganglionectomy on the parameters under study was the same as that employed in vagotomized rats. Relative to sham operation, coeliac ganglionectomy tended to attenuate the MAP reaction to intermittent distension both in run 1 (not shown) and in run 2 (Figure 6A). However, this inhibition of the MAP reaction was statistically significant only at an IG pressure of 15 mm Hg during run 1 (not shown). Gastric compliance in run 1 (Figure 6B) and run 2 (not shown) also tended to be reduced by coeliac ganglionectomy, albeit without statistical significance. The facilitation of gastric compliance following repeated AML runs remained unaltered by coeliac ganglionectomy (Figure 6C).
Figure 6.
Effect of coeliac ganglionectomy, relative to sham operation, on MAP and compliance during repeated gastric distension. (A) Change in MAP during the second run of intermittent gastric distension from 5 to 50 mm Hg. (B) Gastric compliance during the first run of intermittent gastric distension from 5 to 20 mm Hg. (C) Facilitation of gastric compliance on repeated gastric distension as expressed by the difference (D) in the gastric compliance at each step of distension runs 1 and 2 applied at an inter-run interval of 20 min. Ganglionectomy and sham operation were performed during setup of the animals for the experiments. The values are means ± SEM; n = 5.
DISCUSSION
The current study has shown that intermittent distension of the rat stomach elicits changes in MAP and gastric compliance the magnitude of which is related to the IG pressure and the number of distension runs applied. A systematic comparison of the two responses revealed that different mechanisms and pathways underlie both types of responses, since they differ in a number of characteristics. While the MAP response to distension may increase as long as IG pressure is raised to a maximum of 50 mm Hg, gastric compliance becomes maximal at IG pressures of 20 mm Hg or below. Furthermore, facilitation of gastric compliance and the MAP response upon repeated distension occurs at different IG pressure ranges.
The MAP response was recorded in order to assess the stimulation of distension-sensitive extrinsic afferent neurons of the stomach, to demonstrate the activation of the autonomic nervous system and, via this pseudoaffective reflex, to estimate distension-induced nociception. In the absence of techniques to directly assess visceral nociception in animals, visceromotor reactions and changes in MAP or heart rate are frequently used to estimate gastrointestinal pain (9,10,11,12,13,14,15,16,17). While visceromotor responses to distension of the rat stomach are seen only when IG pressures beyond 30 mm Hg are applied (29), changes in MAP were observed here in response to IG pressures as low as 10 mm Hg. This differential pressure sensitivity of the cardiovascular and visceromotor reactions to gastric distension awaits to be further analyzed. The type of cardiovascular reaction to gastrointestinal distension, comprising often biphasic changes in MAP and heart rate (9,10,11,12,13,15), depends on the experimental conditions and the type of anaesthetic used. While the gastrointestinal distension-evoked change in MAP in awake rats is typically a pressor response, the reaction in anaesthetized rats is rather a depressor response (13). Of 60 phenobarbital-anaesthetized rats analyzed in the current study, 83 % responded to gastric distension with a depressor response followed by a MAP increase, while the remaining rats either exhibited only a pressor response or failed to respond at all. For this reason, the MAP reactions were evaluated as absolute changes in MAP, incorporating both MAP decreases and increases.
The gastric distension-evoked change in MAP was inhibited by subdiaphragmatic vagotomy but only marginally reduced by coeliac ganglionectomy, which indicates that vagal afferents play a more prominent part in the afferent arc of the MAP reflex reaction than spinal afferents. This inference is consistent with the observation that the bradycardia evoked by gastric distension in rats anaesthetized with ketamine plus xylazine is also mediated by vagal afferents (15). The MAP reaction to gastric distension was enhanced by the NO synthase inhibitor L-NAME at the effective dose of 10 mg/kg (30). This effect cannot be explained as a consequence of the hypertension caused by L-NAME, because the enhancement was also evident when the MAP responses in rats pretreated with vehicle and L-NAME were expressed as a percentage of the baseline MAP recorded immediately before each distension step. It follows that endogenous NO dampens the MAP reaction to gastric distension, and it awaits to be examined whether it does so by interfering with the afferent arc, the central transmission process or the efferent arc of the cardiovascular reflex. If the MAP reaction to gastric distension were to reflect nociception, our finding would be in keeping with other reports that NO can counteract pain mechanisms (31,32,33). Inhibition of NO synthase in humans, though, does not alter fasting sensitivity to gastric distension but enhances meal-induced satiety (34).
While gastric distension-evoked sensation and gastric compliance in humans is amplified by prior exposure to HCl (20), pretreatment of the rat stomach with supraphysiological HCl concentrations failed to alter gastric compliance and the MAP reaction to gastric distension. Our observations agree with the finding of Lamb et al. (29) that IG administration of HCl in the rat does not alter the visceromotor response to gastric distension. In the rat, such an interaction is unlikely to occur because chemical and mechanical nociception in the rat stomach are mediated by different afferent pathways (29).
At IG pressure levels that were submaximal to change MAP, gastric distension caused a marked rise of gastric compliance, which reflects a decrease in wall tension as it occurs in the gastric accommodation reflex. Proximal gastric accommodation is thought to involve vago-vagal pathways which cause relaxation of the gastric musculature through activation of inhibitory enteric motor neurons releasing NO and vasoactive intestinal polypeptide (2,3,4,21,22,23,34,35). In our experiments, though, gastric compliance recorded during the first run of intermittent isobaric distension was not significantly altered by subdiaphragmatic vagotomy, coeliac ganglionectomy and pretreatment with L-NAME. From these data it would appear that extrinsic neural pathways and NO do not participate in the distension-evoked rise of gastric compliance. It needs to be considered, however, that the IG bag, although placed in the fundus, may have also distended more distal parts of the stomach. If so, the failure of vagotomy to significantly alter the distension-induced gastric compliance may be the net result of inhibitory and excitatory vago-vagal reflexes whereby the proximal stomach is relaxed and the distal stomach is contracted (35,36).
The possibility that the dose of L-NAME used here was insufficient to affect gastric motor responses is unlikely because the same dose of L-NAME has previously been found to inhibit gastric emptying of acid (28). In this context it needs to be noted that NO synthase inhibition in humans likewise fails to modify fasting gastric compliance during isobaric distension (34). The lack of effect of vagotomy and coeliac ganglionectomy to modify the rise of gastric compliance during run 1 distension suggests that this relaxant response, if it is mediated by a neural reflex, depends on an intrinsic nerve circuit. This inference is in keeping with the finding that enteric motor reflexes sensitize in response to repeated muscle stretching (37).
The conclusion to be drawn from the current data is that the distension-evoked rise of gastric compliance involves multiple mechanisms, some of which have not yet been elucidated (2). As a consequence, a comparative analysis of the gastric compliance response to isobaric and isovolumetric distension of the rat stomach is needed. Attention will also have to be paid to the possibility that test species, experimental conditions and distension protocols account for the differences between our and other studies. We also hypothesize that, in gastric accommodation to food intake, the chemical nature of the ingested food, apart from the volume, may have an impact on which pathways of the accommodation reflex are activated.
The most striking finding of this study was that repeated gastric distension facilitated both the cardiovascular and gastric compliance reactions, although sensitization of these two reactions occurred at different stimulus intensities and with a different time course. Since the MAP response was facilitated at IG pressures higher than 35 mm Hg, which may be considered as noxious (17,29), it could be hypothesized that the facilitation of the MAP reaction reflects sensitization of a nociceptive afferent pathway. The facilitation of the MAP response cannot be explained by changes in the mechanosensory gain of gastric distension receptors, because compliance changes during repeated runs of gastric distension were observed only at intragastric pressures lower than 35 mm Hg. Our observation is in keeping with reports that the visceromotor response to colorectal distension in the rat is enhanced by repeated stimulation (38) as is the sensation elicited by distension of the sigmoid colon in humans (39). Other studies in humans failed to observe consistent sensitization to repeated colonic distension (40,41) and repeated distension of the human stomach was actually found to elevate the gastric mechanosensory threshold (7).
Unlike the MAP reaction, the distension-evoked increase in gastric compliance was most pronouncedly facilitated by repeated mechanical stimulation of the stomach at IG pressure levels of 5 - 10 mm Hg. The observation that prior exposure to high IG pressures up to 50 mm Hg amplifies the magnitude of the facilitation process implies that either autonomic nervous influences triggered by noxious levels of gastric distension enhance gastric compliance or that overdistension of the gastric wall can predispose the gastric musculature to a more effective relaxation upon subsequent distension. Since, however, considerable facilitation of gastric compliance was still seen when the first distension run was stopped at 20 mm Hg, we hypothesize that the enforcement of gastric compliance represents to a large extent a functional change that is triggered by comparatively low distension forces and that persists for a limited period of time, at least for 60 min.
This functional change may be accounted for by a number of mechanisms. Apart from modifications in the mechanical properties of gastric smooth muscle, sensitization of afferent pathways involved in the compliance response, facilitation of intrinsic and extrinsic reflexes mediating gastric relaxation or suppression of contractile influences on the stomach are possible explanations. While NO as well as afferent and efferent neurons passing through the coeliac ganglion have been ruled out as relevant factors, vagal pathways contribute in a complex manner, given that they dampen the sensitization in response to low IG pressures (5 to 10 mm Hg) and facilitate it in response to higher IG pressures (20 mm Hg). This finding appears to reflect the dual nature of the vagus in governing gastric motor activity: providing a drive to maintain gastric tone at low IG pressure and facilitating compliance at higher IG pressures. Our results do not permit to characterize the precise reflex pathways of this sensitization phenomenon, because nerve transection interupts both afferent and efferent neurons.
Taken together, the present findings show that gastric distension elicits MAP and gastric compliance responses that are governed by different mechanisms and pathways. This conclusion is supported by the observation that repeated distension amplifies both types of responses, but at completely different ranges of IG pressure. The discovery that repeated runs of distension facilitate gastric compliance is of both physiological and pathophysiological relevance. Physiologically, the sensitization process will support the storage of large amounts of food without an increase in IG pressure that may be felt as uncomfortable. Pathophysiologically, it may be speculated that in patients with functional dyspepsia in which gastric relaxation is impaired (3,5,6,7,8), this sensitization process may also be defective and contribute to early satiety and gastric discomfort. If so, investigation of the underlying mechanisms is of prime relevance to understanding this type of functional dyspepsia and to identifying novel targets for its treatment.
ACKNOWLEDGEMENTS
This work was supported by the Austrian Research Funds (FWF grant P14295) and by the Zukunftsfonds of the Province of Styria (grant 262). The technical help of Thomas Wultsch is appreciated.
REFERENCES
- 1.Mayer EA. The physiology of gastric storage and emptying. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 3rd edn Raven Press; New York: 1994. pp. 929–976. [Google Scholar]
- 2.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 4.Hennig GW, Brookes SJH, Costa M. Excitatory and inhibitory motor reflexes in the isolated guinea-pig stomach. J Physiol. 1997;501:197–212. doi: 10.1111/j.1469-7793.1997.197bo.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troncon LE, Thompson DG, Ahluwalia NK, Barlow J, Heggie L. Relations between upper abdominal symptoms and gastric distension abnormalities in dysmotility like functional dyspepsia and after vagotomy. Gut. 1995;37:17–22. doi: 10.1136/gut.37.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilja OH, Hausken T, Wilhelmsen I, Berstad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci. 1996;41:689–696. doi: 10.1007/BF02213124. [DOI] [PubMed] [Google Scholar]
- 7.Holtmann G, Gschossmann J, Neufang-Huber J, Gerken G, Talley NJ. Differences in gastric mechanosensory function after repeated ramp distensions in non-consulters with dyspepsia and healthy controls. Gut. 2000;47:332–336. doi: 10.1136/gut.47.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellow JE, Delvaux M, Azpiroz F, Camilleri M, Thompson DG, Quigley EMM. Principles of applied neurogastroenterology: physiology/motility-sensation. In: Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE, editors. Rome II - The Functional Gastrointestinal Disorders. Degnon Associates; McLean: 2000. pp. 91–156. [Google Scholar]
- 9.Timar-Peregrin A, Kumano K, Khalil Z, Sanger GJ, Furness JB. The relationship between propagated contractions and pseudoaffective changes in blood pressure in response to intestinal distension. Neurogastroenterol Motil. 2001;13:575–584. doi: 10.1046/j.1365-2982.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 10.Longhurst JC, Spilker HL, Ordway GA. Cardiovascular reflexes elicited by passive gastric distension in anesthetized cats. Am J Physiol. 1981;240:H539–H545. doi: 10.1152/ajpheart.1981.240.4.H539. [DOI] [PubMed] [Google Scholar]
- 11.Pozo F, Fueyo A, Esteban MM, Rojo-Ortega JM, Marin B. Blood pressure changes after gastric mechanical and electrical stimulation in rats. Am J Physiol. 1985;249:G739–G744. doi: 10.1152/ajpgi.1985.249.6.G739. [DOI] [PubMed] [Google Scholar]
- 12.Pittam BS, Ewart WR, Appia F, Wingate DL. Physiological enteric stimulation elicits cardiovascular reflexes in the rat. Am J Physiol. 1988;255:G319–G328. doi: 10.1152/ajpgi.1988.255.3.G319. [DOI] [PubMed] [Google Scholar]
- 13.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 14.Rouzade ML, Fioramonti J, Bueno L. A model for evaluation of gastric sensitivity in awake rats. Neurogastroenterol Motil. 1998;10:157–163. doi: 10.1046/j.1365-2982.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 15.Tougas G, Wang L. Pseudoaffective cardioautonomic responses to gastric distension in rats. Am J Physiol. 1999;277:R272–R278. doi: 10.1152/ajpregu.1999.277.1.R272. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki N, Bielefeldt K, Sengupta JN, Gebhart GF. Models of gastric hyperalgesia in the rat. Am J Physiol. 2002;283:G666–G676. doi: 10.1152/ajpgi.00001.2002. [DOI] [PubMed] [Google Scholar]
- 17.Gebhart GF, Sengupta JN. Evaluation of visceral pain. In: Gaginella TS, editor. Methods in Gastrointestinal Pharmacology. CRC Press; Boca Raton: 1996. pp. 359–373. [Google Scholar]
- 18.Villanova N, Azpiroz F, Malagelada JR. Gastrogastric reflexes regulating gastric tone and their relationship to perception. Am J Physiol. 1997;273:G464–G469. doi: 10.1152/ajpgi.1997.273.2.G464. [DOI] [PubMed] [Google Scholar]
- 19.Piessevaux H, Tack J, Wilmer A, Coulie B, Geubel A, Janssens J. Perception of changes in wall tension of the proximal stomach in humans. Gut. 2001;49:203–208. doi: 10.1136/gut.49.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffin B, Chollet R, Flourie B, Lemann M, Franchisseur C, Rambaud JC, Jian R. Intraluminal modulation of gastric sensitivity to distension: effects of hydrochloric acid and meal. Am J Physiol. 2001;280:G904–G909. doi: 10.1152/ajpgi.2001.280.5.G904. [DOI] [PubMed] [Google Scholar]
- 21.Li CG, Rand MJ. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre RA. Non-adrenergic non-cholinergic neurotransmission in the proximal stomach. Gen Pharmacol. 1993;24:257–266. doi: 10.1016/0306-3623(93)90301-d. [DOI] [PubMed] [Google Scholar]
- 23.Desai KM, Zembowicz A, Sessa WC, Vane JR. Nitroxergic nerves mediate vagally induced relaxation in the isolated stomach of the guinea pig. Proc Natl Acad Sci USA. 1991;88:11490–11494. doi: 10.1073/pnas.88.24.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meulemans AL, Eelen JG, Schuurkes JA. NO mediates gastric relaxation after brief vagal stimulation in anesthetized dogs. Am J Physiol. 1995;269:G255–G261. doi: 10.1152/ajpgi.1995.269.2.G255. [DOI] [PubMed] [Google Scholar]
- 25.Toma TP, Zighelboim J, Phillips SF, Talley NJ. Methods for studying intestinal sensitivity and compliance: in vitro studies of balloons and a barostat. Neurogastroenterol Motil. 1996;8:19–28. doi: 10.1111/j.1365-2982.1996.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead WE, Delvaux M, The Working Team Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. Dig Dis Sci. 1997;42:223–241. doi: 10.1023/a:1018885028501. [DOI] [PubMed] [Google Scholar]
- 27.Roelofs JMM, Clemens C, Akkermans LMA. Barostat review. Dig Dis Sci. 1997;42:1957–1958. doi: 10.1023/a:1018879630648. [DOI] [PubMed] [Google Scholar]
- 28.Holzer P, Painsipp E, Jocic M, Heinemann A. Acid challenge delays gastric pressure adaptation, blocks gastric emptying and stimulates gastric fluid secretion in the rat. Neurogastroenterol Motil. 2003;15:45–55. doi: 10.1046/j.1365-2982.2003.00382.x. [DOI] [PubMed] [Google Scholar]
- 29.Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Rees DD, Palmer RMJ, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo ZD, Cizkova D. The role of nitric oxide in nociception. Curr Rev Pain. 2000;4:459–466. doi: 10.1007/s11916-000-0070-y. [DOI] [PubMed] [Google Scholar]
- 32.Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil-induced peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Brain Res. 2001;909:170–178. doi: 10.1016/s0006-8993(01)02673-7. [DOI] [PubMed] [Google Scholar]
- 33.Ishide T, Maher TJ, Ally A. Role of nitric oxide in the ventrolateral medulla on cardiovascular responses and glutamate neurotransmission during mechanical and thermal stimuli. Pharmacol Res. 2003;47:59–68. doi: 10.1016/s1043-6618(02)00265-7. [DOI] [PubMed] [Google Scholar]
- 34.Tack J, Demedts I, Meulemans A, Schuurkes J, Janssens J. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut. 2002;51:219–224. doi: 10.1136/gut.51.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews PL, Scratcherd T. The gastric motility patterns induced by direct and reflex excitation of the vagus nerves in the anaesthetized ferret. J Physiol. 1980;302:363–378. doi: 10.1113/jphysiol.1980.sp013248. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews PL, Grundy D, Scratcherd T. Reflex excitation of antral motility induced by gastric distension in the ferret. J Physiol. 1980;298:79–84. doi: 10.1113/jphysiol.1980.sp013068. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furness JB, Kumano K, Larsson H, Murr E, Kunze WA, Vogalis F. Sensitization of enteric reflexes in the rat colon in vitro. Auton Neurosci. 2002;97:19–25. doi: 10.1016/s1566-0702(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 38.Gschossmann JM, Coutinho SV, Miller JC, Huebel K, Naliboff B, Wong HC, Walsh JH, Mayer EA. Involvement of spinal calcitonin gene-related peptide in the development of acute visceral hyperalgesia in the rat. Neurogastroenterol Motil. 2001;13:229–236. doi: 10.1046/j.1365-2982.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 39.Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- 40.Bradette M, Delvaux M, Staumont G, Fioramonti J, Bueno L, Frexinos J. Evaluation of colonic sensory thresholds in IBS patients using a barostat. Definition of optimal conditions and comparison with healthy subjects. Dig Dis Sci. 1994;39:449–457. doi: 10.1007/BF02088327. [DOI] [PubMed] [Google Scholar]
- 41.Hammer HF, Phillips SF, Camilleri M, Hanson RB. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol. 1998;274:G584–G590. doi: 10.1152/ajpgi.1998.274.3.G584. [DOI] [PubMed] [Google Scholar]