Abstract
The inflammatory response to intraarticular urate crystals is known to be variable in gouty arthritis. One source of variability may be the modulation of cellular responses by crystal-bound proteins. We have identified three apolipoproteins among the polypeptides bound to urate crystals exposed to plasma. Identification was first based on their coelectrophoresis with polypeptides from isolated lipoproteins and diminution in the protein coat of crystals exposed to lipoprotein-depleted plasma. The apoproteins were immunochemically identified by the Western blotting technique as apoprotein A-I, apoprotein B (apo B), and apoprotein E. Because neutrophils play a central role in acute gout, we investigated the potential effects of lipoproteins on neutrophil-urate crystal interactions. Plasma profoundly inhibited urate crystal-induced neutrophil luminol-dependent chemiluminescence (CL). Lipoprotein depletion by KBr density gradient centrifugation completely abrogated the inhibitory effect of plasma on urate-induced CL. The inhibitory activity of lipoprotein-depleted plasma was restored by adding back the d less than or equal to 1.25 g/cm3 lipoprotein fraction. Plasma also inhibited urate crystal-induced neutrophil superoxide generation and cytolysis (lactic dehydrogenase loss). This inhibition was significantly diminished by lipoprotein depletion, indicating that the lipoprotein effect was not limited to CL. Lipoprotein-depleted plasma reconstituted with very low, intermediate, and low density lipoproteins (LDL) inhibited crystal-induced CL. High density lipoprotein reconstitution was without effect. Immunodepletion from plasma of all apo B lipoproteins by agarose-bound apo B-specific antibody also removed all inhibitory activity for urate-induced CL. Thus, apo B lipoproteins were shown to be the inhibitory species in plasma. Binding of apo B lipoproteins to urate crystals and inhibition of CL was also seen in the absence of other plasma proteins. In addition, the binding of whole lipoprotein particles to the crystals was verified by detection of crystal-associated cholesterol in addition to the apoprotein. The effects of LDL on urate crystal-induced CL were stimulus specific. Coincubation of urate crystals and neutrophils in the presence of 10 micrograms/ml LDL resulted in 83% inhibition. In contrast, CL responses to a chemotactic hexapeptide, opsonized zymosan, and Staphylococcus aureus were not inhibited by LDL. The effects of depletion of apo B lipoproteins on plasma suppression of urate crystal-induced CL appeared to be unique. Plasma or sera depleted of other urate crystal-binding proteins including fibrinogen, fibronectin, C1q, and IgG retained virtually all their CL inhibitory activity. Lipoproteins containing apo B are thus a major regulator of neutrophil responses to urate crystals. These lipoproteins are present in variable concentration in synovial fluid and may exert an important influence on the course of gout.
Full text
PDF



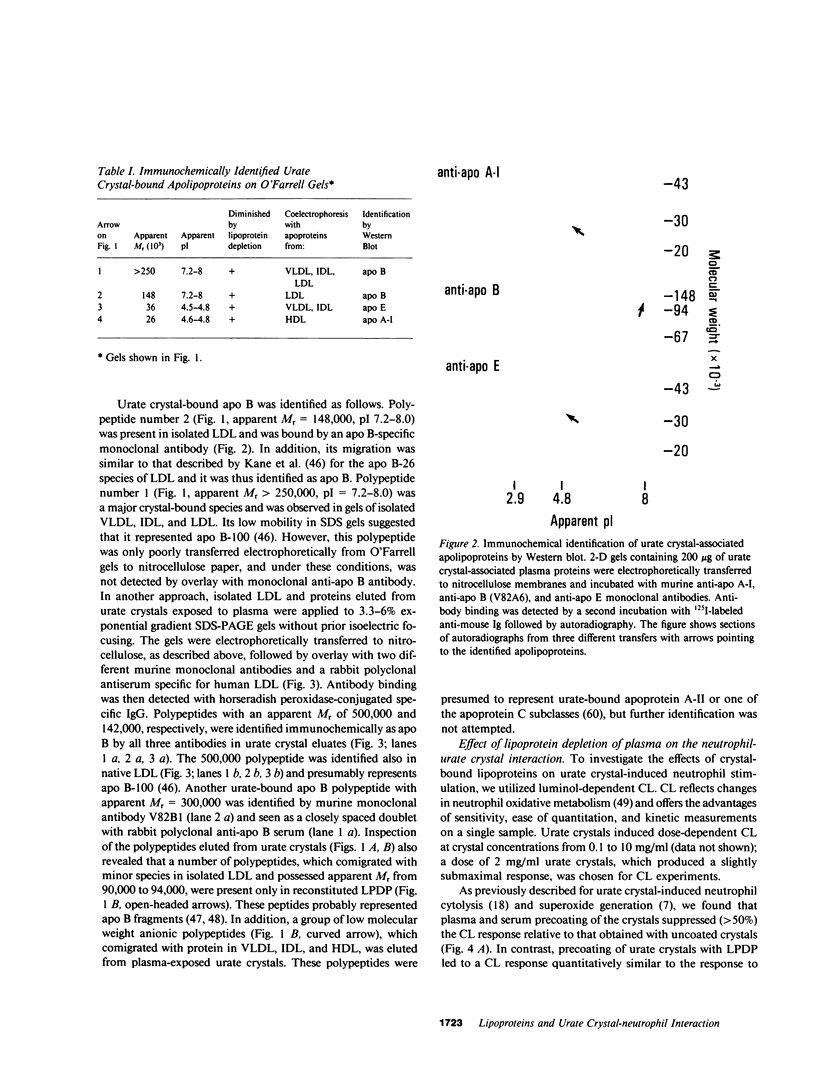

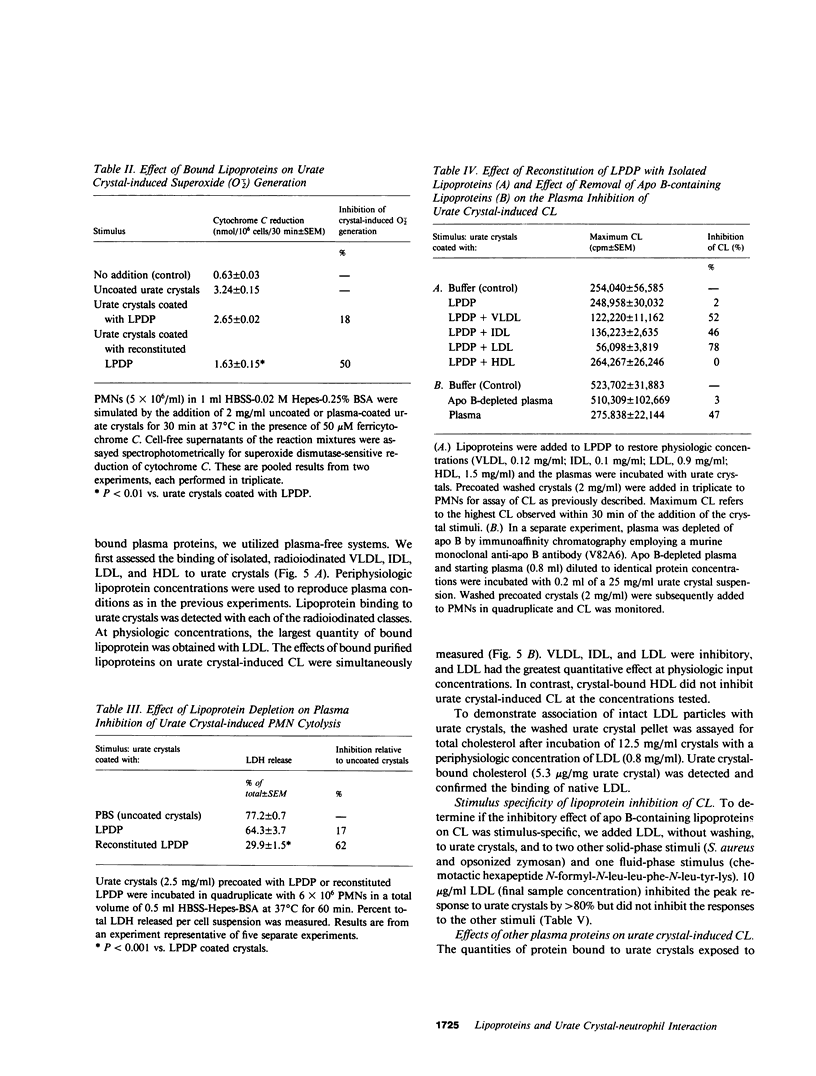
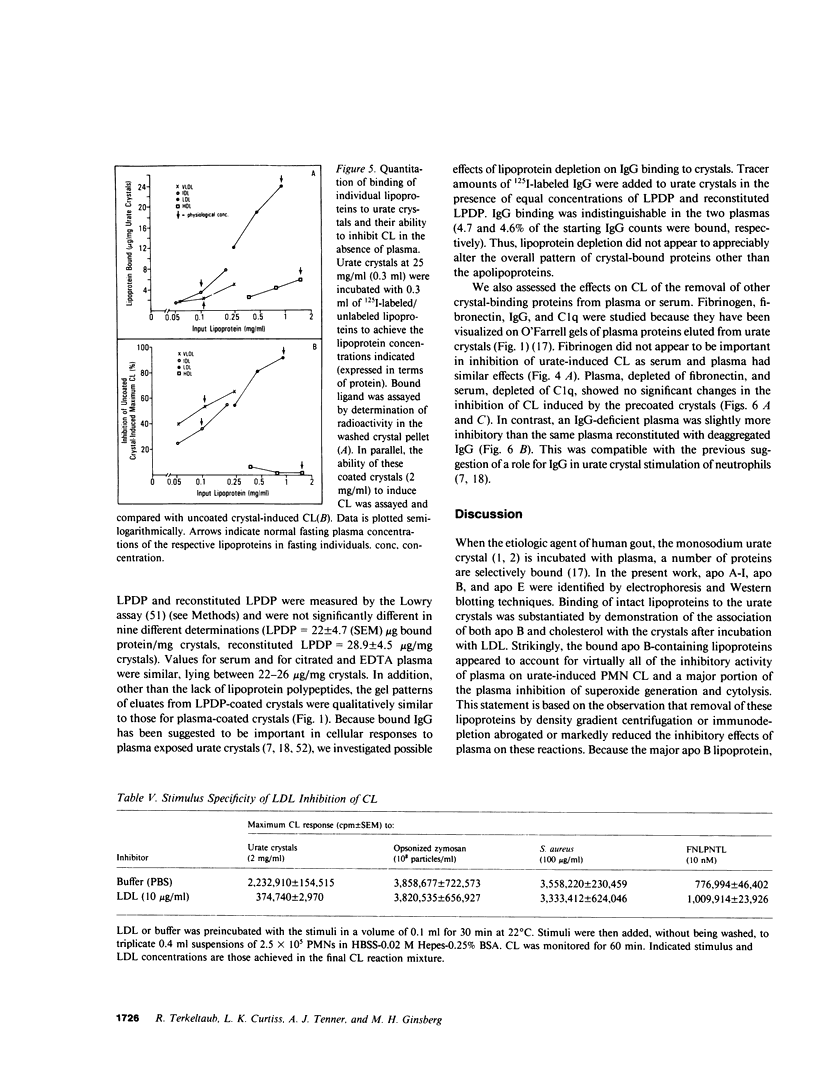




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Hoffstein S. T., Weissmann G. Superoxide anion generation by human neutrophils exposed to monosodium urate. Arthritis Rheum. 1982 Feb;25(2):174–180. doi: 10.1002/art.1780250210. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- BOLE G. G. Synovial fluid lipids in normal individuals and patients with rheumatoid arthritis. Arthritis Rheum. 1962 Dec;5:589–601. doi: 10.1002/art.1780050606. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Chapman M. J., Goldstein S., Mills G. L. Limited tryptic digestion of human serum low-density lipoprotein: isolation and characterisation of the protein-deficient particle and of its apoprotein. Eur J Biochem. 1978 Jul 3;87(3):475–488. doi: 10.1111/j.1432-1033.1978.tb12398.x. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Curtiss L. K., Jensen F. C. Physiologic concentrations of normal human plasma lipoproteins inhibit the immortalization of peripheral B lymphocytes by the Epstein-Barr virus. J Clin Invest. 1981 Aug;68(2):329–336. doi: 10.1172/JCI110260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Differential sensitivity of lymphocyte subpopulations to suppression by low density lipoprotein inhibitor, an immunoregulatory human serum low density lipoprotein. J Clin Invest. 1979 Feb;63(2):193–201. doi: 10.1172/JCI109289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Immunochemical heterogeneity of human plasma apolipoprotein B. I. Apolipoprotein B binding of mouse hybridoma antibodies. J Biol Chem. 1982 Dec 25;257(24):15213–15221. [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- Denko C. W., Whitehouse M. W. Experimental inflammation induced by naturally occurring microcrystalline calcium salts. J Rheumatol. 1976 Mar;3(1):54–62. [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Giclas P. C., Ginsberg M. H., Cooper N. R. Immunoglobulin G independent activation of the classical complement pathway by monosodium urate crystals. J Clin Invest. 1979 Apr;63(4):759–764. doi: 10.1172/JCI109360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Kozin F., Chow D., May J., Skosey J. L. Adsorption of polymorphonuclear leukocyte lysosomal enzymes to monosodium urate crystals. Arthritis Rheum. 1977 Nov-Dec;20(8):1538–1542. doi: 10.1002/art.1780200815. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Kozin F. Mechanisms of cellular interaction with monosodium urate crystals. IgG-dependent and IgG-independent platelet stimulation by urate crystals. Arthritis Rheum. 1978 Nov-Dec;21(8):896–903. doi: 10.1002/art.1780210805. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Painter R. G., Birdwell C., Plow E. F. The detection, immunofluorescent localization, and thrombin induced release of human platelet-associated fibronectin antigen. J Supramol Struct. 1979;11(2):167–174. doi: 10.1002/jss.400110206. [DOI] [PubMed] [Google Scholar]
- Gordon T. P., Bertouch J. V., Walsh B. R., Brooks P. M. Monosodium urate crystals in asymptomatic knee joints. J Rheumatol. 1982 Nov-Dec;9(6):967–969. [PubMed] [Google Scholar]
- HOWELL R. R., SEEGMILLER J. E. Uricolysis by human leukocytes. Nature. 1962 Nov 3;196:482–483. doi: 10.1038/196482a0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hasselbacher P., Schumacher H. R. Immunoglobulin in tophi and on the surface of monosodium urate crystals. Arthritis Rheum. 1978 Apr;21(3):353–361. doi: 10.1002/art.1780210311. [DOI] [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Hoffstein S., Weissmann G. Mechanisms of lysosomal enzyme release from leukocytes. IV. Interaction of monosodium urate crystals with dogfish and human leukocytes. Arthritis Rheum. 1975 Mar-Apr;18(2):153–165. doi: 10.1002/art.1780180213. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozin F., Ginsberg M. H., Skosey J. L. Polymorphonuclear leukocyte responses to monosodium urate crystals: modification by adsorbed serum proteins. J Rheumatol. 1979 Sep-Oct;6(5):519–526. [PubMed] [Google Scholar]
- Kozin F., McCarty D. J. Molecular orientation of immunoglobulin G adsorbed to microcrystalline monosodium urate monohydrate. J Lab Clin Med. 1980 Jan;95(1):49–58. [PubMed] [Google Scholar]
- Kozin F., McCarty D. J. Protein binding to monosodium urate monohydrate, calcium pyrophosphate dihydrate, and silicon dioxide crystals. I. Physical characteristics. J Lab Clin Med. 1977 Jun;89(6):1314–1325. [PubMed] [Google Scholar]
- Kushner I., Somerville J. A. Permeability of human synovial membrane to plasma proteins. Relationship to molecular size and inflammation. Arthritis Rheum. 1971 Sep-Oct;14(5):560–570. doi: 10.1002/art.1780140503. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCCARTY D. J., Jr, FAIRES J. S. A comparison of the duration of local anti-inflammatory effect of several adrenocorticosteroid esters--a bioassay technique. Curr Ther Res Clin Exp. 1963 May;5:284–290. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Squatrito P. M. The role of superoxide anion in peroxidase-catalyzed chemiluminescence of luminol. Arch Biochem Biophys. 1982 Apr 15;215(1):59–65. doi: 10.1016/0003-9861(82)90278-8. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Myllylä G., Häyry P., Penttinen K., Saxén E. Serum lipoproteins in primary cell attachment and growth behaviour of cells on glass. Ann Med Exp Biol Fenn. 1966;44(2):171–176. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Phelps P., McCarty D. J., Jr Crystal-induced inflammation in canine joints. II. Importance of polymorphonuclear leukocytes. J Exp Med. 1966 Jul 1;124(1):115–126. doi: 10.1084/jem.124.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps P. Polymorphonuclear leukocyte motility in vitro. 3. Possible release of a chemotactic substance after phagocytosis of urate crystals by polymorphonuclear leukocytes. Arthritis Rheum. 1969 Jun;12(3):197–204. doi: 10.1002/art.1780120306. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Prendergast E., Mosher D. F. Fibronectin mediates attachment of Staphylococcus aureus to human neutrophils. Blood. 1982 Apr;59(4):681–687. [PubMed] [Google Scholar]
- Rosenfeld S. I., Packman C. H., Leddy J. P. Inhibition of the lytic action of cell-bound terminal complement components by human high density lipoproteins and apoproteins. J Clin Invest. 1983 Apr;71(4):795–808. doi: 10.1172/JCI110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID K., MACNAIR M. B. Characterization of the proteins of human synovial fluid in certain disease states. J Clin Invest. 1956 Jul;35(7):814–824. doi: 10.1172/JCI103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMALL D. M., COHEN A. S., SCHMID K. LIPOPROTEINS OF SYNOVIAL FLUID AS STUDIED BY ANALYTICAL ULTRACENTRIFUGATION. J Clin Invest. 1964 Nov;43:2070–2079. doi: 10.1172/JCI105081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Bjornson A. B., Brothers M. A., Müller-Eberhard H. J. The role of C3 fragments in endocytosis and extracellular cytotoxic reactions by polymorphonuclear leukocytes. Clin Immunol Immunopathol. 1982 May;23(2):335–357. doi: 10.1016/0090-1229(82)90119-2. [DOI] [PubMed] [Google Scholar]
- Schwartz B. S., Levy G. A., Curtiss L. K., Fair D. S., Edgington T. S. Plasma lipoprotein induction and suppression of the generation of cellular procoagulant activity in vitro: two procoagulant activities are produced by peripheral blood mononuclear cells. J Clin Invest. 1981 Jun;67(6):1650–1658. doi: 10.1172/JCI110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. W., Scanu A. M., Kézdy F. J. Structure of human serum lipoproteins inferred from compositional analysis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):837–841. doi: 10.1073/pnas.74.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. C., Jr The effect of plasmin on human plasma low density lipoprotein. Thromb Res. 1979;15(3-4):573–579. doi: 10.1016/0049-3848(79)90165-8. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Analysis of receptor-mediated C1q binding to human peripheral blood mononuclear cells. J Immunol. 1980 Oct;125(4):1658–1664. [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Stimulation of a human polymorphonuclear leukocyte oxidative response by the C1q subunit of the first complement component. J Immunol. 1982 Jun;128(6):2547–2552. [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Terkeltaub R., Tenner A. J., Kozin F., Ginsberg M. H. Plasma protein binding by monosodium urate crystals. Analysis by two-dimensional gel electrophoresis. Arthritis Rheum. 1983 Jun;26(6):775–783. doi: 10.1002/art.1780260612. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao B. P., Curtiss L. K., Edgington T. S. Immunochemical heterogeneity of human plasma apolipoprotein B. II. Expression of apolipoprotein B epitopes on native lipoproteins. J Biol Chem. 1982 Dec 25;257(24):15222–15228. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente A. J., Walton K. W. Studies on increased vascular permeability in the pathogenesis of lesions of connective tissue diseases: I. Experimental hyperlipidaemia and immune arthropathy. Ann Rheum Dis. 1980 Oct;39(5):490–499. doi: 10.1136/ard.39.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger A., Schumacher H. R., Agudelo C. A. Urate crystals in asymptomatic metatarsophalangeal joints. Ann Intern Med. 1979 Jul;91(1):56–57. doi: 10.7326/0003-4819-91-1-56. [DOI] [PubMed] [Google Scholar]
- Wigley F. M., Fine I. T., Newcombe D. S. The role of the human synovial fibroblast in monosodium urate crystal-induced synovitis. J Rheumatol. 1983 Aug;10(4):602–611. [PubMed] [Google Scholar]







