Abstract
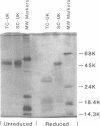
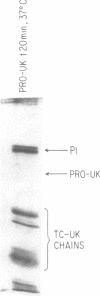
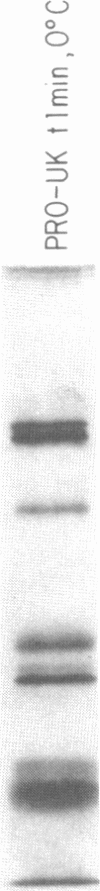
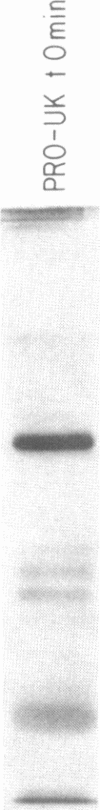
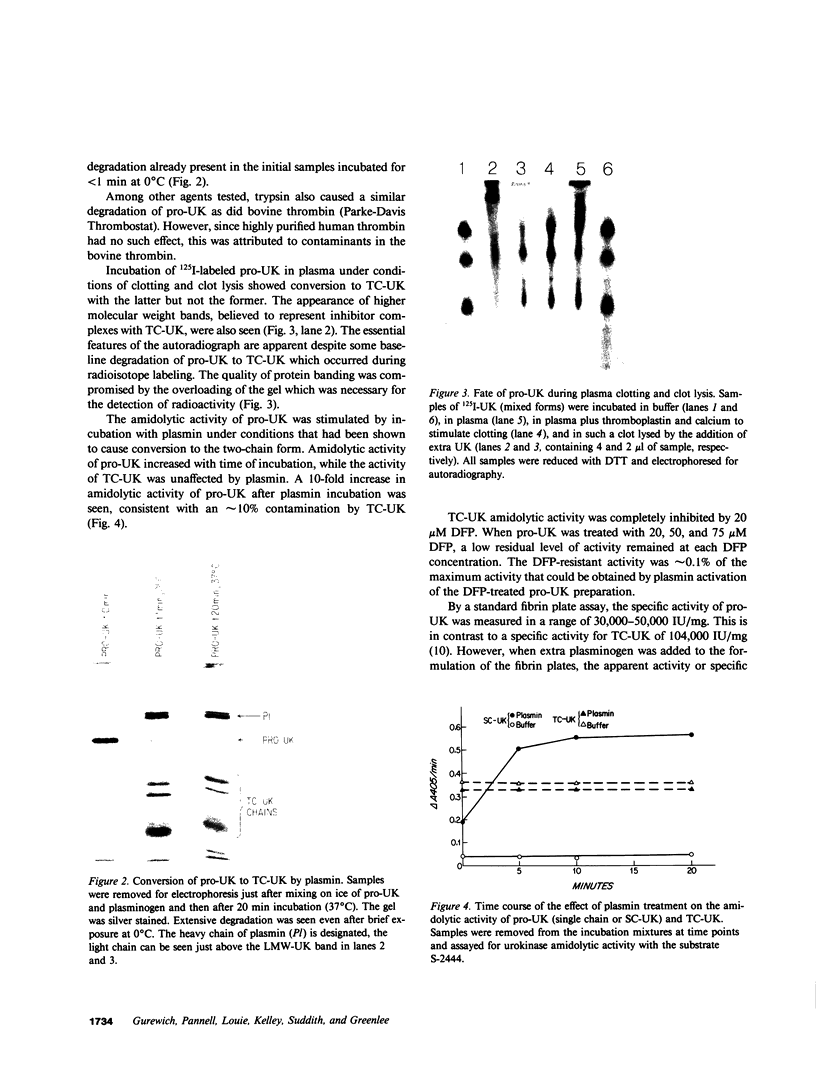
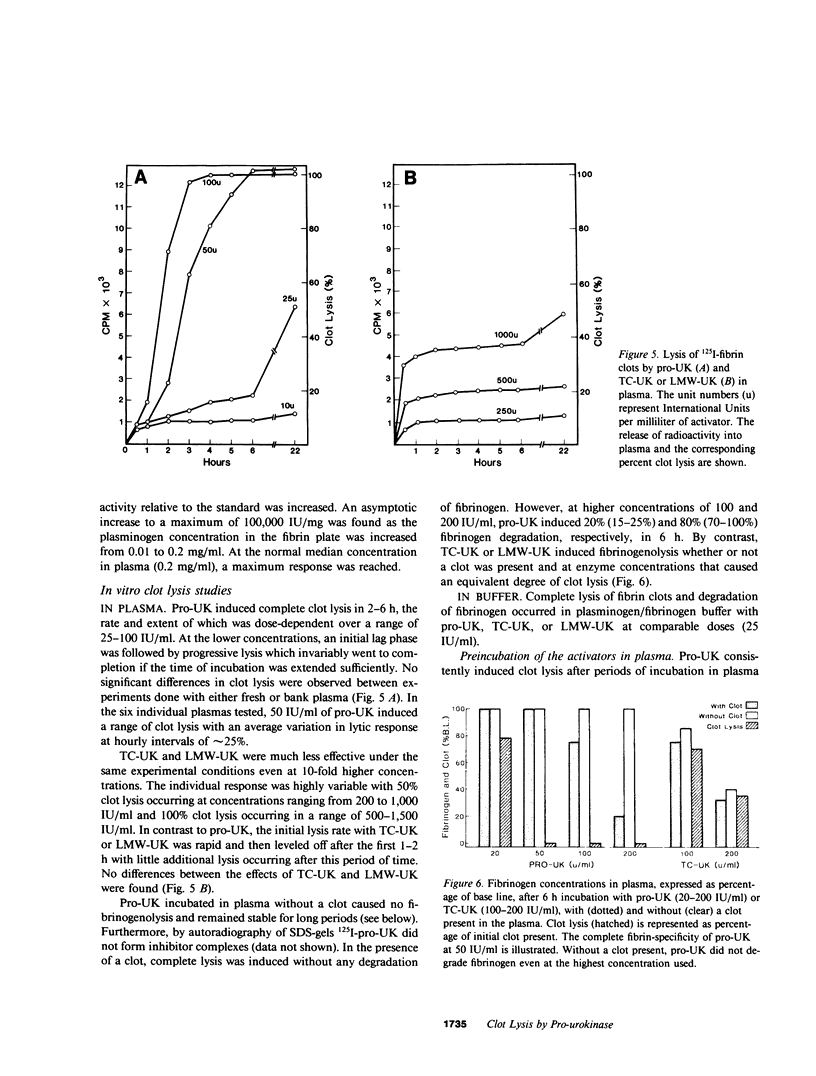
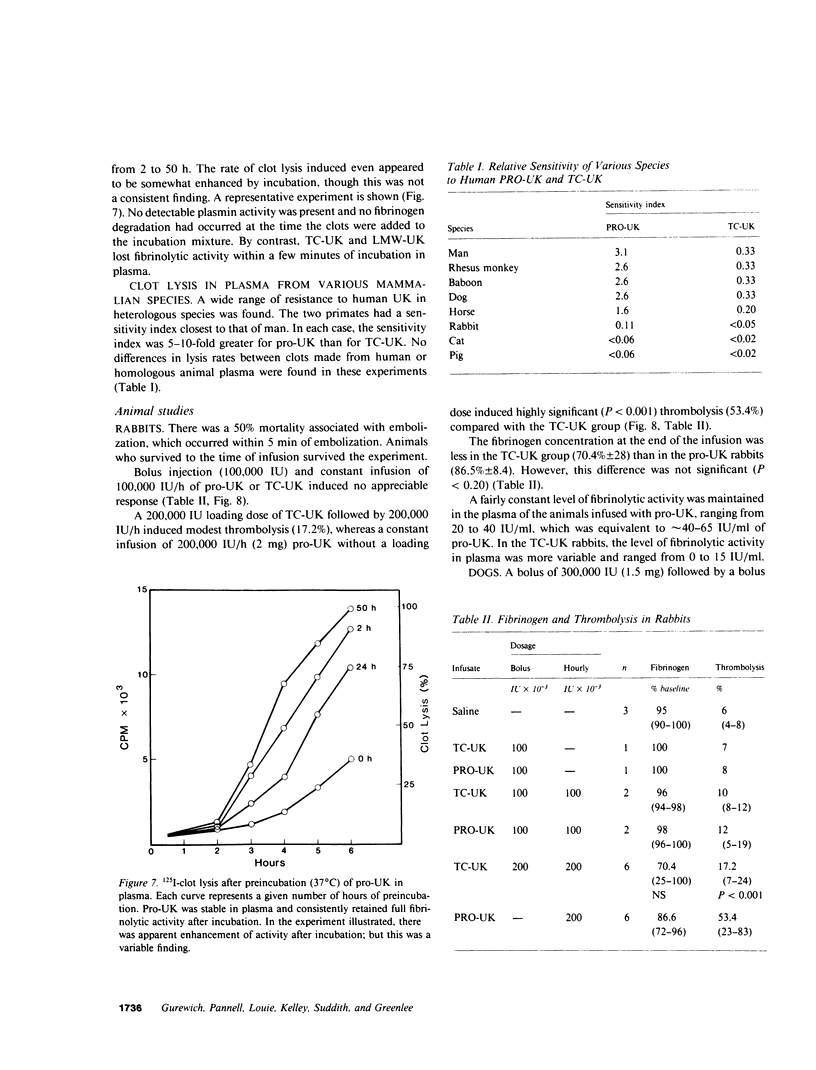
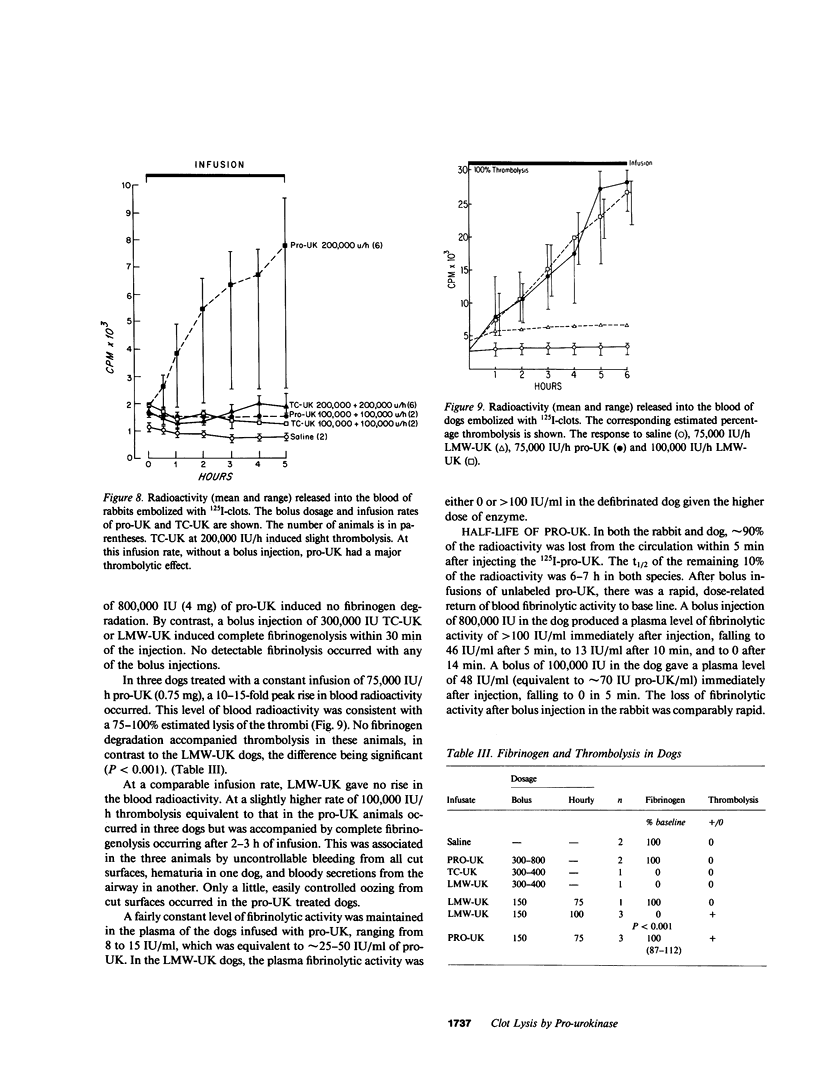
A single-chain 55,000-mol wt form of urokinase (UK), similar to that previously isolated from urine, was purified from a transformed kidney cell culture medium and characterized; and its fibrinolytic properties were evaluated. The preparation immunoprecipitated with UK antiserum, had a low intrinsic amidolytic activity that was 0.1% of its active derivative, and resisted diisopropyl fluorophosphate treatment and inactivation by plasma inhibitors. The single-chain UK was therefore designated pro-UK. In the presence of plasmin and during clot lysis, activation by conversion to two-chain, 55,000-mol wt UK (TC-UK) was demonstrated. This did not occur during blood clotting nor on incubation with purified thrombin. Clot lysis in plasma consistently occurred in 2-5 h with 50-100 IU per ml of pro-UK, whereas comparable lysis was inconsistently achieved by 500-1,000 IU of UK. Pro-UK, in sharp contrast to UK, caused no fibrinogen degradation at fibrinolytic concentrations. In the absence of a clot, pro-UK in plasma was stable for more than 2 d. When a clot was added after incubation (37 degrees C) for 50 h, activation to full lytic activity took place. The findings in vivo were comparable but the rapid clearance of pro-UK required that it be given by a constant infusion despite its plasma stability. In rabbits, a UK-resistant species, pro-UK was significantly (P less than 0.001) more efficacious than TC-UK but neither induced significant fibrinogen degradation. In dogs, a more sensitive species, the high specificity of thrombolysis by pro-UK contrasted with the defibrinogenation and uncontrollable bleeding that accompanied thrombolysis by UK. It was concluded that clot lysis by pro-UK is more effective and specific than UK. The advantage of pro-UK is in the limitation of its activation to the site of a clot. This can be explained by an activation mechanism that is dependent, under physiological conditions, on fibrin-stabilized plasmin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernik M. B. Increased plasminogen activator (urokinase) in tissue culture after fibrin deposition. J Clin Invest. 1973 Apr;52(4):823–834. doi: 10.1172/JCI107246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur J Biochem. 1976 Oct 1;69(1):209–216. doi: 10.1111/j.1432-1033.1976.tb10875.x. [DOI] [PubMed] [Google Scholar]
- Collen D., Stassen J. M., Verstraete M. Thrombolysis with human extrinsic (tissue-type) plasminogen activator in rabbits with experimental jugular vein thrombosis. Effect of molecular form and dose of activator, age of the thrombus, and route of administration. J Clin Invest. 1983 Feb;71(2):368–376. doi: 10.1172/JCI110778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Gurewich V., Lipinski B. Purification and partial characterization of a single-chain high-molecular-weight form of urokinase from human urine. Arch Biochem Biophys. 1983 Jan;220(1):31–38. doi: 10.1016/0003-9861(83)90383-1. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lipinski B., Greuwich V. Rapid purification of a high-affinity plasminogen activator from human blood plasma by specific adsorption on fibrin/Celite. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4265–4269. doi: 10.1073/pnas.78.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Korninger C., Collen D. Neutralization of human extrinsic (tissue-type) plasminogen activator in human plasma: no evidence for a specific inhibitor. Thromb Haemost. 1981 Oct;46(3):662–665. [PubMed] [Google Scholar]
- Korninger C., Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981 Aug 28;46(2):561–565. [PubMed] [Google Scholar]
- LESUK A., TERMINIELLO L., TRAVER J. H. CRYSTALLINE HUMAN UROKINASE: SOME PROPERTIES. Science. 1965 Feb 19;147(3660):880–882. doi: 10.1126/science.147.3660.880. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Collen D. Interaction of plasminogen activators and inhibitors with plasminogen and fibrin. Semin Thromb Hemost. 1982 Jan;8(1):2–10. doi: 10.1055/s-2007-1005038. [DOI] [PubMed] [Google Scholar]
- MOHLER S. R., CELANDER D. R., GUEST M. M. Distribution of urokinase among the common mammals. Am J Physiol. 1958 Jan;192(1):186–190. doi: 10.1152/ajplegacy.1957.192.1.186. [DOI] [PubMed] [Google Scholar]
- Matsuo O., Mihara H. Threshold phenomena in blood fibrinolysis. Thromb Res. 1977 May;10(5):753–758. doi: 10.1016/0049-3848(77)90057-3. [DOI] [PubMed] [Google Scholar]
- Nowak A., Gurewich V. Thrombolysis with streptokinase in rabbits. Dose response, fibrin-clot specificity and laboratory evaluation of fibrinolytic effect. Thromb Diath Haemorrh. 1974 May 15;31(2):265–272. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Rijken D. C., Hoylaerts M., Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982 Mar 25;257(6):2920–2925. [PubMed] [Google Scholar]
- Wun T. C., Ossowski L., Reich E. A proenzyme form of human urokinase. J Biol Chem. 1982 Jun 25;257(12):7262–7268. [PubMed] [Google Scholar]
- Wun T. C., Schleuning W. D., Reich E. Isolation and characterization of urokinase from human plasma. J Biol Chem. 1982 Mar 25;257(6):3276–3283. [PubMed] [Google Scholar]