Abstract
Curcumin has diverse biological activities including antioxidant and anti-inflammatory activity. However, its clinical use for topical application is limited due to its poor aqueous solubility and thus, minimal cutaneous bioavailability. Elastic vesicles (EVs) of curcumin were prepared to improve its cutaneous bioavailability and to use it for topical anti-inflammatory effect. Ex vivo skin permeation and retention studies were performed to check if incorporation of curcumin into EVs could improve its permeation into and retention in the skin. Evaluation of acute and chronic anti-inflammatory effect was done using xylene-induced acute ear edema in mice and cotton pellet-induced chronic inflammation in rats, respectively. A significant improvement in flux (nine times) across murine skin was observed when aqueous dispersion of curcumin (flux − 0.46 ± 0.02 μg/h/cm2) was compared with curcumin-loaded EVs (flux − 4.14 ± 0.04 μg/h/cm2 ). Incorporation of these curcumin-loaded EVs into a hydrophilic ointment base resulted in higher skin retention (51.66%) in contrast to free curcumin ointment (1.64%) and a marketed formulation (VICCO® turmeric skin cream). The developed ointment showed an effect similar (p < 0.05) to the marketed diclofenac sodium ointment (Omni-gel®) in suppression of acute inflammation in mouse; a significant inhibition (28.8% versus 3.91% for free curcumin) of cotton pellet-induced chronic inflammation was also observed. Thus, curcumin-loaded EVs incorporated in hydrophilic ointment is a promising topical anti-inflammatory formulation.
KEY WORDS: anti-inflammatory, curcumin, elastic vesicles, topical formulation
INTRODUCTION
The use of medicinal plants or their active components in the prevention and treatment of chronic diseases is based on experience from traditional systems of medicine of different ethnic societies. Polyphenols, a class of dietary compounds, are currently being evaluated extensively as potential therapeutic agents. Curcuma longa Linn. is one of several medicinal plants to have attracted the interest of scientists. Curcumin, a bis-alpha, beta-saturated beta-diketone, possesses diverse antioxidant and anti-inflammatory properties (1–3). It significantly decreases lipid peroxidation; increases intracellular antioxidant, GSH; regulates antioxidant enzymes; and scavenges hyperglycemia-induced ROS (4,5). In addition, curcumin is shown to inhibit the pro-inflammatory transcriptional factor, NFkβ, and prevent upregulation of VEGF messenger RNA (mRNA) and microvascular angiogenesis (6–8). However, this lipidic bioactive compound suffers from low bioavailability (9) due to low water solubility and low stability including photodegradation. These observations facilitate the doorway of novel delivery systems in the development of curcumin. These systems are developed so as to work in all areas of the delivery and thus can be applied to improve the solubility, permeability, and stability of antioxidant molecules like curcumin (10–13).
Among the vast number of novel drug delivery options available in today’s times, vesicular systems have good potential for topical application. Curcumin-loaded vesicular systems have shown to improve the skin permeability of curcumin and also protect it from degradation (14,15). Elastic vesicular systems (EVs) have a special role in improving poor cutaneous bioavailability, and though they resemble liposomes in morphology, functionally, they are (quasi) metastable, which makes their membrane ultraflexible and the vesicles highly deformable. EVs have been reported to significantly improve the skin deposition and photostability of α-tocopherol (16). Flexible membrane vesicles or elastic vesicles (EVs) loaded with curcumin have shown encouraging results upon topical use for wound healing, skin cancers, psoriasis, and aging (17–19).
Keeping this rationale in mind, EVs of curcumin were developed to augment the cutaneous bioavailability and anti-inflammatory effect of curcumin in the skin. We have already reported the usefulness of developed EVs of curcumin to protect against extrinsic aging or photoaging and a patent for the same has been filed in the Indian Patent Office (2335/DEL/2010, dated 29 September 2010) (20). There are two great advantages of applying an active formulation directly onto the skin; firstly, the skin attains far higher levels of the drug which cannot be achieved by taking these agents orally and secondly, topical application arms the skin with a reservoir of active drug that cannot be washed or rubbed off (21).
MATERIALS AND METHODS
Materials
Curcumin used in the study (containing 95% curcumin and 5% other curcuminoids) was a generous gift from Sanat Products Ltd., India. Phospholipon 90H were gift samples from Phospholipids GmbH, Germany; Sephadex G-50 and sodium deoxycholate were gifts obtained from Panacea Biotec, Lalru, India; sodium cholate and sodium taurocholate from New Zealand Pharmaceutical Ltd. and Labrafac CC (medium chain triglycerides) from Colorcon Asia Pacific Pvt. Ltd., Singapore. All other reagents or chemicals used in the study were of A.R. grade.
Animals—female Laca mice and male Wistar rats—were obtained from Central Animal House, Panjab University, Chandigarh, and were housed in plastic bottom cages and allowed free access to standard animal feed and water. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC), Panjab University, Chandigarh, India.
Standard Plot and Validation of Spectrophotometric Method for Analysis of Curcumin
Standard plot of curcumin was prepared in methanol and 30% isopropyl alcohol. A stock solution was prepared by dissolving 5 mg in 100 mL of the solvent resulting in the concentration of 50 μg/mL. The stock was diluted to obtain a range of concentrations 0.2 to 20 μg/mL, which were analysed spectrophotometrically at the wavelength maxima of 430 nm, using respective solvents as blank. The mean of extinction coefficient, E1%1 cm of drug was calculated. The method was further validated with respect to the linearity, accuracy, and precision.
Preparation of Elastic Vesicles
The EVs were prepared by a method reported earlier (22). Precisely, phospholipid, surfactant, and the drug were taken in a clean, dry, and round-bottom flask, and the lipid mixture was dissolved in chloroform. The organic solvent was removed by rotary evaporation under reduced pressure at 45°C–50°C. The deposited lipid film was hydrated with saline phosphate buffer (pH 6.4) by rotation at 100 rev min−1 for 15–20 mins at 40°C–45°C (23). The resulting vesicles were left overnight for swelling at room temperature. The obtained vesicular dispersion was characterized and used as such without further sonication. Different edge activators/surfactants viz. sodium cholate, Span 80, tween 80, sodium deoxycholate, and sodium taurocholate were evaluated for the preparation of EVs at a variable ratio of phospholipid/edge activators of 95:05 to 75:25.
Characterization of Elastic Vesicles
Incorporation efficiency was determined using the mini centrifugation method (24). Briefly, Sephadex G-50 was allowed to swell in distilled water at room temperature, with occasional shaking, for at least 5 h (after which it gels). The gel was stored at 4°C. To prepare the mini column, Whatman paper pads were placed at the bottom of the barrels of 1.0-mL syringes, which were filled with the gel. Excess water was removed by centrifugation at 3000 rev min−1 for 3 min. Then, 200-μL EVs dispersion was loaded on to the column. Care was taken to apply the dispersion dropwise to the center of the column to avoid trapping of sample in sephadex sticking to the sides of the column. The column was centrifuged and washed once again with 200-μL of buffer. The samples collected in the centrifuge tube (syringe/mini column was placed in a centrifuge tube during centrifugation) were pooled. The un-incorporated drug remained bound to the gel, while vesicles traversed the gel and were collected in the centrifuge tube. The amount of drug incorporated in the vesicles was then determined by disrupting the vesicles using methanol, followed by filtration, appropriate dilution, and spectrophotometric analysis of the sample.
Number of EVs per cubic millimeter or vesicle number per milliliter was determined microscopically using a hemocytometer. Shape and type of EVs were visualized using a Philips transmission electron microscope (TEM), with an accelerating voltage of 100 kV and phase-contrast optical microscope. The size and size distribution of vesicles was determined by dynamic light scattering method (DLS), using Malvern Zetamaster, ZEM 5002, Malvern, UK degree of deformability of EVs or liposomal suspension was determined by passing the dispersions through polycarbonate filter of 1-μ pore size employing vesicle extruder and determining size and size distribution before and after extrusion. Differential scanning calorimetry (DSC) thermograms of pure drug, excipients, and EVs were evaluated for a shift or disappearance/appearance of new peaks.
Incorporation into Secondary Vehicle
Since the formed EV dispersion was very thin, for the topical application, the EVs were lyophilised and incorporated into a suitable quantity of an appropriate base. One milliliter of EV dispersion containing 500 μg of curcumin was added to 100 mg of base. For this purpose, three different bases were selected:
-
Hydrophilic ointment base
PEG 4000 (500 mg) was melted over a water bath maintained at a temperature not exceeding 65°C. To this, PEG 400 (450 mg) and stearyl alcohol (50 mg) were added. The mixing was continued without heating to allow uniform congealing. Freeze-dried EV dispersion (1 mL) was added per 100 mg of the formed base near congealing.
-
Hydrophobic ointment base
Lanolin and white soft paraffin (1:6) were melted on a water bath at 65°C until completely melted. Mixing was continued to cooling and lyophilised EV dispersion was incorporated as above.
-
Hydrogel base
Triethanolamine (1.0 g) was dissolved in 10 g of water. Carbopol 940F (1.0 g) was dispersed in warm water (80°C) using high-speed agitation (800–1500 rpm). Once the dispersion was complete, triethanolamine solution was added to the polymer dispersion in a thin stream under slow agitation to affect gel formation (due to neutralization of Carbopol). Stirring was continued to form a transparent gel and the requisite amount of prepared and lyophilised EVs was mixed slowly in the gel (at quantities described above) to form a homogenous mixture.
Ex Vivo Penetration Cum Retention Studies
Jacketed Franz glass diffusion cells were used for the determination of permeability of curcumin and its respective formulations through mice skin. These cells consist of the donor and the receptor chambers between which mice skin is positioned. Average area of skin in contact with the receptor medium was 3.462 cm2 (n = 6) and the average receptor chamber volume was 30.0 mL. Laca mice, 6–8 weeks old, were sacrificed by cervical dislocation. A section of the dorsal skin surface was depilated and excised from the animals with surgical scissors. Adhering fat and other visceral debris were removed carefully from underneath surface of the skin sample.
Permeation characteristics of curcumin dispersed in a buffer system was compared with the curcumin incorporated into liposomes (prepared similarly with a corresponding composition replacing edge activators with cholesterol), EV dispersion (each containing 500 μg of curcumin in 1 mL), and 100 mg of a hydrophilic ointment/hydrophobic ointment/hydrogel incorporating 1 mL of EV dispersion, and a hydrophilic ointment containing 500 μg of free curcumin; along with their respective blanks. These were applied evenly onto the donor side of the skin surface, while the receptor medium was replaced with fresh medium. The marketed formulation (VICCO® turmeric skin cream) contained 16% curcumin extract; which may contain up to 90% of pure curcumin. Since loading of quantities less than 25 mg of the ointment on the diffusion cell was not practical (as it will not completely cover the mounted skin sample); so 25 mg of the marketed ointment containing approximately 3700 μg curcumin was used in the experiment.
Isopropyl alcohol (30%) was used as the receptor medium. Aliquots (1.0 mL) were withdrawn at regular intervals for 24 h, and analyzed spectrophotometrically at 430 nm.
After 24 h, skin was wiped to remove residual formulation, minced thoroughly, homogenized to a viscous turbid mixture, and centrifuged at 4000 rpm for 10 min. Supernatants were filtered (0.45 μ) and analysed spectrophotometrically to determine the amount of drug retained in the skin.
In Vitro Release Study: Membrane-free Model
Free curcumin, curcumin-loaded EV dispersion (1 mL containing 2 mg of curcumin) and 100 mg of the ointment into which curcumin-loaded EV dispersion is incorporated, as well as their respective blank formulations, were placed in 15-mL flat bottom culture tubes. Volume was made to 5 mL, with the buffer and 5 mL of medium chain triglycerides (LABRAFAC CC) was carefully placed over it. LABRAFAC CC served as the acceptor medium, proposed to simulate the lipophilic biological membranes, considering that curcumin is soluble in it and the EVs remain intact in this medium. The samples were stirred at 32.0 ± 1°C in a temperature-controlled shaking chamber at 70 rpm. Samples (200 μL) withdrawn from the oily phase at suitable intervals were diluted with methanol and analysed spectrophotometrically.
Pharmacodynamic Evaluation: Anti-inflammatory Effect
Acute Inflammation: Xylene-induced Ear Edema
Effect of curcumin and its EVs on acute topical inflammation was evaluated using xylene-induced ear edema in mice model. The mice were divided into five groups of six each, as described in Table I, and inflammation was induced on the right ear of each animal while maintaining the left ear as an internal control (25). Edema was quantified as the weight difference between earplugs obtained from the two ears of the same animal. Activity was evaluated as follows (26):
Table I.
Grouping of Mice for Xylene-Induced Ear Edema
| S. No. | Group (n = 6) | Formulation applied |
|---|---|---|
| 1) | Control | Positive control group, only xylene application value taken as 100% edema |
| 2) | Free CMN OINT | Hydrophilic curcumin (free) ointment |
| 3) | LIPO OINT | Curcumin-loaded liposome in hydrophilic ointment |
| 4) | EVs OINT | Curcumin-loaded EVs in hydrophilic ointment |
| 5) | MKTD | Marketed formulation of diclofenac sodium (Omni-gel®) |
CMN OINT curcumin in ointment, LIPO OINT liposome in ointment, EVs OINT Elastic Vesicles in ointment, MKTD marketed
Where Rt and Lt = mean weight of right and left ear plug of treated animals;
Rc and Lc = mean weight of right and left ear plug of positive control animals.
Chronic Inflammation: Cotton Pellet-induced Granuloma
Male rats of 180–220 g body weight were divided into six groups of five animals each (Table II).
Table II.
Grouping of Animals for Cotton Pellet-Induced Granuloma
| S. No. | Group (n = 5) | Formulation applied |
|---|---|---|
| 1) | Control | No formulation applied |
| 2) | Free CMN OINT | Free curcumin in hydrophilic ointment |
| 3) | LIPO OINT | Curcumin-loaded liposomes in hydrophilic ointment |
| 4) | Blank EVs OINT | Blank EVs in hydrophilic ointment |
| 5) | EVs OINT | Curcumin-loaded EVs in hydrophilic ointment |
| 6) | MKTD | Marketed formulation of diclofenac sodium (Omni-gel®) |
CMN OINT curcumin in ointment, LIPO OINT liposome in ointment, EVs OINT Elastic Vesicles in ointment, MKTD marketed
Two sterile pellets of cotton wool weighing 20 ± 1 mg were implanted subcutaneously, one on each side of the abdomen of the animal under light ether anesthesia aseptically (27,28). Two hours after surgery, topical application of the test drugs (Table II) was initiated on the implanted region, once daily for 7 days. On day 8, animals were sacrificed using an overdose of ether. The pellets were dissected out, freed of tissue attachments, and dried in an oven at 60°C overnight. The dry pellets were weighed and the mean weight of the granuloma tissue formed around each pellet was determined by subtracting the initial weight of each pellet from the final dry weight. Percent inhibition of granuloma tissue development was calculated as:
Where Tc and Tt = weight of granuloma tissue of control and treated group, respectively.
RESULTS
Standard Plot and Validation of Spectrophotometric Method for Analysis of Curcumin
The E1%1 cm value in methanol was found to be 1588, and in 30% isopropyl alcohol, it was found to be 1587. Solutions of varying concentrations of curcumin were prepared in respective solvents and their absorbances were measured at 430 nm. The concentration range showing a linear relationship (n = 6) between absorbance and concentration, upon repeated observations, was selected both for methanol and 30% isopropyl alcohol. Regression equations y = 0.1588x ± 0.003 with r2 = 0.998 ± 0.002 and y = 0.1587x ± 0.0023 r2 = 0.995 ± 0.0028 were obtained for methanol and 30% isopropyl alcohol, respectively. Accuracy of the UV spectrophotometric method of analysis for curcumin in both the solvents was assessed with mean% recovery of 100.77 and 102.01 for methanol and 30%, IPA respectively. Precision of the UV spectrophotometric method of analysis for curcumin was also confirmed.
Preparation of Elastic Vesicles
EVs prepared using different edge activators in varied ratios, were characterized microscopically for vesicle shape and number (Table III). According to Table III, it is vividly apparent that as concentration of surfactant increases, the number of vesicles per cubic millimeter initially increases but beyond a particular concentration of surfactant, the vesicle number decreases. The highest vesicle number was observed at phospholipid/surfactant ratio of 85:15 for all surfactants. The highest vesicle numbers at 85:15 ratio were observed with sodium cholate, Span 80, and sodium taurocholate which indicated that they were best out of all the surfactants tried.
Table III.
Microscopic Evaluation and Number of Vesicles Formed for Various Lipid/Surfactant Ratios
| Ratio | Abundance | Size | Shape | Lamella | No. of vesicles/mm3 |
|---|---|---|---|---|---|
| Sodium cholate | |||||
| 95:05 | Few vesicles | Small | Circular | Mostly unilamellar and bilamellar | 16,000 |
| 90:10 | Few vesicles | Medium | Circular | Mostly unilamellar and bilamellar | 27,300 |
| 85:15 | Abundant | Medium to large | Circular | Bilamellar | 45,120 |
| 80:20 | Abundant | Medium | Circular, oval | More unilamellar, less bilamellar | 33,700 |
| 75:25 | Very few vesicles | Small | Circular | Mostly unilamellar, very few bilamellar | 8900 |
| Span-80 | |||||
| 95:05 | Less vesicles | Small | Irregular, some circular | Mostly unilamellar, very few bilamellar | 15,100 |
| 90:10 | Abundant | Small | Circular | Bilamellar | 30,670 |
| 85:15 | Abundant | Small, medium to some large | Circular | Bilamellar | 43,000 |
| 80:20 | Less vesicles | Small | Circular | More unilamellar, less bilamellar | 17,600 |
| 75:25 | Less vesicles | Small | Circular | Mostly unilamellar, very few bilamellar | 16,300 |
| Tween-80 | |||||
| 95:05 | Few vesicles | Small | Irregular | Unilamellar | 14,300 |
| 90:10 | Few vesicles | Small | Circular | Unilamellar | 15,000 |
| 85:15 | Abundant | Small | Circular | More unilamellar, less bilamellar | 27,500 |
| 80:20 | Very few vesicles | Small | Circular, oval | Mostly unilamellar, very few bilamellar | 6900 |
| 75:25 | No defined vesicles | – | – | – | – |
| Sodium deoxycholate | |||||
| 95:05 | Fairly abundant | Small | Circular, oval, irregular | Mostly unilamellar, very few bilamellar | 23,400 |
| 90:10 | Fairly abundant | Small to big | Circular | More unilamellar, less bilamellar | 22,000 |
| 85:15 | Abundant | Small to moderate | Circular, oval | More unilamellar, less bilamellar | 36,800 |
| 80:20 | Less vesicles | Small | Irregular in shape | Mostly unilamellar, very few bilamellar | 12,900 |
| 75:25 | No defined vesicles | – | – | – | – |
| Sodium taurocholate | |||||
| 95:05 | Few vesicles | Medium | Circular, irregular | More unilamellar, less bilamellar | 14,300 |
| 90:10 | Abundant | Small, medium to large | Circular, oval | Bilamellar | 29,800 |
| 85:15 | Abundant | Medium | Circular | Bilamellar | 47,300 |
| 80:20 | Abundant | Medium | Circular, oval | More unilamellar, less bilamellar | 35,800 |
| 75:25 | No defined vesicles | – | – | – | – |
Characterization of Elastic Vesicles
Incorporation Efficiency
EVs prepared using different phospholipid and surfactant concentration were analysed for % incorporation efficiency (% EE) of curcumin (Table IV). Maximum incorporation of 76.55% was achieved with Span 80 (phospholipid/Span 80 = 85:15), with the maximum drug that could be loaded being 2 mg/mL of the dispersion. This composition with a drug content of 2.20 ± 0.23 mg/mL (n = 6; taken as 2 mg/mL for all calculations) was used for subsequent studies.
Table IV.
Drug Entrapment Efficiency of EVs Prepared with Various Combinations of Phospholipid and Surfactant
| S. No. | Phospholipid | Surfactant | Drug (mg) | % Entrapment |
|---|---|---|---|---|
| 1 | 85 mg | SET-15 mg | 10 | 65.47 |
| 2 | 85 mg | SET-15 mg | 20 | 76.55 |
| 3 | 170 mg | SET-30 mg | 20 | 43.3 |
| 4 | 85 mg | NaC-15 mg | 10 | 59.43 |
| 5 | 85 mg | NaC-15 mg | 20 | 33.59 |
| 6 | 170 mg | NaC-30 mg | 20 | 10.8 |
| 7 | 85 mg | NaTC-15 mg | 10 | 45.23 |
| 8 | 85 mg | NaTC-15 mg | 20 | 49.2 |
| 9 | 170 mg | NaTC-30 mg | 20 | 34.78 |
SET Span-80, NaC sodium cholate, NaTC sodium taurocholate
Particle Size and Size Distribution
Electron microscopy showed multilamellar, spherical EVs (Fig. 1) and their average size was 11.39 μm, with 10% vesicles smaller than 3.77 μm while 90% of the vesicles were equal to or smaller than 64.45 μm (Fig. 2). The vesicle size distribution followed log normal distribution pattern, indicating that the selected formulation comprises of almost uniform monodispersed vesicles.
Fig. 1.
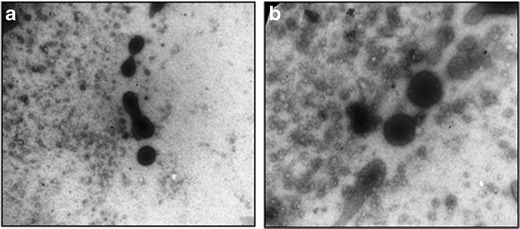
Visualization of EVs by transmission electron microscopy (magnification a ×40,000 and b ×60,000, respectively)
Fig. 2.
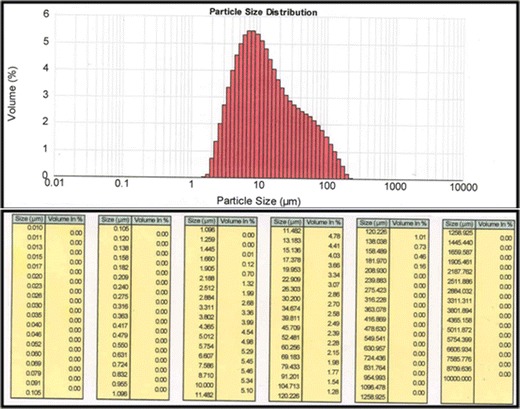
Particle size distribution of EVs
Degree of Deformability
EVs showed a much smaller change (17.75%) in average particle size (Table V) in comparison to liposomes which showed a significant decrease in size of almost 40% (Table V) after passage through 1-μm membrane. This confirms the flexibility of developed EVs (29–31). The particle size distribution of EVs after extrusion showed a bimodal curve and also an increased d value (0.9) (Fig. 3, Table V). This was accounted for by the extreme deformation/squeezing of large particles in such a way that they were elongated along one of their dimensions and were thus not spherical anymore.
Table V.
Particle Size of EVs and Liposomes Before and After Extrusion
| Group | Particle size (μm) (before) | Particle size (μm) (after) | ||||
|---|---|---|---|---|---|---|
| d (0.1) | d (0.5) | d (0.9) | d (0.1) | d (0.5) | d (0.9) | |
| Elastic vesicles | 2.99 | 6.59 | 24.06 | 2.57 | 5.42 | 36.92 |
| Liposomes | 4.92 | 13.14 | 33.18 | 3.42 | 7.98 | 27.83 |
Fig. 3.
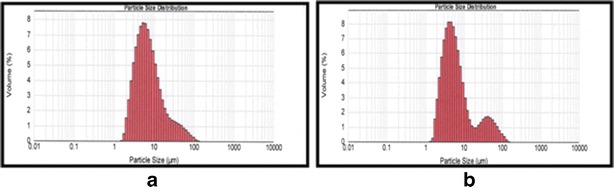
Particle size distribution of EVs before extrusion (a) and after extrusion (b)
Differential Scanning Calorimetry
Figure 4 depicts DSC thermograms of pure curcumin (a), Span 80 (b), phospholipid (Phospholipon 90H) (c), and the developed EV dispersion (d). It is evident from the thermograms that the original peak of curcumin, Span 80, and phospholipids disappear from the thermogram of EV dispersion confirming incorporation of curcumin into EVs. Further, a lower phase transition temperature of EVs in comparison to the phospholipid indicates a higher mobility (elasticity) of vesicles.
Fig. 4.
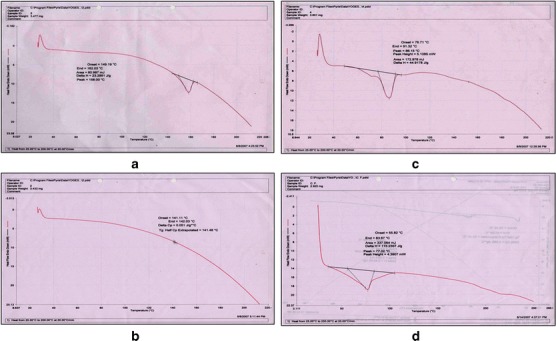
DSC of pure curcumin (a), Span 80 (b), phospholipid (c), and EV dispersion (d)
Ex Vivo Penetration cum Retention Studies
Permeation of curcumin when incorporated in liposomes and EVs was compared with permeation of free curcumin aqueous dispersion (Fig. 5). The flux value obtained when curcumin was applied as an aqueous dispersion (0.46 ± 0.02 μg/h/cm2) was significantly lower than the flux values obtained for curcumin entrapped in liposomes and EVs (6.46 ± 0.22 and 4.14 ± 0.04 μg/h/cm2, respectively) (Table VI).
Fig. 5.
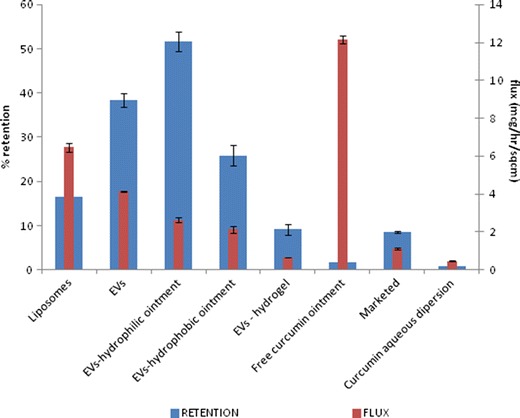
Comparison of flux and % skin retention of curcumin from different systems
Table VI.
Average Flux and Retention Values Expressed As Mean ± S.D. of Various Test Formulations
| S. NO. | Formulation | Average flux (μg/h/cm2) | % Retention |
|---|---|---|---|
| 1) | Liposomes | 6.46 ± 0.22 | 16.41 ± 0.35 |
| 2) | EVs | 4.14 ± 0.04 | 38.30 ± 1.55 |
| 3) | EVs-hydrophilic ointment | 2.63 ± 0.14 | 51.66 ± 2.26 |
| 4) | EVs-hydrophobic ointment | 2.13 ± 0.18 | 25.87 ± 2.30 |
| 5) | EVs-hydrogel | 0.67 ± 0.01 | 9.16 ± 1.25 |
| 6) | Free curcumin ointment | 12.14 ± 0.21 | 1.65 ± 0.30 |
| 7) | Marketed | 1.11 ± 0.05 | 8.55 ± 0.22 |
| 8) | Curcumin aqueous dispersion | 0.46 ± 0.02 | 0.84 ± 0.16 |
EVs Elastic Vesicular Systems
Figure 5 depicts the influence of different types of ointment bases on the penetration of curcumin through excised mice skin and its % skin retention in the skin tissue. Flux of curcumin across the murine skin was significantly higher when EVs were incorporated into the hydrophilic ointment base (p < 0.05). Further, the extent of skin retention of curcumin when its EVs were incorporated into a hydrophilic ointment base was approximately 2 and 5.6 times higher than respective hydrophobic base or hydrogel (Table VI). Hence, the hydrophilic ointment was selected for incorporation of curcumin-loaded EVs. The marketed formulation (VICCO® turmeric skin cream containing 16% w/w of curcumin extract) was also included as a control. The flux value of marketed formulation was significantly less (p < 0.05) than the liposomes as well as EVs.
In Vitro Release Study: Membrane-free Model
The cumulative amount of drug released after 24 h in case of EV dispersion and EVs incorporated into the hydrophilic ointment base was found to be 1320.62 ± 34.86 and 1085.38 ± 36.28 μg, respectively. Both the systems showed first-order release kinetics (r2 = 0.9826 and 0.9759, respectively; Fig. 6). Curcumin is known to be soluble (17.75 ± 0.11 mg/mL) in Labrafac CC (32).
Fig. 6.
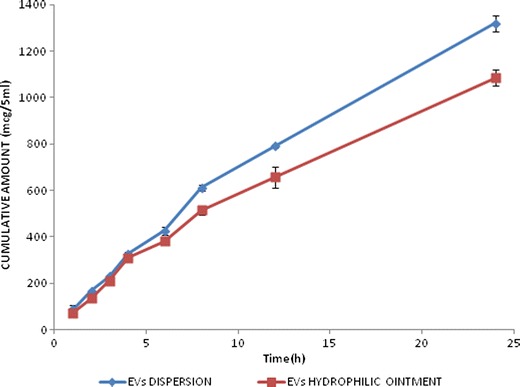
Release profiles of curcumin from EVs dispersion and the EVs-hydrophilic ointment
Pharmacodynamic Anti-inflammatory Effect
Acute Inflammation: Xylene-induced Ear Edema
Diclofenac sodium gel (Omni-gel®) was taken as a positive control in the study and it showed 86% inhibition of the induced inflammatory response (Table VII). EVs of curcumin incorporated into hydrophilic ointment base showed significantly similar inhibition of the inflammatory response (p < 0.05). The free curcumin ointment and liposomal formulation, at same dose, could reduce the edematous response by only 20% and 59%, respectively. Developed formulation showed a 1.5 times better effect than corresponding liposomal formulation.
Table VII.
Effect of Free Curcumin and Its Formulation(s) in Acute Xylene-Induced Ear Edema Model
| S. No. | Group (n = 6) | Right ear weight (mg) | Left ear weight (mg) | Diff | % of control |
|---|---|---|---|---|---|
| 1) | Control | 27.75 ± 2.42 | 14.47 ± 2.71 | 13.28 | 100 |
| 2) | Free CMN OINT | 23.93 ± 1.99 | 13.33 ± 1.95 | 10.6 | 79.79 |
| 3) | LIPO OINT | 18.77 ± 1.62 | 13.2 ± 1.61 | 5.57 | 41.91 |
| 4) | EVs OINT# | 17.35 ± 1.47 | 15.37 ± 1.19 | 1.98 | 14.93 |
| 5) | MKTD# | 21.93 ± 4.71 | 20.08 ± 4.65 | 1.85 | 13.92 |
CMN OINT curcumin in ointment, LIPO OINT liposome in ointment, EVs OINT Elastic Vesicles in ointment, MKTD marketed, DIFF difference
#No significant difference between EVs Ointment and MKTD (p < 0.05)
Chronic Inflammation: Cotton Pellet-induced Granuloma
Significant effects were observed in all the groups under test (Fig. 7). Results indicate an inhibition of 28.79% when EVs incorporated in hydrophilic ointment base were applied topically. However, liposomes and free curcumin ointment showed 19.85% and 10.69% inhibition, respectively. Diclofenac sodium gel (Omni-gel®) showed significantly better results with an inhibition of 37.16%.
Fig. 7.
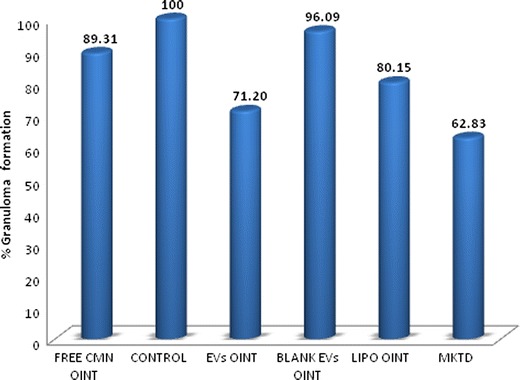
Percent granuloma formation in cotton pellet-induced granuloma test (n = 5). All groups are significantly different from each other (p < 0.05)
DISCUSSION
Elastic vesicles (EVs) were chosen as the drug delivery system of choice for topical curcumin delivery. The marked advantage of EV formulations, over conventional liposomes, is their flexibility, achieved by judicious combination of at least two lipophilic/amphiphilic components (phospholipid plus edge activator), with sufficiently different packing characteristics, into a single bilayer. Latter permits EVs to squeeze themselves through pores much smaller than their own diameter and thus penetrate the skin spontaneously with a minimal risk of vesicle rupture.
In the present study, we prepared curcumin-loaded EVs using different combinations of phospholipid and surfactant/edge activator. The results showed that vesicle number per cubic millimeter increased with increase in surfactant concentration to a limit, beyond which the vesicle number decreased. This observation may be ascribed to the fact that an increase in concentration of surfactant, up to a certain level, favors the formation of vesicles; any increase thereafter causes lysis of the phospholipid bilayers. In other words, beyond a certain threshold concentration, a further increase in the surfactant concentration probably dissolves the phospholipid bilayer. This supposition is an extension of the established concept that maximum micelle formation takes place at critical micellar concentration (CMC).
Analyses of vesicle shape, vesicle number, and % incorporation efficiency showed that span 80 was the most efficient surfactant when used with the phospholipid in the ratio 85:15. The prepared EVs formulation was investigated for in vitro permeation and skin deposition behavior. Results confirmed their superiority over conventional liposomes and significant flexibility was also demonstrated.
It is known that full-thickness skin tends to underestimate the in vivo permeability of hydrophobic compounds, when tested in vitro, because these essentially insoluble compounds may not partition freely from excised skin into an aqueous receptor fluid (33,34). However, they may readily pass the barrier layers because of their solubility in biological fluids (35) upon in vivo application. To obviate the poor in vitro/in vivo correlations when using full-thickness skin for hydrophobic compounds, solubilizing agents may be added to the receptor fluid. In this study, curcumin, which is essentially insoluble in water, may partition only slightly from excised skin into pH 6.4 phosphate buffer. Hence, 30% isopropyl alcohol was chosen as the receptor medium for the in vitro studies, using horizontal glass diffusion cell and murine skin as the barrier. A lower flux value obtained with aqueous dispersion of curcumin can be attributed to its poor solubility and limited cutaneous permeability; the latter improved significantly upon incorporation in EVs and liposomes. Significant flux and higher retention of curcumin were observed in the skin when it was entrapped within EVs. This improved performance can be attributed to the deformable nature of the vesicles and their ability to retain their integrity and thus, the entrapped curcumin during skin permeation (36,37). The flux value and skin retention of curcumin from marketed formulation (VICCO® turmeric skin cream) was significantly less than liposomes and EVs dispersion. EVs were further incorporated into different secondary vehicles, namely, hydrophilic ointment, hydrophobic ointment, and hydrogel. Hydrophilic ointment base emerged as the secondary vehicle of choice with maximum drug flux.
Significant topical anti-inflammatory effect suggests that curcumin-loaded EVs may relieve rheumatism and offer the additional advantage of suppressing inflammatory response initiated by tissue injury. The liposomal formulation could also reduce the edematous response but the effect shown by curcumin containing EVs was significantly (1.5 times) better in acute inflammation studies. EVs also showed promising results (28.8% inhibition) in cotton pellet-induced chronic inflammation in comparison to both liposomes (19.85% inhibition) and free curcumin ointment (3.91% inhibition). This establishes the usefulness of EVs as a better delivery system for topical targeting of curcumin vis á vis liposomes.
CONCLUSION
EVs of curcumin were successfully prepared with desired profile using Span-80 as an edge activator. Ex vivo permeation studies indicated a better penetration and retention of curcumin in the skin when incorporated into EVs. Further, high retention was also observed when compared to the marketed formulation (VICCO® turmeric skin cream). Acute inflammation animal model data showed curcumin EVs to be almost similar in effect to the marketed topical preparation of diclofenac sodium (Omni-gel®). Chronic inflammation studies showed % inhibition of 28.79% for curcumin EVs and 37.16% for marketed formulation; again showing highly promising results with respect to the diclofenac gel formulation.
REFERENCES
- 1.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223(2):181–90. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Hsuuw YD, Chang CK, Chan WH, Yu JS. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J Cell Physiol. 2005;205(3):379–86. doi: 10.1002/jcp.20408. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–98. [PubMed] [Google Scholar]
- 4.Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One. 2013;8(6):e67078. doi: 10.1371/journal.pone.0067078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–25. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 6.Deng S-L, Chen W-F, Zhou B, Yang L, Liu Z-L. Protective effects of curcumin and its analogues against free radical-induced oxidative haemolysis of human red blood cells. Food Chem. 2006;98(1):112–9. doi: 10.1016/j.foodchem.2005.05.063. [DOI] [Google Scholar]
- 7.Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann N Y Acad Sci. 2005;1043:440–51. doi: 10.1196/annals.1333.050. [DOI] [PubMed] [Google Scholar]
- 8.Strasser EM, Wessner B, Manhart N, Roth E. The relationship between the anti-inflammatory effects of curcumin and cellular glutathione content in myelomonocytic cells. Biochem Pharmacol. 2005;70(4):552–9. doi: 10.1016/j.bcp.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2010;55(3):495–503. doi: 10.1002/mnfr.201000310. [DOI] [PubMed] [Google Scholar]
- 10.Kaur IP, Kapila M, Agrawal R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res Rev. 2007;6(4):271–88. doi: 10.1016/j.arr.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila) 2011;4(8):1158–71. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol. 2011;49(11):2906–13. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Bhushan S, Kakkar V, Pal HC, Guru SK, Kumar A, Mondhe DM, et al. Enhanced anticancer potential of encapsulated solid lipid nanoparticles of TPD: a novel triterpenediol from Boswellia serrata. Mol Pharm. 2012;10(1):225–35. doi: 10.1021/mp300385m. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17(5):5972–87. doi: 10.3390/molecules17055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao YZ, Lu CT, Zhang Y, Xiao J, Zhao YP, Tian JL, et al. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int J Pharm. 2013;454(1):302–9. doi: 10.1016/j.ijpharm.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 16.Gallarate M, Chirio D, Trotta M, Eugenia Carlotti M. Deformable liposomes as topical formulations containing alpha-tocopherol. J Dispers Sci Technol. 2006;27(5):703–13. doi: 10.1080/01932690600662588. [DOI] [Google Scholar]
- 17.Castangia I, Nacher A, Caddeo C, Valenti D, Fadda AM, Diez-Sales O, et al. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2013;10(3):1292–300. doi: 10.1016/j.actbio.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary H, Kohli K, Kumar V. Nano-transfersomes as a novel carrier for transdermal delivery. Int J Pharm. 2013;454(1):367–80. doi: 10.1016/j.ijpharm.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–83. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal R, Kaur IP. Inhibitory effect of encapsulated curcumin on ultraviolet-induced photoaging in mice. Rejuvenation Res. 2010;13(4):397–410. doi: 10.1089/rej.2009.0906. [DOI] [PubMed] [Google Scholar]
- 21.Cevc G, Blume G, Schätzlein A. Transfersomes-mediated transepidermal delivery improves the regio-specificity and biological activity of corticosteroids in vivo. J Control Release. 1997;45(3):211–26. doi: 10.1016/S0168-3659(96)01566-0. [DOI] [Google Scholar]
- 22.El Maghraby GM, Williams AC, Barry BW. Skin hydration and possible shunt route penetration in controlled estradiol delivery from ultradeformable and standard liposomes. J Pharm Pharmacol. 2001;53(10):1311–22. doi: 10.1211/0022357011777800. [DOI] [PubMed] [Google Scholar]
- 23.El Maghraby GMM, Williams AC, Barry BW. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int J Pharm. 2004;276:143–61. doi: 10.1016/j.ijpharm.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Essa EA, Bonner MC, Barry BW. Iontophoretic estradiol skin delivery and tritium exchange in ultradeformable liposomes. Int J Pharm. 2002;240(1–2):55–66. doi: 10.1016/S0378-5173(02)00107-2. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Li Y, Li X, Wu Y. Anti-inflammatory effects of 4-methylcyclopentadecanone on edema models in mice. Int J Mol Sci. 2013;14(12):23980–92. doi: 10.3390/ijms141223980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu X, Li Y, Li W, Hu H, Yao H, Li H, et al. The anti-inflammatory effects of Caragana tangutica ethyl acetate extract. J Ethnopharmacol. 2014;152(1):99–105. doi: 10.1016/j.jep.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Swingle KF, Shideman FE. Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. J Pharmacol Exp Ther. 1972;183(1):226–34. [PubMed] [Google Scholar]
- 28.Fabri RL, Garcia RA, Florencio JR, de Castro Campos Pinto N, de Oliveira LG, Aguiar JA, et al. Anti-inflammatory and antioxidative effects of the methanolic extract of the aerial parts of Mitracarpus frigidus in established animal models. J Pharm Pharmacol. 2013;66(5):722–33. [DOI] [PubMed]
- 29.Ntimenou V, Fahr A, Antimisiaris SG. Elastic vesicles for transdermal drug delivery of hydrophilic drugs: a comparison of important physicochemical characteristics of different vesicle types. J Biomed Nanotechnol. 2012;8(4):613–23. doi: 10.1166/jbn.2012.1426. [DOI] [PubMed] [Google Scholar]
- 30.Uchino T, Lefeber F, Gooris G, Bouwstra J. Physicochemical characterization of drug-loaded rigid and elastic vesicles. Int J Pharm. 2011;412(1–2):142–7. doi: 10.1016/j.ijpharm.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 31.van den Bergh BA, Wertz PW, Junginger HE, Bouwstra JA. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements. Int J Pharm. 2001;217(1–2):13–24. doi: 10.1016/S0378-5173(01)00576-2. [DOI] [PubMed] [Google Scholar]
- 32.Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm. 2010;76:475–85. doi: 10.1016/j.ejpb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies. VI: preparation of the barrier layer. J Pharm Sci. 1986;75(5):487–91. doi: 10.1002/jps.2600750513. [DOI] [PubMed] [Google Scholar]
- 34.Catz P, Friend DR. Transdermal delivery of levonorgestrel. VIII. Effect of enhancers on rat skin, hairless mouse skin, hairless guinea pig skin, and human skin. Int J Pharm. 1990;58(2):93–102. doi: 10.1016/0378-5173(90)90245-Y. [DOI] [Google Scholar]
- 35.Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies III: hydrophobic compounds. J Pharm Sci. 1984;73(9):1255–8. doi: 10.1002/jps.2600730916. [DOI] [PubMed] [Google Scholar]
- 36.Cevc G, Gebauer D, Stieber J, Schatzlein A, Blume G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim Biophys Acta. 1998;1368(2):201–15. doi: 10.1016/S0005-2736(97)00177-6. [DOI] [PubMed] [Google Scholar]
- 37.Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29(9):1013–26. doi: 10.1081/DDC-120025458. [DOI] [PubMed] [Google Scholar]


