Abstract
Cerebral tissues possess highly selective and dynamic protection known as blood brain barrier (BBB) that regulates brain homeostasis and provides protection against invading pathogens and various chemicals including drug molecules. Such natural protection strictly monitors entry of drug molecules often required for the management of several diseases and disorders including cerebral vascular and neurological disorders. However, in recent times, the ischemic cerebrovascular disease and clinical manifestation of acute arterial thrombosis are the most common causes of mortality and morbidity worldwide. The management of cerebral Ischemia requires immediate infusion of external thrombolytic into systemic circulation and must cross the blood brain barrier. The major challenge with available thrombolytic is their poor affinity towards the blood brain barrier and cerebral tissue subsequently. In the clinical practice, a high dose of thrombolytic often prescribed to deliver drugs across the blood brain barrier which results in drug dependent toxicity leading to damage of neuronal tissues. In recent times, more emphasis was given to utilize blood brain barrier transport mechanism to deliver drugs in neuronal tissue. The blood brain barrier expresses a series of receptor on membrane became an ideal target for selective drug delivery. In this review, the author has given more emphasis molecular biology of receptor on blood brain barrier and their potential as a carrier for drug molecules to cerebral tissues. Further, the use of nanoscale design and real-time monitoring for developed therapeutic to encounter drug dependent toxicity has been reviewed in this study.
KEY WORDS: blood brain barrier (BBB), cerebral ischemic disorders, drug delivery, earthworm protease, neurodegenerative disorder, thrombolytic
INTRODUCTION
The blood brain barrier (BBB) is a highly dynamic biological membrane interface between blood and brain, providing selective transport to various biomolecules (1). The selective transport facilitates uptake of ions, amino acids, glucose, and other nutrient from blood to fulfil nutrient and energy demand (2). Simultaneously, BBB also restricts entry of pathogens, toxic chemicals, and metabolic products into neuronal tissue, maintain integrity of vital tissue (3). Such dynamic nature of the BBB has been a major challenge to drug delivery systems in delivering drug molecules into neuronal tissue for the management of several life-threatening diseases and disorders (4). These additional biological protections are not limited only to the brain but also extended into the spinal cord. There are numerous diseases and disorders with higher mortality that are associated with brain and other neuronal tissue and additional protection to neuronal tissue that made them even more devastating (5,6). The diseases such as Alzheimer, Parkinson, brain tumor, and ischemic cerebral disorder are few needs to encounter first. To combat these life-threatening diseases, there is an immense need for an efficient and selective method for the delivery of therapeutics from external sources (7,8).
The ischemic cerebrovascular disorders and associated complications have emerged as a major cause of mortality and physical deformity in the recent time (9). The major cause of cerebral ischemia is thrombus/plaque formation within the fine vascular pipeline of the cerebral tissue that restricts supply of blood and nutrient subsequently (10). The blood coagulation is a dynamic process regulated by a series of enzyme-catalyzed reactions running concurrently in blood plasma (11). A healthy homeostatic system governs blood coagulation and clot dissolution under highly regulated process of defensive and aggressive component of blood plasma (12). The failure of any one of the components results in clot formation and bring several life-threatening consequences. As for the concern to the neuronal tissue, abnormal behavior of blood coagulation mechanism brings most devastating consequence as these tissues need a continuous supply of nutrients and oxygen (Fig. 1) (14). To combat cerebral vascular ischemia, an external clot dissolving agent, thrombolytic essentially required from external sources (15). The major challenge with an external thrombolytic agent for clinical use in cerebral tissue is their low affinity towards the blood brain barrier and poor diffusion into the brain and neuronal tissue by conventional means of delivery (16).
Fig. 1.
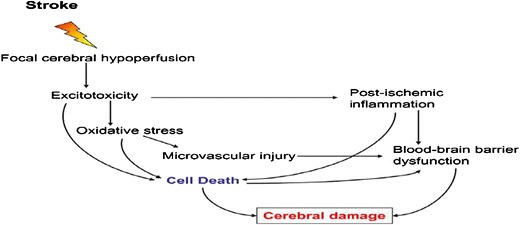
Impact and consequence of stroke on blood brain barrier and neuronal tissue under ischemic condition (13)
STRUCTURE AND BIOLOGY OF BBB
The blood brain barrier (BBB) comprised of a monolayer of brain microvascular endothelial cells (BMVEC) joined together by much tighter junctions than peripheral vessels and formed a cellular membrane that is known as the main physical barrier of BBB (4,17). The other elements playing an important role as a building block in designing of blood brain barrier are claudins and occludin supported by tight junction and adherence (18,19). The cellular membrane provides unique features such as uniform thickness, low pinocytosis activity, and continuous membrane with an overall negative charge (20). In addition to endothelial cells, the BBB is composed of the capillary basement membrane enriched in cells like pericytes, astrocytes, and microglia building neurovascular tissue with a highly selective affinity for biomolecules (21,22). The building blocks of the BBB, brain micro-vascular endothelial cells are further supported by another layer of natural structural protein, including collagen and elastin, specialized proteins such as fibronectin and laminin with a significant amount of proteoglycans (Fig. 2) (23,24).
Fig. 2.
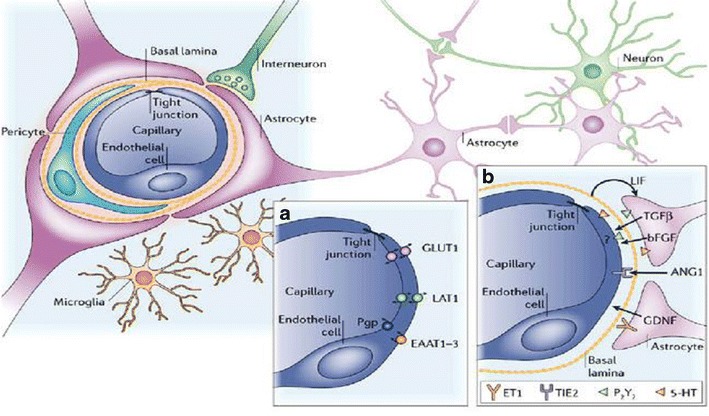
An overview of cellular structural organization in blood brain barrier. Cells, tight junction, and adhesion molecules define protection and selective transport (25)
Recent investigations have shown that the lipophilic nature (lipophilcity of drug), charge (net ionic concentration), and molecular weight of molecule are three key factors that decide diffusion from blood into the CNS (26). However, the overwhelming majority of small molecules with average molecular weight 500 Da including proteins and peptides fail to cross the blood brain barrier (27). Approximately, 98% of the small molecules and nearly all large molecules of average molecular weight more than 1 kDa, such as recombinant proteins or gene-based medicines completely fail to cross the blood brain barrier (28). Currently, more emphasis is given to deliver the drug molecules through the interaction with specific transporters and/or receptors expressed on the luminal (blood) side of the endothelial cells. The conventional and novel developed therapeutics will be effective once drug must reach into the brain and other part of neuronal tissue (29). Along with blood brain barrier, a physical protection in restricting entry of drug molecules, there are several additional secondary biological protections that are also running to inhibit the efficiency of drug delivery (30). The existing enzymes in blood brain barrier can be regarded as a second barrier after negative surface charge. These native enzymes involved in disposition of drugs and xenobiotic before entering the endothelial cells of capillaries (31). A series of enzyme, including alkaline phosphatases, acid phosphatase, 5′-nucleotides, adenosine triphosphatase, and nucleoside diphosphatase are among well-studied enzymes distributed within the blood brain barrier constituting a second barrier line against invading molecules (32).
CARRIER PROTEINS AND BLOOD BRAIN BARRIER
The delivery of drug molecules into the brain and cerebral tissue is the main obstacle despite of decade research. The highly protective barriers and selective transport across the blood brain barrier can be conquered by different mechanism depending on physiochemical property of drug molecules (33). Among these mechanisms, the hydrophilic molecules, including amino acids, glucose, and other small sized often uptake by different transporter expressed in BBB (34). In case of larger lipoprotein molecules such as hormone, iron, and insulin and lipophilic molecules target specific receptor for their transport into the brain and cerebral tissue (35). More important lipophilic molecules enter into the brain by passive diffusion using an efflux pump (P-glycoprotein (P-gp), some multidrug resistance proteins (MRP), breast cancer resistance protein (BCRP), and others (Fig. 3). However, all these mechanism will be functional in only one case as once targeted molecules must show its affinity to these carrier proteins towards the luminal side of the BBB (3). In the current research investigations, there is more emphasis that has been given in exploring transporter/receptors expressing on BBB and mediated drug delivery.
Fig. 3.
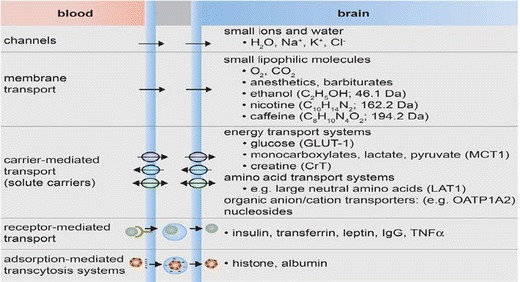
Transport across blood brain barrier, role of channels, transport membrane proteins, selective carriers, and receptors for precise transport (36)
Both, luminal side (blood) and abluminal possess a series of receptors that regulate trafficking of different molecules, including essential nutrient and drug molecules (37). The major proteins expressing on blood brain barrier as transporter or receptor are as follows:
- Carrier-mediated transport (CMT)
- Glucose transporter 1 (GLUT1)
- Organic anion transporting polypeptide (OATP)
- Large neutral amino acid transporter (LAT)
- Receptor-Mediated transport (RMT)
-
d.Transferrin receptor (TFR)
-
e.Insulin receptor (IR)
-
f.Lipoprotein receptor (LPR)
-
g.Diphtheria Toxin receptor (DPTR)
-
d.
CARRIER-MEDIATED TRANSPORT (CMT)
The carrier mediated transport is a natural phenomenon running spontaneously across the BBB for transferring of small biomolecules including nutrients-glucose, hormones, amino acids, bile salts, and monocarboxylic acids (38). The driving force for carrier-mediated transport is a concentration gradient across the BBB. Additional factors, such as affinity of molecules, molecular size, and physiochemical properties further facilitate/inhibits transfer of a wide variety of molecules (39). This passive diffusion transport does not have much scope in developing drug delivery therapeutics (40). However, structural refinements in drug can result the possibility to enter BBB. Among these carrier proteins, glucose transporter 1 (GLUT1) expresses, especially for the uptake of glucose essentially needed to supply energy for brain physiology (41). Further, the glucose transporter is highly specific towards D-glucose and conjugates often fail to transfer via glucose transporter 1 (42).
Several studies have been done to deliver antitumor drug coupled with glucose but fail to enter the brain (3). There are very less evidence and data available demonstrating applications of glucose transport 1 as a carrier for therapeutic agents. Recently, glycosylated drug molecules are under trails for conquering BBB transport via glucose transport 1 (21). Another important carrier protein on BBB recently characterized and has shown tremendous potential in delivery of chemotherapeutic agents is organic anion transporting polypeptides (OATPs). There are several OATPs that have characterized OATP 1Cl, OATP1A, OATP2B1, naturally expressing on endothelial brain cells (25). These transporters were studied as an ideal carrier for the drugs into brain tissue, such as antibiotics, sterols, opioid peptides, and bile salts. Additionally, OATPs are choice to target for hormones (thyroxin), antimicrobial agents (methotrexate), antiviral (saquinavir), and non-steroidal anti-inflammatory drugs NSAIDs (43). There are no evidences for use of OATPs as a carrier of thrombolytics, but their potential in delivering larger peptides surely makes a positive remark in the future.
The large neutral amino acid transporter (LAT1) is a sodium independent exchanger expresses on different tissues, including brain, testis, and placenta. The LAT1 is primarily associated with transport of large amino acids such as tyrosine, thyroid hormones, especially triiodothyronine across the biological membranes in different tissues (44). In the last few decades, LAT1 has emerged as prime targets to deliver several drugs acting on central nervous system such as antiparkinson (L-Dopa), anticonvulsant, e.g., gabapentin and antidepressant (45). The LAT-1, an ideal target for drug delivery of wide variety drugs with slight structural modifications so that they become LAT-1 substrates have enhanced BBB penetration (Fig. 4). The use of LTA1 as a ligand for delivery of recombinant proteins is underway and may be in the future, it comes with clinical practice (46).
Fig. 4.
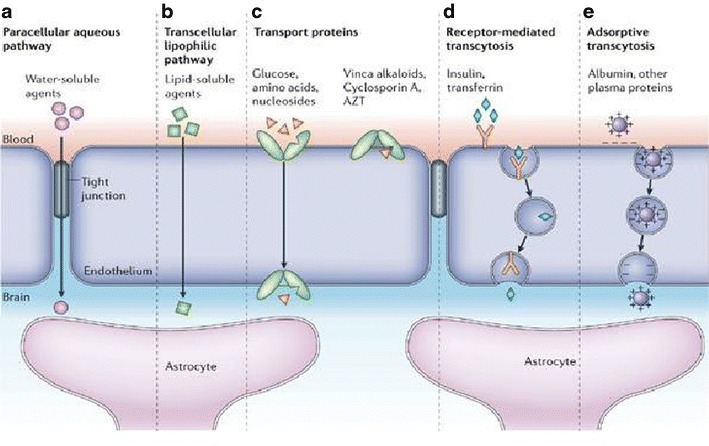
An overview of carrier and receptor-mediated transport across blood brain barrier (25)
RECEPTOR-MEDIATED TRANSPORT (RMT)
In contrast to carrier-mediated transport, receptor-mediated transport is an active transport and highly specific (47). The receptor-mediated transport facilitates entry of larger molecules by transcytosis. The RMT runs against concentration gradient, and hence, its active transport needs large amount of energy. The governing factor in RMT is the affinity of drug molecule towards receptor (48). This is an ideal platform for the large size of molecules, including drugs and recombinant proteins as part of therapeutics. The receptor-bound molecules undergo endocytosis and forming intracellular transport vesicles (49). There are different pathways for trafficking of therapeutic vesicles either by lysosomal or ubiquitin-protease cascade. There is another mechanism delivering therapeutics vesicles into neuronal tissue by exocytosis transport intracellular in the abluminal side of the BBB (50). The most important and vital protein expressing on the BBB is transferrin receptor which is basically a transmembrane glycoprotein with two subunits (51). The transferrin receptor generally expresses on the luminal side of the BBB and associated with transport of ions into brain parenchyma in conjugation with transferrin, a circulating iron binding protein (52).
Under normal circumstances, transferrin receptors do not allow binding and entry of any drug/recombinant protein due to high concentration of endogenous transferrin (53). There are two ways to target drugs to via transferrin receptor, one using endogenous transferrin as ligand and raising antibodies against TR and targeting. Rather than targeting endogenous transferrin as a ligand for drug/recombinant protein, receptor-specific antibodies and drug targeting are more practical (54). The TR specific antibodies bind to the receptors on endothelial cells and conjugated drugs, recombinant proteins uptake by brain parenchyma by endocytosis (55). Among the several receptors expressed abundantly on BBB, insulin receptor expressed on the luminal side of the base membrane of BBB and associated with receptor-mediated transport (RMT) for the transport of large size molecules, including drugs and recombinant proteins (56). The insulin receptor does not allow entry of any molecules in conjugation with the insulin due to the high specificity of IR and substrate insulin (57). On the other hand, using antibodies against IR is an ideal option for delivering candidate drug and recombinant protein into brain parenchyma through the BBB.
Coloma et al. (2000) have demonstrated the scope of IR-specific antibodies in the delivery of various drugs (58). Further, Boada et al. (2007) developed chimeric and humanized antibodies against IR for the transport of drugs and recombinant proteins in conjugation with antibodies (59). Currently, lipoprotein receptor family had great attention to researcher as an ideal target for delivery of large drug molecules and a variety of proteins in brain and neuronal tissue. The lipoprotein receptor protein (LRP) is 600 kDa synthesize as precursor protein and cleaved by furin in trans-Golgi into two fragments, larger one 515 kDa and smaller unit 86 kDa lined non-covalently (60). The LRP is a multifunctional endocytic receptor associated with internalization and degradation of different ligands involved in diverse metabolic pathways (61). The LRP is associated with internalization of a series of proteins, including tissue plasminogen activators (t-PA), plasminogen activators inhibitors 1, amyloid precursor protein (APP), factor VIII, α2 macroglobulin, and apolipoprotein E (62). In the LRP family, two individual receptors have been characterized as LRP1 and LRP2 and are the ideal target for transferring drugs and recombinant proteins in brain parenchyma (63).
Over the year, low-density lipoprotein receptor protein, LRP1 and LRP2 tremendously explored for delivery of drugs in conjugation with nanodesigns. Different drugs, including tubocurarine, loperamide, 8-chloro-4-hydroxy-1-oxol, 2-dihydropyridazino, quinoline-5-oxide choline salt fail to pass BBB in native form under conventional modes of administration shown tremendous scope in the conjugation with nanoparticle mediated by LRP (60,64). The diphtheria toxin receptor (DTR) is a transmembrane heparin binding growth factor (HB-EGE) constitutively expressed in BMEC, neuron, and glial cells. The DTR unregulated under hypoxic conditions like ischemic stroke, inflammatory conditions, and seizures (65,66). The diphtheria toxin readily binds to the receptor and internalized by endocytosis but cannot be used as such for a ligand is toxic in nature. A mutant variant of toxin CRM 197 was studied and shown potential as a ligand and carrier for drug into brain tissue. The mutant toxin showed its affinity towards HB-EGF analyzed by conjugation between CRM197 and horseradish peroxidase (HRP) were transported across the in vitro model of the BBB using bovine brain capillary endothelial cells in co-culture with newborn rat astrocytes. Interestingly, intravenous administration of CRM197-HRP that was reported in the brain parenchyma in guinea pigs suggests the scope of thrombolytic therapy in brain (67).
RECENT TRENDS IN THROMBOLYTIC THERAPY TO BBB
Tissue Plasminogen Activator (t-PA)
The recombinant variant of tissue plasminogen activators (rt-PA) is the only drug available as external thrombolytic clinically approved for the management of cerebral ischemia (68). The human tissue plasminogen activator (EC 3.4.21.68) is a protease of the S1 family (trypsin family) and is found in a wide variety of mammalian tissues, especially endothelial cells (67). The t-PA (70 kDa) is secreted as a single chain precursor, which is cleaved to a two-chain form by plasmin. The tissue plasminogen activator (t-PA) is routinely given intravenously to treat acute stroke (69). The t-PA has shown tremendous scope in the management of cerebral ischemia due to its thrombolytic activity and its ability to restore circulation to the brain (Table I) (70). Simultaneously, t-PA is associated with neuronal damage after intracerebral infusion stimulates excitotoxins such as glutamate, a major challenge with t-PA. The clinical application of t-PA brings intracranial hemorrhage immediate after infusion into neuronal tissues (71).
Table I.
List of Thrombolytic Drugs Clinically Approved and Available to Combat Cardiac Ischemia and will be Potential External Thrombolytic for Cerebral Ischemia with Advancement in Selective Drug Delivery
| S no | Thrombolytic | Molecular weight (kDa) and half-life (min) | Mechanism and plasmin specificity | Sources | Scope and affinity towards cerebral tissue |
|---|---|---|---|---|---|
| 1. | Streptokinase | 47 and 30 | Indirect and no | Streptococcus | Toxic to neuronal tissue with moderate affinity |
| 2. | Staphylokinase | 16.5 and 6 | Indirect and yes | Staphylococcus | Moderate affinity |
| 3. | Tissue plasminogen activator (t-PA) | 72 and 4 | Direct and yes | Human | Only approved drug available with significant affinity |
| 4. | Urokinase (u-PA) | 55 and 15 | Indirect and no | Human | Moderate affinity can be used in combination with other drugs |
| 5. | Earthworm serine protease EFE | 24–33 and 30 | Direct, indirect, and yes | Earthworm | Significant affinity with great scope in management of cerebral ischemia |
| 6. | Recombinant reteplase | 40 and 20 | Indirect and no | Chimeric | Moderate affinity can be used in combination with other drugs |
| 7. | Anistreplase | 131 and 90 | Indirect and no | Chimeric | Moderate affinity can be used in combination with other drugs |
| 8. | Alteplase (rt-PA) | 70 and 70 | Indirect and no | Chimeric | Moderate affinity can be used in combination with other drugs |
EFE earthworm fibrinolytic enzyme
Streptokinase and Staphylokinase
The microbial-based external thrombolytic including streptokinase (SK), staphylokinase (SAK), and their recombinant variants has shown therapeutic potential in cardiac ischemia, pulmonary embolism, and myocardial infarction (72). The application of these microbial agents for cerebral tissue in combating cerebral ischemia has not been reported. The major challenge associated with these agents towards neuronal tissue is their poor diffusion across the BBB. The drug instability, short half-life, rapid tissue clearance, and immunogenicity are subsequent limitations associated with microbial-based external thrombolytic (73). However, recombinant variants of staphylokinase with enhanced half-life and reduced immunogenicity will be possibly future medicine for cerebral ischemia (74). An advantage using staphylokinase for further study in developing novel neuronal anti-ischemic drug molecule is its molecular weight. The staphylokinase possesses least molecular weight 16.5 kDa among available external thrombolytic ideal for crossing blood brain barrier (75).
Earthworm Fibrinolytic Enzyme
The earthworm was known for its therapeutic potential since ancient time, and several potent biomolecules were isolated and purified in the last two decades (76). The earthworm fibrinolytic enzyme (EFE), a group of serine protease has shown tremendous potential in combating vascular diseases not only cardiac but also cerebral disorders (77). The EFE is a fibrin-specific serine protease that exists as isoform of six protease of molecular weight 24–33 kDa with different fibrinolytic activity (78). The EFE acts on circulating plasminogen leading to activation into plasmin and also dissolves clot directly by acting on fibrin. The dual mechanism of fibrinolysis EFE emerged as amazing thrombolytic molecule (79). The EFE differs from other available external thrombolytics, one is it can easily get absorbed from intestinal mucosa and it possesses stability in different pH and temperatures (80).
Further, one of the fractions of the earthworm fibrinolytic enzyme had shown great potential in combating cerebral ischemia. In a study, carried out in 2008, Hongrui Ji et al. has demonstrated EFE fraction managing cerebral ischemia by regulating JAK1/STAT1 pathway (9). The role of platelets in the initiation and development of ischemic vascular disease has been studied, and antiplatelet therapy has become the useful means of preventing or treating ischemic cerebrovascular diseases (81). The onset of cerebral ischemia leads to activation of Janus tyrosine kinase (JAK1), promote the development of procerebrum, and offer protection to neuronal tissue. However, ischemic conditions lower down signal transducer and activator of transcription (STAT 1) expression for a reason not known yet. Hongrui Ji et al. have identified messenger RNA (mRNA) level of JAK1/STAT1 after administration of EFE to animal model (rat) (82). The expression was significantly increased in case of JAK1 mRNA while STAT1 was remarkably falling down.
The management of cerebral ischemia requires immediate infusion of anti-ischemic drug to conquer blood flow restrictions (83). However, the ischemic condition driven consequences, such as cerebral hypoxia or cerebral infarction and subarachnoid hemorrhage or intracerebral hemorrhage need extra protection (84). The ideal anti-ischemic drugs for brain also include protection of neuronal tissue (85). Unfortunately, available external thrombolytic offer only anti-ischemic property and often fail in protecting damage of vital tissue from damage caused by higher doses (86). Moreover, many external thrombolytic leads to drug depending toxicity to brain tissue as prescribed in higher concentration to cross the BBB (87). The EFE has shown tremendous scope in regulating several metabolic pathways and offering protection to the brain.
Receptor-Based Therapeutics—Challenges and Limitations
One of the major challenges with current drugs, including thrombolytic is their poor diffusion across the blood brain barrier. Several attempts have been made to design novel therapeutics especially for drug delivery to neuronal tissue, and very few results bought preliminary success (88). Numerous problems were reported while delivering drug molecule across BBB including the hydrophilic nature of drug molecules, larger molecular size, and poor affinity for receptor expressed on BBB, kinetics parameters, and lack of real-time monitoring (89).
Physiochemical Property of Drug Molecule
One basic prerequisite for any drug intended to cross BBB is that drug must be lipophilic. The hydrophilic drugs and biomolecules often fail to bind receptor expressed on BBB. Biomolecules, including protein and peptide drugs, being large and mostly polar show minimal passive uptake into neuronal tissue. Further, charged drugs and other molecules also fail to cross biological barriers on neuronal tissues (90). Research finding has explored more than 20 different transporter shuttles on BBB that governs entry of essential molecules into or outside of brain tissue under through the precise mechanism (91). The molecular size and weight of candidate drug is another crucial factor to decide fate of drug into neuronal tissues. The average molecular weight less than 500 Da is ideal for transport shuttle running in the brain, and higher molecular weight fails to diffuse.
Optimization of Kinetic Parameters
To achieve threshold plasma therapeutic concentration is a crucial factor for any drug after administration by any route defining fate of therapy. The pharmacokinetic parameters (ADME) include absorption from the site of administration, drug distribution in targeted tissue, metabolism, or biotransformation of candidate drug, and elimination of tissue is crucial for targeted therapy (92). Moreover, drug targeting to neuronal tissue is much complicated, as it consists of additional biological barriers. In order to achieve success in drug delivery to neuronal tissue, several factors are mandatory, including ease in attaining a required therapeutic concentration of drug at the site of action for an appropriate period of time (93). Further, availability of drug to candidate tissue and volume of drug distribution (Vd) that includes cellular uptake, intracellular compartmentalization is essential to optimize for neuronal drug delivery. Additionally, cerebrospinal fluid enriches in catabolic enzymes facilitate drug metabolism and lack of plasma protein in cerebrospinal fluid further potentiate drug metabolism (94).
Real-Time Imaging
Lack of real-time monitoring is another major challenge for thrombolytic drug delivery and in vivo evaluation of thrombolysis in neuronal tissue. There has been an over-reliance towards development of cell culture systems for evaluation of novel drug targeting systems (95). Novel drugs and brain targeting systems are often primarily evaluated with cell culture models of the BBB in vitro with a number of limitations. This is much more difficult to design a therapeutic along with a reporter molecule (cy3 and cy5) to neuronal tissue for real-time monitoring due to many reasons, one is diffusion limitations and other toxicity offered by reporter molecules. Though, several attempts have been made and shown positive results but need to refine for precise monitoring (96).
Advancement Towards BBB Targeting for Thrombolytic
To achieve efficient therapeutic concentration of candidate drug into neuronal tissue, one can aim for by refining of existing thrombolytic drugs to increase BBB penetration by promising strategies. However, developing a new chemical entity that already possesses the desired permeability properties will be an advantage (67). Several achievements have been made in thrombolytic therapy exclusively for brain by refining existing external thrombolytic drugs. Claude R. Benedict et al. (2014) developed a novel variant of t-PA (T103N, N117Q, KHRR 296-299 AAAA, or TNK-TPA) with higher affinity longer plasma half-life, enhanced fibrin specificity, and increased resistance to inhibition by plasminogen activator inhibitor (PAI-1) in a rabbit thrombosed carotid artery model (97). Similarly, t-PA-S481A, another variant of t-PA, has shown tremendous potential for combating cerebral ischemia. The t-PA-S481A efficiently prevents neuronal toxicity by activation of NMDA receptor that plays a crucial role in impairment of cerebral hemodynamic and enhances excitotoxic neuronal death (98). Further, novel variant of t-PA (t-PA-S481A) offers selective thrombolysis by competing wild type t-PA present in the systemic circulation.
The low-density lipoprotein receptor-related protein 1 (LRP1) emerges to play fundamental roles in cellular signalling pathways in the neuronal tissue (99). Research studies carried out over a decade were designed with more emphasis on revealing the mechanism of low-density lipoprotein receptor-related protein 1 (LPR1) and ideal carrier for t-PA. Research investigations suggested LRP1, and the NMDA receptor might eventually act in a combined fashion to mediate t-PA downstream signalling (100). In this study, t-PA after binding to LRP1 and utilizing the receptor-associated protein resulted in complete inhibition of NMDA receptor and its activation. Additionally, inhibition of NMDA receptor calcium influx with MK-801 resulted in dramatic reduction of t-PA-mediated downstream signalling. The Angiopep-2 possesses higher BBB permeability and emerged as an ideal vehicle for the delivery of small molecules, DNA, and proteins (101). Recently, a dual-drug delivery system for brain tumor was developed based on PEGylated oxidized multi-walled carbon nanotubes (O-MWNTs) modified with Angiopep-2 (O-MWNTs-PEG-ANG), possibly a tool for thrombolytic drug delivery (102).
The application of nanoscale technology in delivering candidate drug has become an integral part of modern medicine (103). The nanodesigns are not limited to only delivering drugs but also contribute real-time monitoring (104). In the context of nanodesigned based thrombolytic drug delivery, it was Marsh JN, et al. (2011) who developed a fibrin-specific, liquid perfluorocarbon nanoparticle with modified surface to deliver the plasminogen activator streptokinase to neuronal tissue. The results from targeted thrombolysis were evaluated in vitro using quantitative acoustic microscopy, and 1% surface targeting of streptokinase nanoparticles produced significant decreases in clot volumes (approximately 30%) in 1 h (105). Further, the use of ultrasonic waves in clot dissolution had shown potential for future vascular medicine. In a study, carried out in 2002, recombinant tissue plasminogen activator (rt-PA) was employed for ischemic tissue in conjugation with ultrasonic waves (106). The ultrasonic waves are known to have several biological effects with their energy characteristics. Interestingly, an ultrasonic wave at higher energy levels alone has a thrombolytic effect and such waves were already used for clinical purposes in interventional therapy using ultrasonic catheters (107).
Recently, more emphasis was given in exploring the waves at lower energy levels (<2 W/cm) which facilitates enzymatic-mediated thrombolysis, most probably by breaking molecular linkages of fibrin polymers and therefore, increasing the working surface for the thrombolytic drug (108). Gene therapy is one of the most advances arenas of current medicine and next most suitable technology for combating neurological disorder including cerebral ischemia (109). The gene therapy works on the principle of repair or replacement of defective gene/s responsible for several life-threatening diseases. In a study, 2001, defective herpes simplex viral vectors were designed and evaluated for delivery of potential carrier towards several genes that lead to neuronal damage (110). The study suggested that genetically refined herpes simplex virus can be an experimental model to combat stroke, cardiac arrest, and excitotoxicity. Further, in 2003, adenovirus-mediated gene delivery was carried out for cerebral ischemia with significant outcomes (111). There are numerous studies carried out, and researchers are looking for a new generation delivering cargoes for gene therapy to neuronal tissue. The gene therapy-based studies are still in clinical trials phase and associated with several limitations, including ethical violation along with higher risk of failure of therapy. More emphasis are needed at molecular research to refine existing tools of gene therapy and develop novel carrier for gene delivery to vital tissues.
DISCUSSION
The CNS disorders have become a major challenge for modern therapeutics and associated with millions of deaths all around the world (112,113). The CNS infection, brain tumor, and cerebral ischemic disorders are the leading along with neurodegenerative disorders (114–116). Disease burden because of CNS disorders, including infectious, tumor, and vascular is anticipated to rise to 14.7% by the year 2020, owing to an increase in the aged population (117). The major problem for the treatment of these diseases and disorders is the lack of precise drug delivery system (118–120). The existing conventional therapeutically options often get fail to deliver drugs to neuronal tissue and also contribute drugs dependent toxicity that led to damage of vital tissue (121). However, lack of real-time monitoring system for the evaluation of drug delivery to brain tissue further potentiated treats (122,123). The available therapeutics for vascular disorders have shown great potential to combat cardiac ischemia and associated outcome, including cardiac infraction and thromboembolism (124). The overriding goal of modern vascular therapeutics is to develop drug molecules with broad spectrum. Development of new generation vascular medicine also emphasizes to conquer socio-economical boundaries across the globe.
However, conventional thrombolytic therapeutics fail in case of cerebral ischemia and ischemia-driven pathological consequences (125,126). Hence, there is an immense need for finding novel options and tools to deliver drugs across the BBB, a major obstacle for CNS disorders (127). To conquer BBB, several attempts have been made, including receptor expressing on BBB, refining the physiochemical property existing drug, and in conjugation with nanovehicles (128). Among these tools, drug refinements leading to a more lipophilic drug are a more convenient option to deliver drug without hampering neuronal tissue homeostasis (129,130). Further, redefining pharmacokinetic parameters of candidate drug and developing into a novel drug delivery system using different carrier had shown great scope in future medicine for CNS disorders (131). Conjugation with reporter molecules is advantageous to regulate the fate of therapy and efficiency. A multidisciplinary approach is essentially needed to conquer BBB and delivering drugs into neuronal tissue in therapeutic concentration. Mechanical thrombectomy emerged as a novel option for selective clot lysis in deep vascular pipelines in cardiac and brain tissues (132–135). Further, sonothrombolysis led is another emerging area of modern vascular medicine with precise clot lysis (136–139). Tremendous research work and massive research funding in developing novel therapeutics for neuronal tissue will transform conventional medicine in the future.
ACKNOWLEDGMENTS
The author thanks the Principal and Management, R.V.R. & J.C. College of Engineering (A), Guntur, Andhra Pradesh, India for their support to carry out the research work. Furthermore, my deep gratitude to the Department of Biotechnology, R.V.R. & J.C. College of Engineering (A) for providing facility utilized in the current study.
REFERENCES
- 1.Abbott NJ. Physiology of the blood–brain barrier and its consequences for drug transport to the brain. Int Congr Ser. 2005;1277:3–18. doi: 10.1016/j.ics.2005.02.008. [DOI] [Google Scholar]
- 2.Jones AR, Shusta EV. Blood–brain barrier transport of therapeutics via receptor mediation. Pharm Res. 2007;24:1759–71. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso FL, Brites D, Brito MA. Looking at the blood–brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–63. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge WM. Blood–brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3:90–105. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 6.Eyal S, Hsiao P, Unadkat JD. Drug interactions at the blood–brain barrier: fact or fantasy? Pharmacol Ther. 2009;123:80–104. doi: 10.1016/j.pharmthera.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shilo M, Motiei M, Hana P, Popovtzer R. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale. 2014;6(4):2146–52. doi: 10.1039/C3NR04878K. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 9.Ji H, Wang L, Bi H, Sun L, Cai B, Wang Y, et al. Mechanisms of lumbrokinase in protection of cerebral ischemia. Eur J Pharmacol. 2008;590:281–9. doi: 10.1016/j.ejphar.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Furlan AJ, Abou-Chebi A. The role of recombinant pro-urokinase (r-pro-UK) and intraarterial thrombolysis in acute ischemic stroke: the PROACT trials. Prolyse in acute cerebral thromboembolism. Curr Med Res Opin. 2002;18(Suppl 2):44–7. doi: 10.1185/030079902125000723. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129(3):307–21. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 12.Huai Q, Mazar A, Kuo A, Parry GC, Shaw DE, Callahan J, et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311:656–9. doi: 10.1126/science.1121143. [DOI] [PubMed] [Google Scholar]
- 13.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards MT, Murphy MM, Geraghty JJ, Wulf JA, Konzen JP. Intraarterial cerebral thrombolysis for acute ischemic stroke in a community hospital. AJNR Am J Neuroradiol. 1999;20(9):1682–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JH, Diamond SL. Tissue plasminogen activator (t-PA) inhibits plasmin degradation of fibrin. A mechanism that slows t-PA-mediated fibrinolysis but does not require alpha 2-antiplasmin or leakage of intrinsic plasminogen. J Clin Invest. 1995;95(6):2483–90. doi: 10.1172/JCI117949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhim T, Lee DY, Lee M. Drug delivery systems for the treatment of ischemic stroke. Pharm Res. 2013;30(10):2429–44. doi: 10.1007/s11095-012-0959-2. [DOI] [PubMed] [Google Scholar]
- 17.Abbott NJ. Prediction of blood–brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today Technol. 2004;1:407–16. doi: 10.1016/j.ddtec.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood brain barrier. Trends Pharmacol Sci. 2010;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer AM. The role of the blood-CNS barrier in CNS disorders and their treatment. Neurobiol Dis. 2010;37:3–12. doi: 10.1016/j.nbd.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Pardridge WM. Introduction to the blood–brain barrier: methodology, biology, and pathology. Cambridge University PRESS. 1998; 24(9):521–81.
- 21.Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Anstrom JA, Brown WR, Moody DM, Thore CR, Challa VR, Block SM. Anatomical analysis of the developing cerebral vasculature in premature neonates: absence of precapillary arteriole to-venous shunts. Pediatr Res. 2002;52:554–60. doi: 10.1203/00006450-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens. 2007;16:459–64. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 24.Findley MK, Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61(4):431–7. doi: 10.1002/iub.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 26.Pardridge WM. Molecular biology of the blood–brain barrier. Mol Biotechnol. 2005;30:57–70. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- 27.Asotra K, Ningaraj N, Black KL. Measurement of blood–brain and blood-tumor barrier permeabilities with (14C)-labeled tracers. Methods Mol Med. 2003;89:177–90. doi: 10.1385/1-59259-419-0:177. [DOI] [PubMed] [Google Scholar]
- 28.Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abulrob A, Sprong H, Bergen Henegouwen PV, Stanimirovic D. The blood–brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem. 2005;95:1201–14. doi: 10.1111/j.1471-4159.2005.03463.x. [DOI] [PubMed] [Google Scholar]
- 30.Schlossauer B, Steuer H. Comparative anatomy, physiology and in vitro models of the blood–brain and blood-retina barrier. Curr Med Chem. 2002;2:175–86. [Google Scholar]
- 31.Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci. 2011;32:708–14. doi: 10.1016/j.tips.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miksys S, Tyndale RF. Brain drug-metabolizing cytochrome P450 enzymes are active in vivo, demonstrated by mechanism-based enzyme inhibition. Neuropsychopharmacology. 2009;34(3):634–40. doi: 10.1038/npp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Born J, Lange T, Kern W, Mc-Gregor GP, Bickel U, Fehm HL. Sniffing neuropeptides a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–6. doi: 10.1038/nn0602-849. [DOI] [PubMed] [Google Scholar]
- 34.Huang CC, Lee CC, Hsu KS. The role of insulin receptor signalling in synaptic plasticity and cognitive function. Chang Gung Med J. 2010;33(2):115–25. [PubMed] [Google Scholar]
- 35.Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood–brain barrier. J Neurochem. 2001;76:1597–600. doi: 10.1046/j.1471-4159.2001.00222.x. [DOI] [PubMed] [Google Scholar]
- 36.Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood–brain barrier: an engineering perspective. Front Neuroeng. 2013;6:1–22. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2001;20:77–95. doi: 10.1023/A:1006948027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. Active transport of high-affinity choline and nicotine analogs into the central nervous system by the blood–brain barrier choline transporter. J Pharmacol Exp Ther. 2003;304:1268–74. doi: 10.1124/jpet.102.045856. [DOI] [PubMed] [Google Scholar]
- 39.Smith QR. A review of blood brain barrier transport techniques. Methods Mol Med. 2003;89:193–208. doi: 10.1385/1-59259-419-0:193. [DOI] [PubMed] [Google Scholar]
- 40.Hawkins RA, O’Kane RL, Simpson IA, Viña JR. Structure of the blood–brain barrier and its role in the transport of amino acids. J Nutr. 2006;136(1):218S–26. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 41.Zhao F-Q, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–28. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Augustin R, Carayannopoules MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9) J Biol Chem. 2004;279:16229–36. doi: 10.1074/jbc.M312226200. [DOI] [PubMed] [Google Scholar]
- 43.Rip J, Schenk GJ, Boer AG. Differential receptor-mediated drug targeting to the diseased brain. Expert Opin Drug Deliv. 2009;6:227–37. doi: 10.1517/17425240902806383. [DOI] [PubMed] [Google Scholar]
- 44.Gynther M, Laine K, Ropponen J, Leppanen J, Mannila A, Nevalainen T, et al. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J Med Chem. 2008;51(4):932–6. doi: 10.1021/jm701175d. [DOI] [PubMed] [Google Scholar]
- 45.Peura L, Malmioja K, Huttunen K, Leppanen J, Hamalainen M, Forsberg MM, et al. Design, synthesis and brain uptake of LAT1-targeted amino acid prodrugs of dopamine. Pharm Res. 2013;30(10):2523–37. doi: 10.1007/s11095-012-0966-3. [DOI] [PubMed] [Google Scholar]
- 46.Peura L, Malmioja K, Laine K, Leppanen J, Gynther M, Isotalo A, et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol Pharm. 2011;8(5):1857–66. doi: 10.1021/mp2001878. [DOI] [PubMed] [Google Scholar]
- 47.Xiao G, Gan L-S. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int J Cell Biol. 2013;2013 doi: 10.1155/2013/703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Pardridge WM. Delivery of β-galactosidase to mouse brain via the blood–brain barrier transferrin receptor. J Pharmacol Exp Ther. 2005;313:1075–81. doi: 10.1124/jpet.104.082974. [DOI] [PubMed] [Google Scholar]
- 49.Temsamani J, Rousselle C, Rees AR, Scherrmann JM. Vector-mediated drug delivery to the brain. Exp Opin Biol Ther. 2001;1:773–82. doi: 10.1517/14712598.1.5.773. [DOI] [PubMed] [Google Scholar]
- 50.Maresh GA, Maness LM, Zadina JE, Kastin AJ. In vitro demonstration of a saturable transport system for leptin across the blood–brain barrier. Life Sci. 2001;69(1):67–73. doi: 10.1016/S0024-3205(01)01093-1. [DOI] [PubMed] [Google Scholar]
- 51.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor mediated endocytosis pathway. Pharmacol Rev. 2002;54(4):561–87. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 52.Li H, Qian ZM. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22(3):225–50. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- 53.Waheed A, Grubb JH, Zhou XY, Tomatsu S, Fleming RE, Costaldi ME, et al. Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci. 2002;99(5):3117–22. doi: 10.1073/pnas.042701499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karsten U, Telli H, Elisabeth H, Jorg K. Transferrin- and transferrin-receptor antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB) Eur J Pharm Biopharm. 2009;71(2):251–6. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Van Hoof D, Rodenburg KW, Van der Horst DJ. Receptor-mediated endocytosis and intracellular trafficking of lipoproteins and transferrin in insect cells. Insect Biochem Mol Biol. 2005;35(2):117–28. doi: 10.1016/j.ibmb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Boado RJ, Zhang Y, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol Bioeng. 2007;96(2):381–91. doi: 10.1002/bit.21120. [DOI] [PubMed] [Google Scholar]
- 57.Coloma MJ, Lee HJ, Landaw EM, Boado RJ, Morrison SL, Pardridge WM. Transport across the primate blood–brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res. 2000;17(3):266–74. doi: 10.1023/A:1007592720793. [DOI] [PubMed] [Google Scholar]
- 58.Boado RJ, Zhang Y, Zhang Y, Wang Y, Pardridge WM. GDNF fusion protein for targeted-drug delivery across the human blood–brain barrier. Biotech Bioeng. 2007;100:387–96. doi: 10.1002/bit.21764. [DOI] [PubMed] [Google Scholar]
- 59.Chung NS, Wasan KM. Potential role of the low-density lipoprotein receptor family as mediators of cellular drug uptake. Adv Drug Deliv Rev. 2004;56(9):1315–34. doi: 10.1016/j.addr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Demeule M, Currie JC, Bertrand Y, Che C, Regina A, Gabathuler R, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106(4):1534–44. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 61.May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39(3):219–28. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- 62.Yoon IS, Chen E, Busse T, Repetto E, Lakshmana MK, Koo EH, et al. Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 2007;21:2742–52. doi: 10.1096/fj.07-8114com. [DOI] [PubMed] [Google Scholar]
- 63.Harris-White ME, Frautschy SA. Low density lipoprotein receptor-related proteins (LRPs), Alzheimer’s and cognition. Curr Drug Targets CNS Neurol Disord. 2005;4(5):469–80. doi: 10.2174/156800705774322102. [DOI] [PubMed] [Google Scholar]
- 64.Gaillard PJ, Brink A, Boer AG. Diphtheria toxin receptor-targeted brain drug delivery. Int Congr Ser. 2005;1277:185–95. doi: 10.1016/j.ics.2005.02.022. [DOI] [Google Scholar]
- 65.Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neuron. J Biochem. 1994;269:18521–8. [PubMed] [Google Scholar]
- 66.Raab G, Klagsbrun M. Heparin-binding EGF like growth factor. Biochim Biophys Acta. 1997;1333:F179–99. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 67.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (t-PA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4(2):228–31. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 68.Chernyshev OY, Martin-Schild S, Albright KC, Barreto A, Misra V, Acosta Grotta JC, et al. Safety of t-PA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology. 2010;74(17):1340–5. doi: 10.1212/WNL.0b013e3181dad5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatcher MA, Starr JA. Role of tissue plasminogen activator in acute ischemic stroke. Ann Pharmacother. 2011;45(3):364–71. doi: 10.1345/aph.1P525. [DOI] [PubMed] [Google Scholar]
- 70.Cronin CA. Intravenous tissue plasminogen activator for stroke: a review of the ECASS III results in relation to prior clinical trials. J Emerg Med. 2010;38(1):99–105. doi: 10.1016/j.jemermed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Nakashima T, Minematsu K. Prospects of thrombolytic therapy for acute ischemic stroke. Brain Nerve. 2009;61(9):1003–12. [PubMed] [Google Scholar]
- 72.Anderson JL. Development and evaluation of anisoylated plasminogen streptokinase activator complex (APSAC) as a second generation thrombolytic agent. J Am Coll Cardiol. 1987;10(5 Suppl B):22B–7. doi: 10.1016/S0735-1097(87)80424-2. [DOI] [PubMed] [Google Scholar]
- 73.Monk JP, Heel RC. Anisoylated plasminogen streptokinase activator complex (APSAC). A review of its mechanism of action, clinical pharmacology and therapeutic use in acute myocardial infarction. Drugs. 1987;34(1):25–49. doi: 10.2165/00003495-198734010-00002. [DOI] [PubMed] [Google Scholar]
- 74.Collen D. Staphylokinase: a potent, uniquely fibrin-selective thrombolytic agent. Nat Med. 1998;4(3):279–84. doi: 10.1038/nm0398-279. [DOI] [PubMed] [Google Scholar]
- 75.Moreadith RW, Collen D. Clinical development of PEGylated recombinant staphylokinase (PEG-Sak) for bolus thrombolytic treatment of patients with acute myocardial infarction. Adv Drug Deliv Rev. 2003;55(10):1337–45. doi: 10.1016/S0169-409X(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 76.Cooper EL, Hrzenjak TM, Grdisa T. Alternative sources of fibrinolytic, anticoagulative, antimicrobial and anticancer molecules. Int J Immunopathol Pharmacol. 2004;17:237–44. doi: 10.1177/039463200401700303. [DOI] [PubMed] [Google Scholar]
- 77.Wang F, Wang C, Li M, Gui L, Zhang J, Chang W. Purification, characterization and crystallization of a group of earthworm fibrinolytic enzymes from Eisenia fetida. Biotechnol Lett. 2003;25:1105–9. doi: 10.1023/A:1024196232252. [DOI] [PubMed] [Google Scholar]
- 78.Inoue T, Yaguchi I, Takayangi K, Hayashi T, Moorrks S, Eguchi Y. A new thrombolytic agent, monteplase, is independent of plasminogen activator inhibitor in patients with acute myocardial infarction: comparison with native t-PA E 610. J Am Coll Cardiol. 1997;29:1447–53. doi: 10.1016/S0735-1097(97)00074-0. [DOI] [PubMed] [Google Scholar]
- 79.Sun HL, Jiao JD, Pan ZW, Dong DL, Yang FB. The cardio-protective effect and mechanism of lumbrokinase. Yao Xue Xue Bao. 2006;41(3):247–51. [PubMed] [Google Scholar]
- 80.Justicia C, Gabriel C, Planas AM. Activation of the JAK/STAT pathway following transient focal cerebral ischemia: signaling through Jak1 and Stat3 in astrocytes. Glia. 2000;30:253–70. doi: 10.1002/(SICI)1098-1136(200005)30:3<253::AID-GLIA5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 81.Katoh M, Karasawa T, Doi H, Odawara A, Takagi M, Ikeo T, et al. Antiplatelet mechanisms of TA-993 and its metabolite MB3 in ADP-induced platelet aggregation. Eur J Pharmacol. 2000;399:91–6. doi: 10.1016/S0014-2999(00)00352-6. [DOI] [PubMed] [Google Scholar]
- 82.Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci. 1998;95:5590–4. doi: 10.1073/pnas.95.10.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–7. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X, Zhang J, Kuang P, Lang S, Wu W, et al. The effect of lumbrokinase on P-selectin and E-selectin in cerebral ischemia model of rat. J Tradit Chin Med. 2003;23:141–6. [PubMed] [Google Scholar]
- 85.Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47(4):462–9. doi: 10.1002/1531-8249(200004)47:4<462::AID-ANA9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 86.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–37. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 87.Schellinger PD, Jansen O, Fiebach JB, Heiland S, Steiner T, Schwab S, et al. Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke. 2000;31(6):1318–28. doi: 10.1161/01.STR.31.6.1318. [DOI] [PubMed] [Google Scholar]
- 88.Kuntz M, Mysiorek C, Pétrault O, Petrault M, Uzbekov R, Bordet R, et al. Stroke-induced brain parenchymal injury drives blood–brain barrier early leakage kinetics: a combined in vivo/in vitro study. J Cereb Blood Flow Metab. 2014;34:95–107. doi: 10.1038/jcbfm.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Collins VE, Macleod MR, Donnan GA, Horky LL, Van Der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 90.Rosenberg N, Chen M, Prabhakaran S. New devices for treating acute ischemic stroke. Recent Pat CNS Drug Discov. 2005;5:118–34. doi: 10.2174/157488910791213176. [DOI] [PubMed] [Google Scholar]
- 91.Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–5. doi: 10.1111/j.1749-6632.2012.06691.x. [DOI] [PubMed] [Google Scholar]
- 92.Franko J, Pomfy M, Prosbova T, Kimakova T. Temporal kinetics of calcium in neocortical neuronal nuclei after global cerebral ischemia and reperfusion. An ultrastructural study. Sb Lek. 2001;102(2):149–52. [PubMed] [Google Scholar]
- 93.Summerfield SG, Read K, Begley DJ, Obradovic T, Hidalgo IJ, Coggon S. Central nervous system drug disposition: the relationship between in situ brain permeability and brain free fraction. J Pharmacol Exp Ther. 2007;322:205–13. doi: 10.1124/jpet.107.121525. [DOI] [PubMed] [Google Scholar]
- 94.Avdeef A. Physicochemical profiling (solubility, permeability and charge state) Curr Top Med Chem. 2001;1:277–351. doi: 10.2174/1568026013395100. [DOI] [PubMed] [Google Scholar]
- 95.Laquintana V. New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6(10):1017–32. doi: 10.1517/17425240903167942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pardridge W. Vector mediated drug delivery to the brain. Adv Drug Deliv Rev. 1999;36:299–321. doi: 10.1016/S0169-409X(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 97.Benedict CR, Refino CJ, Keyt BA, Pakala R, Paoni NF, Thomas GR, et al. New variant of human tissue plasminogen activator (TPA) with enhanced efficacy and lower incidence of bleeding compared with recombinant human TPA. Circulation. 1995;92:3032–40. doi: 10.1161/01.CIR.92.10.3032. [DOI] [PubMed] [Google Scholar]
- 98.Armstead WM, Riley J, Yarovoi S, Cines DB, Smith DH, Higazi AA. TPA-S481A prevents neurotoxicity of endogenous tPA in traumatic brain injury. J Neurotrauma. 2012;29(9):1794–802. doi: 10.1089/neu.2012.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graham EM, Sheldon RA, Flock DL, Ferriero DM, Martin LJ, O’Riordan DP, et al. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17(1):89–98. doi: 10.1016/j.nbd.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Maier W, Bednorz M, Meister S, Roebroek A, Weggen S, Schmitt U, et al. LRP1 is critical for the surface distribution and internalization of the NR2B NMDA receptor subtype. Mol Neurodegener. 2013;8:25. doi: 10.1186/1750-1326-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kanwar JR, Sriramoju B, Kanwar RK. Neurological disorders and therapeutics targeted to surmount the blood–brain barrier. Int J Nanomedicine. 2012;7:3259–78. doi: 10.2147/IJN.S30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pardridge WM. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18(3):157–67. doi: 10.3109/10611860903548354. [DOI] [PubMed] [Google Scholar]
- 103.Ren X, Cui G, Zhao M, Wang C, Peng S. Coordination of thrombolytic pro-ala-lys peptides with Cu (II): leading to nanoscale self-assembly, increase of thrombolytic activity and additional vasodilation. J Phys Chem B. 2008;112(27):8174–80. doi: 10.1021/jp800645g. [DOI] [PubMed] [Google Scholar]
- 104.Marsh JN, Hu G, Scott MJ, Zhang H, Goette MJ, Gaffney PJ, et al. A fibrin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine (London) 2011;6(4):605–15. doi: 10.2217/nnm.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marsh JN, Partlow KC, Abendschein DR, Scott MJ, Lanza GM, Wickline SA. Molecular imaging with targeted perfluorocarbon nanoparticles: quantification of the concentration dependence of contrast enhancement for binding to sparse cellular epitopes. Ultrasound Med Biol. 2007;33(6):950–8. doi: 10.1016/j.ultrasmedbio.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alexandrov AV, Demchuk AM, Burgin WS, Robinson DJ, Grotta JC, CLOTBUST Investigators Ultrasound-enhanced thrombolysis for acute ischemic stroke: phase I. Findings of the CLOTBUST trial. J Neuroimaging. 2004;14(2):113–7. doi: 10.1177/1051228403261462. [DOI] [PubMed] [Google Scholar]
- 107.Alexandrov AV. Ultrasound-enhanced thrombolysis for stroke: clinical significance. Eur J Ultrasound. 2002;16(1–2):131–40. doi: 10.1016/S0929-8266(02)00040-X. [DOI] [PubMed] [Google Scholar]
- 108.Alexandrov AV. Ultrasound enhanced thrombolysis for stroke. Int J Stroke. 2005;1(1):26–9. doi: 10.1111/j.1747-4949.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 109.Eibel B, Rodrigues CG, Giusti II, Nesralla IA, Prates PR, Sant Anna RT, et al. Gene therapy for ischemic heart disease: review of clinical trials. Rev Bras Cir Cardiovasc. 2011;26(4):635–46. doi: 10.5935/1678-9741.20110056. [DOI] [PubMed] [Google Scholar]
- 110.Masumu M, Hata R. Recent advances in adenovirus-mediated gene therapy for cerebral ischemia. Curr Gene Ther. 2003;3(1):43–8. doi: 10.2174/1566523033347516. [DOI] [PubMed] [Google Scholar]
- 111.Yenari MA, Dumas TC, Sapolsky RM, Steinberg GK. Gene therapy for treatment of cerebral ischemia using defective herpes simplex viral vectors. Ann N Y Acad Sci. 2001;939:340–57. doi: 10.1111/j.1749-6632.2001.tb03643.x. [DOI] [PubMed] [Google Scholar]
- 112.Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24(2):202–11. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- 113.Soler EP, Ruiz VC. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev. 2010;6(3):138–49. doi: 10.2174/157340310791658785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.European Stroke Organisation . ESO guidelines for the management of ischaemic stroke. Basel: European Stroke Organisation; 2008. [Google Scholar]
- 115.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–212. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 116.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158(3):1062–73. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 117.Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6(1–2):69–81. doi: 10.1023/A:1009676112184. [DOI] [PubMed] [Google Scholar]
- 118.Goldstein LB. Modern medical management of acute ischemic stroke. Methodist Debakey Cardiovasc J. 2014;10(2):99–104. doi: 10.14797/mdcj-10-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnston DCC, Goldstein LB. Clinical carotid endarterectomy decision making: noninvasive vascular imaging versus angiography. Neurology. 2001;56(8):1009–15. doi: 10.1212/WNL.56.8.1009. [DOI] [PubMed] [Google Scholar]
- 120.Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713–6. doi: 10.1056/NEJMsb020987. [DOI] [PubMed] [Google Scholar]
- 121.Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25(4):338–43. doi: 10.1159/000118379. [DOI] [PubMed] [Google Scholar]
- 122.Kastrup A, Groschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, et al. Early disruption of the blood–brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39(8):2385–7. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- 123.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284(22):2901–6. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 124.Gorelick PB. The burden and management of TIA and stroke in government-funded healthcare programs. Am J Manage Care. 2009;15(6 Suppl):S177–84. [PubMed] [Google Scholar]
- 125.Power A, Epstein D, Cohen D, Bathula R, Devine J, Kar A, et al. Renal impairment reduces the efficacy of thrombolytic therapy in acute ischemic stroke. Cerebrovasc Dis. 2013;35(1):45–52. doi: 10.1159/000345071. [DOI] [PubMed] [Google Scholar]
- 126.Masrur S, Abdullah AR, Smith EE, Hidalgo R, El-Ghandour A, Rordorf G, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis. 2011;20(2):124–30. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 127.Ross OA, Worrall BB, Meschia JF. Advancing stroke therapeutics through genetic understanding. Curr Drug Targets. 2007;8(7):850–9. doi: 10.2174/138945007781077355. [DOI] [PubMed] [Google Scholar]
- 128.Robinson T, Zaheer Z, Mistri AK. Thrombolysis in acute ischaemic stroke: an update. Ther Adv Chronic Dis. 2011;2(2):119–31. doi: 10.1177/2040622310394032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood–brain barrier. Int J Nanomedicine. 2014;9:795–811. doi: 10.2147/IJN.S52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 2012;64(7):640–65. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 131.Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood–brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2(4):554–71. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke. 2008;39:1205–12. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 133.Penumbra Pivotal Stroke Trial Investigators The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–8. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 134.Pereira VM, Gralla J, Davalos A, Bonafé A, Castaño C, Chapot R, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke. Stroke. 2013;44(10):2802–7. doi: 10.1161/STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gralla J, Schroth G, Remonda L, Nedeltchev K, Slotboom J, Brekenfeld C. Mechanical thrombectomy for acute ischemic stroke, thrombus–device interaction, efficiency, and complications in vivo. Stroke. 2006;37:3019–24. doi: 10.1161/01.STR.0000248457.55493.85. [DOI] [PubMed] [Google Scholar]
- 136.Mijajlovic MD, Pavlovic A, Covickovic-Sternic N. Is sonothrombolysis an effective stroke treatment? J Ultrasound Med. 2013;32:1117–23. doi: 10.7863/ultra.32.7.1117. [DOI] [PubMed] [Google Scholar]
- 137.Rubiera M, Alexandrov AV. Sonothrombolysis in the management of acute ischemic stroke. Am J Cardiovasc Drugs. 2010;10(1):5–10. doi: 10.2165/11316850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 138.Barlinn K, Alexandrov AV. Sonothrombolysis in ischemic stroke. Curr Treat Options Neurol. 2013;15(2):91–103. doi: 10.1007/s11940-012-0214-5. [DOI] [PubMed] [Google Scholar]
- 139.Ahadi G, Welch CS, Grimm MJ, Fisher DJ, Zadicario E, Ernstrom K, et al. Transcranial sonothrombolysis using high-intensity focused ultrasound: impact of increasing output power on clot fragmentation. J Ther Ultrasound. 2013;1:22. doi: 10.1186/2050-5736-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]


