Abstract
The present study shows that roller compaction (RC) can successfully be used as a granulation method to prepare hydroxypropyl methylcellulose (HPMC)-based extended release matrix tablets containing a high drug load, both for materials deforming mainly by fragmentation (paracetamol) as for those having mainly plastic deformation (ibuprofen). The combined effect of RC process variables and composition on the manufacturability of HPMC tablets was investigated. Standard wet granulation grade HPMC was compared with a larger particle size direct compressible HPMC grade. Higher roll pressure was found to result in larger paracetamol granules and narrower granule particle size distributions, especially for formulations containing smaller size HPMC. However, for ibuprofen, no clear effect of roll pressure was observed. High roll pressure also resulted in denser ribbon and less bypass fines during RC. Loss of compactibility was observed for granules compared to powder blends, which was found to be related to differences in granule porosity and morphology. Using the large-sized HPMC grade did in some cases result in lower tensile strength tablets but had the advantage to improve the powder flow into the roller compactor. This work also indicates that when the HPMC level lies near the percolation threshold, significant changes can occur in the drug release rate due to changes in other factors (raw material characteristics and processing).
Electronic supplementary material
The online version of this article (doi:10.1208/s12249-014-0219-3) contains supplementary material, which is available to authorized users.
KEY WORDS: dry granulation, extended release, hydroxypropyl methylcellulose, roller compaction, work hardening
INTRODUCTION
Hydrophilic matrix tablets for extended drug release were first described in the mid-1960s by Lapidus and Lordi, and as can be seen from the number of publications today, it is still a relevant and widely employed technology (1–3). The advantages of these matrices are associated with their ease of manufacture and use of low-cost polymers.
Roller compaction has been employed as a dry granulation method since the 1800s, and for pharmaceutical purposes, it has been used since the 1950s (4,5). As this technology is a continuous process, scale-up and post-approval process changes are easy (6). During roller compaction, the powder is compacted between two counter-rotating rolls and milled to granules. The wetting and drying steps are eliminated making the process simpler and more suitable for heat and moisture-sensitive materials. Furthermore, water granulation of hydrophilic matrix formulations based on hydroxypropyl methylcellulose (HPMC) has been found challenging. Hard lumps and unwetted areas are formed due to the hydrophilicity of HPMC (7,8). A dry process could eliminate these problems. Compared to direct compression, granulation with roller compaction will also improve the mixture uniformity of drug- and gel-forming polymers (9).
A limited number of studies have been published evaluating the suitability of dry granulation as the granulation method in the manufacture of HPMC-based hydrophilic matrix tablets (10–12). Hariharan et al. studied the effects of formulation variables of diclofenac and HPMC-based matrix tablets prepared by roller compaction (12). Sheskey and Hendren compared HPMC-based hydrophilic matrix tablets made from dry granulated particles to ones produced via high-shear wet granulation or direct compression (10). It was found that the interplay between the feeder speed and applied pressure affected the density and compactibility of the granulated material. The granule densities were found to decrease with increasing amounts of HPMC in the formulation, and in line with the results of Hariharan et al., tablets with lower tensile strength were obtained (12). The strongest tablets were prepared by direct compaction followed by roller compaction and high-shear granulation (10). Finally, one of the main challenges in using dry granulation is the loss of compactibility during double compacting (13). Two reasons have been identified as the cause: (i) particle size enlargement and (ii) a phenomenon called work hardening (14). This effect has been found especially significant for plastically deforming materials including HPMC as well as drug substances. Therefore, it is important to investigate the combined effect of process and formulation parameters.
The aims of the present study were to investigate the suitability of roller compaction as granulation method for HPMC-based hydrophilic matrix tablets and to deduce combined effects of the preparation method and raw material variables on tablet properties and manufacturability. In the present study, high drug load HPMC-based hydrophilic matrix tablets were produced using two model active substances, paracetamol, which deforms mainly by a fragmenting process, and ibuprofen, which is more plastic than brittle (15–18). The roll pressure and the ratio between the feeder screw speed and the roll speed were the process variables chosen in the present study. The influence of these parameters and particle size of APIs and HPMC on the manufacturability, i.e. the granule flow properties and tablet physical properties, were investigated. A novel larger particle size DC quality of HPMC was used and compared with a standard grade HPMC sample. To our knowledge, the combined effect of both API and HPMC particle size as well as process parameters on the roller compacted granules and tablet performance has not been previously investigated.
MATERIALS AND METHODS
Raw Materials
The model active substances in the study were paracetamol (Sri Krishna pharmaceuticals, Hyderabad, India) and ibuprofen (IOL chemicals and pharmaceuticals limited, Punjab, India). Two different particle size grades of HPMC USP type 2208 (Dow Chemical company, Midland, USA) were used as matrix formers, standard grade Methocel K100LV Premium (HPMC-S) and Methocel K100LV Premium DC Gen I (HPMC-DC) having a larger particle size. Mannitol (Parteck M200, Merck GaA, Darmstadt, Germany) was added as a soluble filler and sodium stearyl fumarate (PRUV, Moehs, Barcelona, Spain) as a lubricant.
Composition of Tested Formulations
Pre-testing of different formulations was conducted to find compositions that were possible to roller compact into ribbons. The compositions used in the study were 30% API (paracetamol/ibuprofen), 32% HPMC-S or HPMC-DC, 35% mannitol and 3% sodium stearyl fumarate (2% added to the powder blend before roller compaction and 1% intergranularly). The center point blend for the HPMC particle size was made by mixing HPMC-S or HPMC-DC in ratio 50:50. Sieving was used to obtain ibuprofen particles of different sizes, and for the centre point blend, the original unsieved ibuprofen was used.
Design of Experiments
The software program MODDE 9 (Umetrics, Umeå, Sweden) was employed for design of experiments (DoE) and statistical analysis of the results (19). Responses probing relevant changes in, e.g. powder flow and compactibility, were identified. All the experiments, factor combinations and three centre points were carried out in a randomized order. The first design of experiments (DoE I) was based on the paracetamol formulations (Table I). Two grades of HPMC with different particle size were used. The design of experiment II (DoE II) was based on the ibuprofen formulations. Two formulation variables, the particle size of the ibuprofen (API) and HPMC, were varied (Table I). The formulation variables were combined with process variables such as the ratio between the feeder screw and the roll speed and roll pressure. Multiple linear regression (MLR) was used for analyzing the multi-dimensional process data and building multivariate correlations.
Table I.
Design of Experiments I and II
| Design of experiment I | Design of experiment II | ||||||
|---|---|---|---|---|---|---|---|
| Experiment no. | Rat | Rol (MPa) | HPMC d50 (μm) | Experiment no. | HPMC d50 (μm) | Rol (MPa) | API d50 (μm) |
| P1 | 6 | 3 | 71 | I1 | 71 | 3 | 15 |
| P2 | 10 | 3 | 71 | I2 | 177 | 3 | 15 |
| P3 | 6 | 8 | 71 | I3 | 71 | 8 | 15 |
| P4 | 10 | 8 | 71 | I4 | 177 | 8 | 15 |
| P5 | 6 | 3 | 177 | I5 | 71 | 3 | 37 |
| P6 | 10 | 3 | 177 | I6 | 177 | 3 | 37 |
| P7 | 6 | 8 | 177 | I7 | 71 | 8 | 37 |
| P8 | 10 | 8 | 177 | I8 | 177 | 8 | 37 |
| P9 | 8 | 5.5 | 124 | I9 | 124 | 5.5 | 21 |
| P10 | 8 | 5.5 | 124 | I10 | 124 | 5.5 | 21 |
| P11 | 8 | 5.5 | 124 | I11 | 124 | 5.5 | 21 |
Rat ratio between feeder screw speed and roll speed, Rol roll pressure, P paracetamol and I ibuprofen
Powder Blend Preparation, Roller Compaction and Tableting
The powder blend components were weighed and mixed in a diffusion blender (Turbula T10B/ T2F, Willy A. Bachofen Ag, Basel, Switzerland) for 10 min at 32–34 rpm, after which 2% lubricant was added and the blend mixed for another 5 min. The API and lubricant were sieved prior to mixing through mesh size of 1.120 and 0.710 mm, respectively. The remaining 1% of lubricant was added intergranularly and blended for 2 min at 34 rpm. To prepare different size fractions of ibuprofen, bulk ibuprofen was sieved using mesh sizes of 125 and 150 μm (Retsch AS 200, Haan, Germany).
Roller compaction was performed using a bench top roll compaction system, Vector TFC-LABO (Freund Vector, Marion, USA). Horizontally mounted concave and convex rolls with a fluted roll surface design were selected in order to have a better gripping power of the powder. Roll dimensions were 50 mm in diameter and 14 mm in width. In line with Teng et al. pre-tests that were performed, the ratio between feeder screw speed and roll speed was varied between 6 and 10 and roll pressure between 3 and 8 MPa (20). In DoE I (Table I), the roll velocity was kept at 2.5 rpm, while the velocity of the vertically mounted screw feeder was varied between 15 and 25 rpm. The compacted ribbons were milled into granules in the vector mill with screen size 965 μm and speed 143 rpm. In DoE II, the ratio between feeder screw speed and roll velocity was kept constant, and the roll pressure was varied (Table I). All starting materials and produced granules were kept in closed plastic containers. All roller compaction trials were performed in normal room relative humidity and lasted no longer than 2 h; hence, exposure to air humidity was consistently at a minimum level. For compactibility studies, a single punch tablet press, Korsch EK0 (Korsch Pressen GmbH, Berlin, Germany), with 8-mm punches was used. Tablet weight was set to 200 mg.
Particle Size Distribution and Morphology
Particle size distribution of the two HPMC grades, APIs and ibuprofen sieve fractions, powder blends and granules was characterized by laser diffraction using a particle size analyzer (Mastersizer 2000 v.5.6, Malvern Instruments Ltd, Worcestershire, UK). The disperser pressure was set to 0–0.2 bar for paracetamol, mannitol, HPMC, powder blends and granules, and for ibuprofen, both 0 and 3 bar were used. The 10, 50 and 90% quantiles were determined. Scanning electron microscope, SEM, (Quanta 200, FEI and Quanta 250, FEI, Quorum Technologies USA/UK) was used to examine the morphology and porosity of the starting materials and granules. The samples were first sputtered with gold or platinum to form a thin conductive layer on top of the particles.
Powder Flow Characterization
Two values indicating the flow properties of powders and granules were investigated, the modified Hausner ratio (mHR) and air permeability of powder. The mHR is calculated from compressed bulk density (CBD) and poured bulk density (PBD) according to Eq. 1 (21).
| 1 |
The values for CBD are higher than the tapped density normally used for Hausner ratio calculations giving somewhat higher values for the mHR compared to the Hausner ratio according to Pharmacopoeia. The permeability of powder and granules was measured using a powder rheometer (FT4, Freeman Technology, Worchestershire, UK). A powder sample of 10–15 ml was introduced into a glass vessel with a split cell. Pre-conditioning was done with a rotating blade in order to have a standardized initial packing condition for all samples. During the permeability test, air was passed up through the vessel at a constant flow rate of 2 mm/s, at the same time as a gradually increasing normal stress of 1–15 kPa was applied on the powder. The air pressure drop across the powder bed, the resistance to air flow through the powder, was measured at each applied normal stress.
Apparent Density and Solid Fraction
Apparent (envelope) density of the ribbons and tablets was characterized using GeoPyc 1360 (Micromeritics, USA). The compaction force was set at 51 N, number of cycles was three, cylinder diameter was 25.4 mm and the sample amount was around 2 g. The solid fraction (SF, relative density) of blends, ribbon and tablets was determined using Eq. 2.
| 2 |
Here, ρapp is the apparent density and ρtrue is the true density as derived from literature (22–24).
Compactibility and Tablet Friability
To obtain information about the relationship between compaction pressure and tablet hardness, tablets pressed at different compaction pressures were tested for their weight, height and breaking force. Tablet compaction pressure (P) and the radial tensile strength (σ t) were calculated according to Pitt et al. and Fell and Newton (25,26). A tablet hardness tester (C50 Holland, Engineering systems, England) was used to measure the radial tablet breaking force. A set of ten tablets from each batch were tested with MultiCheck (Erweka GTB, Pretech Instruments, Sollentuna, Sweden) for tablet weight and thickness. To supplement tablet hardness tests, friability was measured according to USP methodology using a friability tester (Pharmatest, Hainburg, Germany).
RESULTS AND DISCUSSION
Raw Material Characterization
The particle size distributions of the two APIs (including sieved ibuprofen samples), the two HPMC grades, paracetamol and mannitol used in the study were characterized and are summarized in Table II.
Table II.
Particle Size Distribution Measured by Laser Diffraction
| Material | d (0.1) μm | d (0.5) μm | d (0.9) μm |
|---|---|---|---|
| Paracetamol* | 8.0 | 35 | 105 |
| Ibuprofen | 3**(39*) | 28**(136*) | 137** |
| Ibuprofen 125–150 μm** | 2 | 21 | 93 |
| Ibuprofen >150 μm** | 4 | 37 | 169 |
| Mannitol*
(Souihi et al. 2013) (27) |
68.7 | 211 | 522 |
| HPMC small* | 25 | 71 | 184 |
| HPMC large* | 54 | 177 | 429 |
*At 0–0.2 bar
**At 3 bar
Scanning electron micrographs of the two HPMC grades show that both comprise of a mixture of fibrous and irregularly shaped flat particles, Fig. 1, with the notable difference that HPMC-DC contained loosely held agglomerates of these primary particles.
Fig. 1.
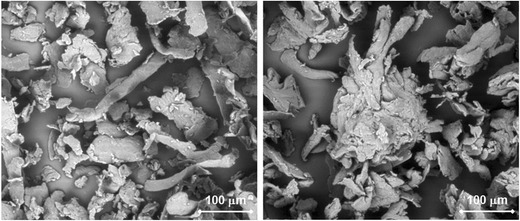
Scanning electron micrographs of HPMC-S(left) and HPMC-DC(right). Measuring bar is 100 μm
Roller Compaction of Paracetamol Compositions
An interesting observation made during roller compaction was that most of produced ribbons had intermittent branching and splitting. The splitting was prominent especially when the roll pressure and ratio between feeder and roll speed were set high. The higher pressure occurring in the middle part of the rolls led to a denser state compared to the edges. The uneven tensile strength might make the particle bonding weaker, which results in splitting after the pressure is released.
Paracetamol Powder Blend and Granule Particle Size Distribution
Dry granulation by roller compaction increased the particle size of the powder blends (Table III). Larger sized granules were obtained using HPMC-S compared to those obtained using HPMC-DC (i.e. higher d50 and d90 in most cases). Herting and Kleinebudde made a similar observation using small-sized raw material (microcrystalline cellulose PH105) (28). However, they observed that when the same sieve fractions of granules were compacted, granules prepared using smaller particles as raw material resulted in tablets with higher tensile strength. This was assumed to be because of the larger area available for binding.
Table III.
Particle Size Distribution (d 0.1, d 0.5 and d 0.9) of Paracetamol Powder Blends (Small, Large and Centre) and Granules (P1-P11) Measured by Laser Diffraction at 0 to 0.1 Bar
| Rol (MPa) | Rat | d0.1(μm) | d0.5(μm) | d0.9(μm) | |
|---|---|---|---|---|---|
| Blend HPMC small | na | na | 16 | 73 | 349 |
| P1 | 3 | 6 | 15 | 153 | 913 |
| P2 | 3 | 10 | 16 | 158 | 870 |
| P3 | 8 | 6 | 22 | 297 | 1,138 |
| P4 | 8 | 10 | 22 | 376 | 1,202 |
| Blend HPMC large | na | na | 18 | 118 | 376 |
| P5 | 3 | 6 | 15 | 162 | 664 |
| P6 | 3 | 10 | 15 | 202 | 881 |
| P7 | 8 | 6 | 19 | 193 | 875 |
| P8 | 8 | 10 | 22 | 282 | 1,044 |
| Blend centre point | na | na | 17 | 98 | 511 |
| P9 (CP) | 6 | 8 | 21 | 288 | 1130 |
| P10 (CP) | 6 | 8 | 19 | 258 | 1031 |
| P11 (CP) | 6 | 8 | 21 | 288 | 1079 |
na not applicable, CP centre point
In most cases, the particle size of granules increased, when roller compacted at higher roll pressure, Fig. 2 and Table III. These results indicate that in terms of particle size enlargement, the dry granulation process is more effective at high roll pressures, producing size distributions more centered around a higher particle size (Fig. 2a, b). Granules based on HPMC-DC did not have as clear difference in particle size (d10, d50, d90) and distribution to corresponding powder blends as did the granules based on HPMC-S (Fig. 2b). This is most likely because the particle size of the initial blend was larger but also since part of the large size HPMC that consists of agglomerates (see Fig. 1) might have been broken down during roller compaction. The ratio between feeder screw and roll speed had no significant effect on the particle size distribution based on the MLR analysis.
Fig. 2.
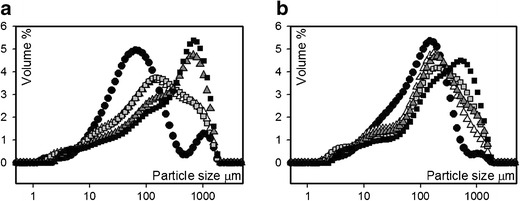
a Particle size distribution of powder blend (black circle) and granules (p1 (white triangle), p2 (grey white square), p3 (grey white triangle) and p4(black square)) based on HPMC-S. b Particle size distribution of powder blend (black circle) and granules (p5 (white triangle), p6 (grey white square), p7 (grey white triangle) and p8(black square)) based on HPMC-DC
Paracetamol Powder Blend and Granule Flow Properties
According to statistical MLR analysis, roll pressure, HPMC particle size and ratio between feeder screw and roll speed all had a significant effect on granule flow properties, Fig. 3. Higher air permeability (indicating decreased cohesivity and increased flowability (29)) was observed with increasing roll pressure and feeder screw/roll speed ratio. Further, increasing HPMC particle size also showed similar trend in the air permeability measurements. However, according to the Hausner ratio classification, all of the powder blends (mHR = 1.76 to 1.85) and granules (mHR = 1.58 to 1.64) would be classified as very poorly flowing or very very poorly flowing. The suitability of this test to poorly flowable powders and granules such as those based on paracetamol can be questioned since this method was not able to differentiate between the granules in the same way as the permeability test. Also, flow rate through an orifice was found unsuitable for characterizing paracetamol blends and powders as this test is more applicable mainly to more freely flowing to more freely flowable materials. In this respect, the results indicate that the less cohesive granules (P5, P7 and P8) also flew better in the funnel. Finally, it is important to note that, according to the permeability test and as expected, the flow properties of powder blends containing HPMC-DC were significantly better than those of HPMC-S. This was also clearly seen during the roller compaction process as this material exhibited more even and flowability into the feeder of the instrument.
Fig. 3.
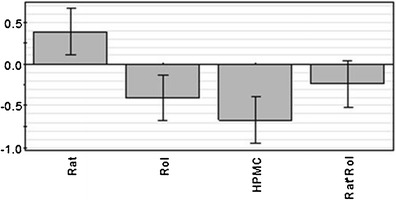
Coefficient plot of permeability test (pressure drop at 15 kPa); R2 = 0.914, Q2 = 0.775
Apparent Densities and Solid Fraction of Paracetamol Powders, Ribbons and Granules
The calculated true density values were used to calculate the solid fraction of each material, see Tables IV and V (9). Envelope density measured using the fluid-displacement technique was used as the apparent density for ribbons and tablets. The apparent densities for powder blends and granules were taken as the conditioned and compressed bulk densities measured by powder rheometer. The ribbon envelope density and solid fraction increased as the roll pressure or ratio between feeder and roll speed was increased. Denser ribbon produced also somewhat larger granules with larger solid fraction. These effects of roller compactor parameters were also statistically significant according to MLR analysis. This result is in accordance with literature, e. g. denser ribbon has been related to increased granule size (28).
Table IV.
Apparent Density (ρapp) and Solid Fraction (SF) of Powder Blends and Direct Compressed Paracetamol Tablets
| Experiment | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF |
|---|---|---|---|---|---|---|
| Powder (conditioned) | Powder (compressed) | Tablets | ||||
| HPMC-S | 0.39 | 0.28 | 0.52 | 0.38 | 1.16 | 0.85 |
| HPMC-DC | 0.35 | 0.25 | 0.47 | 0.34 | 1.18 | 0.86 |
| Centre point | 0.37 | 0.27 | 0.50 | 0.36 | 1.15 | 0.84 |
Table V.
Apparent Density (ρapp) and Solid Fraction (SF) of Ribbon, Granules and Roller Compacted Tablets (Paracetamol)
| Exp | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF |
|---|---|---|---|---|---|---|---|---|
| ribbons | granules (conditioned) | granules (compressed) | tablets (RC) | |||||
| P1 | 0.97 | 0.70 | 0.46 | 0.34 | 0.61 | 0.45 | 1.20 | 0.87 |
| P2 | 0.98 | 0.71 | 0.45 | 0.33 | 0.60 | 0.44 | 1.21 | 0.88 |
| P3 | 1.02 | 0.74 | 0.50 | 0.36 | 0.64 | 0.46 | 1.23 | 0.89 |
| P4 | 1.13 | 0.82 | 0.50 | 0.36 | 0.65 | 0.47 | 1.22 | 0.89 |
| P5 | 0.98 | 0.71 | 0.45 | 0.33 | 0.59 | 0.43 | 1.21 | 0.88 |
| P6 | 1.00 | 0.73 | 0.47 | 0.34 | 0.62 | 0.45 | 1.22 | 0.89 |
| P7 | 1.00 | 0.73 | 0.44 | 0.32 | 0.57 | 0.41 | 1.20 | 0.87 |
| P8 | 1.07 | 0.78 | 0.51 | 0.37 | 0.64 | 0.47 | 1.22 | 0.89 |
| P9 | 1.04 | 0.75 | 0.48 | 0.35 | 0.63 | 0.46 | 1.20 | 0.87 |
| P10 | 1.01 | 0.73 | 0.49 | 0.36 | 0.64 | 0.46 | 1.22 | 0.89 |
| P11 | 1.05 | 0.76 | 0.48 | 0.35 | 0.62 | 0.45 | 1.24 | 0.90 |
Compactibility of Paracetamol Compositions
The pressure curves for tablets containing paracetamol are shown in Fig. 4a, b. Tablets compacted from granules show relatively low tensile strengths (<2 MPa) at compaction pressures of 200 to 300 MPa, whereas the primary powder blends produced harder tablets. Most tablets produced had an acceptable friability (all except P4 gave <1%). This loss in compactibility for plastic materials during double compaction has been argued not only to be due to the size enlargement during granulation but also to be linked to the work hardening of the material, since the resistance to further plastic deformation is increased (28). The loss of compactibility seems greater in the case of compositions containing HPMC-S. This is likely due to the fact that RC increased the particle size of these compositions to a greater extent compared to those containing HPMC-DC, see Table III. On the other hand, the direct compressed compositions containing HPMC-S gave tablets with significantly higher tensile strengths than all roller compacted material and the directly compressed compositions containing HPMC-DC (30). This observation is in accordance with that of Nokhodchi et al. who found that for direct compressed tablets, a decrease in HPMC particle size results in decreased elastic recovery associated with an increased compact tensile strength (31). At the same compaction pressure, all roller compacted tablets were found to have quite similar solid fraction and apparent density independently of processing. The direct compressed tablets were found to be only slightly less dense, see Tables IV and V.
Fig. 4.
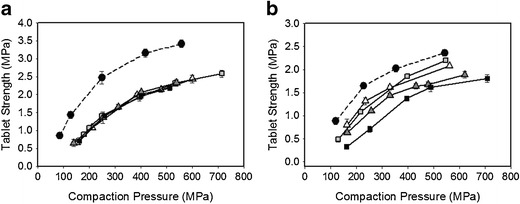
a Average tablet tensile strength (n = 5) and standard deviation at increasing compaction pressures for paracetamol powder blends (black circle) and granules (p1 (white triangle), p2 (grey white square),p3 (grey white triangle) and p4(black square)) based on HPMC-S. b Average tablet tensile strength (n = 5) and standard deviation at increasing compaction pressures for paracetamol powder blends (black circle) and granules (p5 (white triangle), p6 (grey white square), p7 (grey white triangle) and p8(black square)) based on HPMC-DC
No considerable difference in compaction properties is seen between the granules made with HPMC-S using different roller compactor parameters. The formulations containing HPMC-DC were more sensitive to changes in the roller compactor parameters. Here, granulation at high roll pressure produced tablets with significantly lower tensile strength. This may be due to that denser granules were produced at high roll pressure resulting in loss of compactibility.
Increased porosity of granules has been related to increase in compact strength (32). Compaction of granulated material will lead to a pore structure with intragranular and intergranular pores. In this study, roller compaction at high roll pressure produced denser ribbon and granules. Granules compacted at high pressures, e.g. with lower fragmentation propensity, have been found to have relatively larger intergranular pores and to give lower strength tablets (33). When granules with higher fragmentation propensity are compacted, the intergranular pore size is reduced, and the pore size distribution of the system becomes unimodal. This results in harder tablets. Pore structure could therefore explain the higher tensile strength of tablets of granules compacted at lower roll pressure, e.g. lower granule density gave harder tablets, see Table V for ribbon densities of P1, P2, P5 and P6.
The granule morphology may also affect the compression properties (32). Spherical granules may have smaller intergranular voids than irregular granules, leading to weaker compacts. The surface morphology affects the compaction properties. Granules with smooth surfaces have smaller specific surface area and therefore less binding points. Roller compacted granules have been found to have rather smooth surfaces compared to those of wet granulated (10). Figure 5a–d shows SEM images of granules made at the extremes of DoE I, e.g. L-Rol/L-Rat and H-Rol/H-Rat for both HPMC particle sizes. The SEM images confirm the results from laser diffraction showing a clear increase in particle size with increased roll pressure as well as ratio between the feeder screw and the roll speed. The roller compacted granulate P8, which had the poorest compaction properties, was observed to contain a larger amount of dense particles with smooth surfaces compared to granules containing HPMC-S (Fig. 5b, d).
Fig. 5.
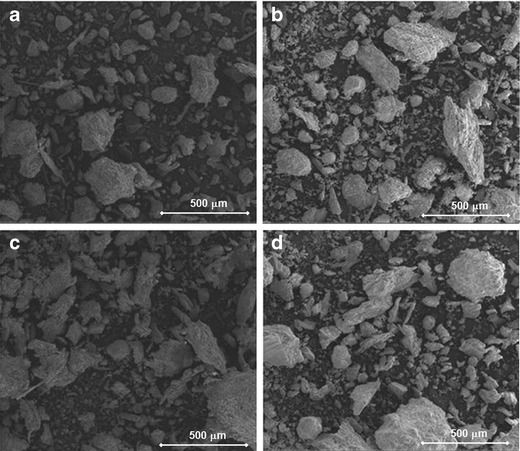
a–d SEM images of paracetamol granules (P1, P4, P5 and P8). a P1 (HPMC-S and L-Rol/L-Rat). b P4 (HPMC-S and H-Rol/H-Rat). c P5 (HPMC-DC and L-Rol/L-Rat). d P8 (HPMC-DC and H-Rol/H-Rat)
In this study, all the granulated HPMC-S formulations produced tablets with equal or higher tensile strengths compared to HPMC-DC formulations. Only the HPMC-DC formulation roller compacted at low roll pressures had tensile strengths approaching that of granules with HPMC-S. It could be seen that the low roll pressure is just enough to break down the larger agglomerated HPMC particles to smaller primary particles. At high roll pressures, however, HPMC-DC particles exhibit higher elastic recovery, which results in compacts of lower tensile strength. It has also been suggested in other studies that even after roller compaction small particles have more binding points and can form stronger tablets compared to large-sized raw material (28).
Roller Compaction of Ibuprofen Compositions
In general, the roller compaction processing of ibuprofen composition was more sensitive to changes in the processing parameters than the corresponding paracetamol composition. Higher roll speeds resulted in ribbons that crumbled. This is a common seen feature for plastically deforming materials, where the particle bonding is depended on the dwell time at the nip area (34). Further, the ribbon achieved was not continuous and the material stuck to the rolls due to the adhesiveness of the powder blend. Roller compaction of pure ibuprofen with only lubricant resulted in a ribbon that was very adhesive to the equipment and the resulting material contained as much as 15% of granulation bypass fines.
Ibuprofen Powder Blend and Granule Particle Size Distribution and Shape
Roller compaction of the highly deformable ibuprofen produced granules with only a slight increase in particle size, see Table VI. The granules containing HPMC-DC had a slightly larger mean particle size and narrower size distribution than the respective granules made with HPMC-S. This is likely caused by the fact that the granule blend is a mixture of granules and unagglomerated material, e.g. size of unagglomerated particles is larger. Roller compaction also made the particle size distribution narrower for the powder blends consisting of HPMC-S, blends 1 and 2. However, the opposite was observed for the HPMC-DC, powder blends 3 and 4. The size distribution was broader after dry granulation. This is because the initial particle size distribution was more even to begin with and because of the formation of bypass fines during roller compaction. Unlike the paracetamol compositions (DoE I), no clear effect of roll pressure on the granule size could be observed. This might be related to the deformation properties and better elastic recovery of ibuprofen (17). The particle size distribution was, however, narrower for granules compacted at high roll pressure, an observation similar to that made in DoE I. The SEM pictures show that the granules are irregularly shaped and have more coarse surfaces, which makes the intergranular voids and specific surface area larger, Fig. 6a, b. A large number of binding points is known to contribute to a better compactibility.
Table VI.
Particle Size Distribution (d 0.1, d 0.5 and d 0.9) of Ibuprofen Powder Blends (1–5) and Granules (I1-I11) Measured by Laser Diffraction at 0.1 and 0 Bar, Respectively
| Ibuprofen | HPMC | Rol (MPa) | d0.1(μm) | d0.5(μm) | d0.9(μm) | |
|---|---|---|---|---|---|---|
| Blend 1 | small | small | na | 24 | 99 | 774 |
| I1 | 3 | 20 | 137 | 805 | ||
| I3 | 8 | 19 | 119 | 643 | ||
| Blend 2 | small | large | na | 32 | 146 | 730 |
| I2 | 3 | 22 | 207 | 937 | ||
| I4 | 8 | 29 | 318 | 1089 | ||
| Blend 3 | large | small | na | 27 | 121 | 422 |
| I5 | 3 | 21 | 151 | 849 | ||
| I7 | 8 | 24 | 212 | 915 | ||
| Blend 4 | large | large | na | 44 | 183 | 645 |
| I6 | 3 | 25 | 226 | 1067 | ||
| I8 | 8 | 24 | 196 | 817 | ||
| Blend 5 | CP | CP | na | 31 | 140 | 920 |
| I9 | 5.5 | 22 | 185 | 817 | ||
| I10 | 5.5 | 20 | 129 | 860 | ||
| I11 | 5.5 | 22 | 174 | 917 |
na not applicable, CP centre point
Fig. 6.
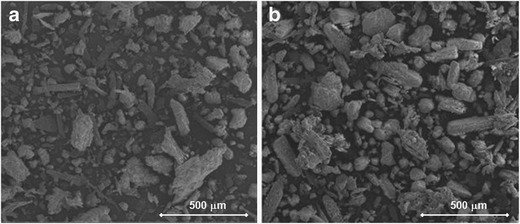
a, b SEM images of ibuprofen granules (I3 and I4). a I3 (HPMC-S/Ibuprofen and H-Rol). b I4 (HPMC-DC/Ibuprofen and H-Rol)
Flow Properties of Ibuprofen Powder Blends and Granules
In general, the ibuprofen granules and powder blends had lower air permeability (indicating better flowability) than the paracetamol granules. This was seen to an even a higher extent in the powder blends as larger particle sizes of ibuprofen and HPMC were used in these compositions. This observation is consistent with the results in DoE I. Small particles are able to pack tighter together and resist the airflow to a greater extent which is normally related to poor flow. As for the paracetamol compositions, this suggests advantages of using HPMC-DC grade to enhance the powder flow into the roller compactor. Ibuprofen granules that were roller compacted at high roll pressure had lower permeability indicating better flow. This contradicts the observations of Weyenberg et al. who showed that granules based on plastically deforming material produced at the high roll speed and with low compaction force had the best flow properties (35). Roller compaction increased the bulk density and decreased the values for mHR indicating enhanced flow properties by granulation (mHR = 1.18 to 1.21 for granules). The formulations containing with large-sized HPMC and API had the lowest values. Ibuprofen granules had somewhat lower values for the permeability and the mHR than the paracetamol granules of same composition, and their flow could be classified as good or fair in the common classification system. The modified Hauner ratio gave a slightly better discrimination of the ibuprofen granules than what was obtained for the paracetamol granules. This is due to the fact that the flow of the ibuprofen granules generally is better than that of the paracetamol granules.
Apparent Densities and Solid Fraction of Ibuprofen Powders, Ribbons, Granules and Tablets
The true densities for ibuprofen powder blends and granules were 1.32 g/cm3 as calculated from literature (22,24). The measured apparent densities were used to calculate the solid fraction of the powder blends, granules, ribbons and tablets, see Tables VII and VIII. The solid fraction increases during the process from powder to granules and tablets. The ribbon envelope densities were significantly higher when the higher roll pressure of 8 MPa was applied during roller compaction, which was also noted in DoE I. No significant difference, however, could be seen in the solid fraction of the different granule blends.
Table VII.
Apparent Density (ρapp) and Solid Fraction (SF) of Powder Blends and Direct Compressed Tablets (Ibuprofen)
| Experiment | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF |
|---|---|---|---|---|---|---|
| Powder (conditioned) | Powder (compressed) | Tablets | ||||
| Blend 1 | 0.43 | 0.32 | 0.53 | 0.40 | 1.10 | 0.83 |
| Blend 2 | 0.36 | 0.27 | 0.45 | 0.34 | 1.13 | 0.85 |
| Blend 3 | 0.45 | 0.34 | 0.55 | 0.42 | 1.11 | 0.84 |
| Blend 4 | 0.36 | 0.27 | 0.44 | 0.33 | 1.13 | 0.85 |
Table VIII.
Apparent Density (ρapp) and Solid Fraction (SF) of Ribbon, Granules and Roller Compacted Tablets (Ibuprofen)
| Exp | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF | ρapp (g/cm3) | SF |
|---|---|---|---|---|---|---|---|---|
| ribbons | granules (conditioned) | granules (compressed) | tablets (RC) | |||||
| I1 | 0.91 | 0.69 | 0.46 | 0.35 | 0.56 | 0.42 | 1.14 | 0.86 |
| I2 | 0.93 | 0.70 | 0.47 | 0.36 | 0.56 | 0.42 | 1.16 | 0.88 |
| I3 | 0.97 | 0.73 | 0.47 | 0.35 | 0.56 | 0.42 | 1.17 | 0.89 |
| I4 | 0.94 | 0.71 | 0.49 | 0.37 | 0.58 | 0.44 | 1.16 | 0.88 |
| I5 | 0.90 | 0.68 | 0.47 | 0.35 | 0.56 | 0.42 | 1.17 | 0.89 |
| I6 | 0.90 | 0.68 | 0.48 | 0.36 | 0.56 | 0.43 | 1.12 | 0.85 |
| I7 | 0.95 | 0.72 | 0.48 | 0.36 | 0.57 | 0.43 | 1.12 | 0.85 |
| I8 | 0.92 | 0.70 | 0.47 | 0.35 | 0.55 | 0.42 | 1.08 | 0.82 |
| I9 | 0.95 | 0.72 | 0.48 | 0.36 | 0.57 | 0.43 | 1.11 | 0.84 |
| I10 | 0.91 | 0.69 | 0.44 | 0.33 | 0.53 | 0.40 | 1.14 | 0.86 |
| I11 | 0.93 | 0.70 | 0.47 | 0.35 | 0.56 | 0.42 | 1.21 | 0.92 |
Compactibility of Ibuprofen Compositions
The tablet tensile strength at different compaction forces is presented in Fig. 7a, b. It is noticeable that the loss of compactibility is not as great as in DoE I. Two ibuprofen granules, I1 and I2, reach the same tablet tensile strength as the powder blends at compaction pressures of around 200 MPa. This observation differs from the expected. Ibuprofen deforms mainly plastically, and it would be expected to be more susceptible to the phenomenon of work hardening (4,17). One reason for the observed compaction behaviour might be that the difference in particle size distribution between the initial powder blend and granules was not as great as in DoE I (30). The granulation of ibuprofen compositions did not produce as many large particles. Therefore, the small-sized particles could compact to the same extent as the powder blend. Porous materials are known to compact better (32,36). Ibuprofen granules (compressed) were somewhat more porous compared to the paracetamol granules, which would contribute to a better compactibility. Using small ibuprofen particles, granules compacted at low roll pressure produced tablets with higher tensile strengths comparable to that of powder blends, Fig. 7a–d. For higher roll pressure on the other hand, a small reduction of tensile strength could be observed. This is slightly unexpected as it could not clearly be associated to granules of larger size and lower porosity being produced at high roll pressure (compare Tables VI and VIII). On the other hand, one factor could dominate for one blend (I3 ribbons have lower porosity than I1) and another for the other blend (I4 granules are larger than I1). For blends based on large particle sized ibuprofen (blends 3 and 4), both roll pressures reduce compactibility to the same extent.
Fig. 7.
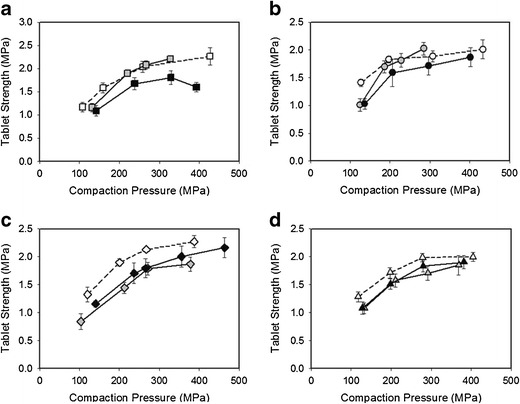
a–d Average tablet tensile strength (n = 5) and standard deviation at increasing compaction pressures for ibuprofen powder blends (white) and granules (I1/I2/I5/I6 (grey) and I3/I4/I7/I8 (black)) based on HPMC and ibuprofen (a), large-sized HPMC and small-sized ibuprofen (b), small-sized HPMC and large-sized ibuprofen (c) and large-sized HPMC and large-sized ibuprofen (d)
Release of Paracetamol and Ibuprofen ER Matrix Compositions
Tablet dissolution testing indicates that, when directly compressed, the HPMC-DC particles were unable to percolate through the tablet and form a consistent network at contents of 22 to 32% (w/w) (Supplementary 1). Roller compaction helped to break down the large HPMC agglomerates and distribute them more evenly within the tablets and gave robust compositions at 32% HPMC content but not at 22%. This result indicates that when the HPMC level lies near the percolation threshold, significant changes can occur in the drug release rate due to changes in other factors (raw material characteristics and processing).
CONCLUSIONS
The present work shows that roller compaction can successfully be used as a granulation method to overcome the difficulties associated with water granulation of HPMC based compositions. The combined effect of API and HPMC particle size on the roller compacted hydrophilic matrix granules and tablet performance has been investigated and show clearly that tablet with acceptable tensile strength can be manufactured at high load of both brittle and plastically deformable drug substances. Increase in roll pressure was found to result in larger and denser granules with improved flow properties. For compositions of the more brittle drug substance (paracetamol), a significant loss of compactibility was observed after roller compaction. However, for compositions of the more plastically deforming drug substance (ibuprofen), the granule size distributions were broad also after roller compaction leading to a less pronounced loss in compactibility. In general, the loss in compactibility was larger at higher roll pressure. Large-sized HPMC particles generally resulted in slightly lower, but still acceptable, tensile strengths. However, an advantage of the novel large-sized HPMC grade was the improvement of the powder flow into the roller compactor with potential for better weight variation and content uniformity. This work also indicates that when the HPMC level lies near the percolation threshold, significant changes can occur in the drug release rate due to changes in other factors (raw material characteristics and processing). The large-sized HPMC particles were found unable to percolate through the tablet to form a consistent network at contents of ≤32% (w/w) and to provide a robust release behaviour for the direct compressed pacacetamol and ibuprofen tablet compostions of the present study.
Electronic supplementary material
(DOCX 134 kb)
ACKNOWLEDGMENTS
We acknowledge Lars Johnson (AZ) for his assistance with the permeability tests, Mervi Lindman (University of Helsinki) for taking SEM images of granules and Pirjo Tajarobi (AZ) for providing particle size data for paracetamol. HPMC DC Gen I was kindly supplied by Dow Pharma and Food Solutions.
REFERENCES
- 1.Lapidus H, Lordi NG. Some factors affecting the release of a water-soluble drug from a compressed hydrophilic matrix. J Pharm Sci. 1966;55:840–3. doi: 10.1002/jps.2600550818. [DOI] [PubMed] [Google Scholar]
- 2.Alderman DA. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int J Pharm Technol Prod. 1984;5:1–9. [Google Scholar]
- 3.Larsson A, Abrahmsén-Alami S, Juppo A. Oral extended-release formulations. In: Gad S C, editors. Pharmaceutical manufacturing handbook: production and processes. John Wiley & Sons Inc; 2008. p. 1191–222.
- 4.Kleinebudde P. Roll compaction/dry granulation: pharmaceutical applications. Eur J Pharm Biopharm. 2004;58:317–26. doi: 10.1016/j.ejpb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Augsburger LL, Vuppala MK. In: Theory of granulation. Book: handbook of pharmaceutical granulation technology, 2nd ed. Parikh DM, editor. New York: Marcel Dekker Inc; 1997. pp. 7–24. [Google Scholar]
- 6.Leuenberger H. New trends in the production of pharmaceutical granules: batch versus continuous processing. Eur J Pharm Biopharm. 2001;52:289–96. doi: 10.1016/S0939-6411(01)00199-0. [DOI] [PubMed] [Google Scholar]
- 7.Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol. 2005;57:533–46. doi: 10.1211/0022357055957. [DOI] [PubMed] [Google Scholar]
- 8.Herder J, Adolfsson Å, Larsson A. Initial studies of water granulation of eight grades of hypromellose (HPMC) Int J Pharm. 2006;313:57–65. doi: 10.1016/j.ijpharm.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Am Ende MT, Moses SK, Carella AJ, Gadkari RA, Graul TW, Otano AL. Improving the content uniformity of a low-dose tablet formulation through roller compaction optimization. Pharm Dev Technol. 2007;12:391–404. doi: 10.1080/10837450701369253. [DOI] [PubMed] [Google Scholar]
- 10.Sheskey PJ, Hendren J. The effects of roll compaction equipment variables, granulation technique, and HPMC polymer level on a controlled-release matrix model drug formulation. Pharm Technol. 1999;23:90–106. [Google Scholar]
- 11.Saravanan M, Sri Nataraj K, Ganesh KS. Hydroxypropyl methylcellulose based cephalexin extended release tablets: influence of tablet formulation, hardness and storage on invitro release kinetics. Chem Pharm Bull. 2003;51:978–83. doi: 10.1248/cpb.51.978. [DOI] [PubMed] [Google Scholar]
- 12.Hariharan M, Wowchuk C, Nkansah P, Gupta VK. Effect of formulation composition on the properties of controlled release tablets prepared by roller compaction. Drug Dev Ind Pharm. 2004;30:565–72. doi: 10.1081/DDC-120037487. [DOI] [PubMed] [Google Scholar]
- 13.Malkowska S, Khan K. Effect of re-compression on the properties of tablets preprared by dry granulation. Drug Dev Ind Pharm. 1983;9:331–47. doi: 10.3109/03639048309044678. [DOI] [Google Scholar]
- 14.Sun C, Himmelspach MW. Reduced tabletability of roller compacted granules as a result of granule size enlargement. J Pharm Sci. 2006;95:200–6. doi: 10.1002/jps.20531. [DOI] [PubMed] [Google Scholar]
- 15.Shaw LR, Irwin WJ, Grattan TJ, Conway BR. The effect of selected water-soluble excipients on the dissolution of paracetamol and ibuprofen. Drug Dev Ind Pharm. 2005;31:515–25. doi: 10.1080/03639040500215784. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Peck GE, Miller RW, Morris KR. Effect of the variation in the ambient moisture on the compaction behavior of powder undergoing roller-compaction and on the characteristics of tablets produced from the post-milled granules. J Pharm Sci. 2005;94:2314–26. doi: 10.1002/jps.20414. [DOI] [PubMed] [Google Scholar]
- 17.Rowe RC, Roberts RJ. In: Mechanical properties in pharmaceutical powder compaction technology. Alderborn G, Nyström C, editors. New York: Marcel Dekker; 1996. pp. 283–322. [Google Scholar]
- 18.Nokhodchi A, Rubinstein MH, Larhrib H, Guyot JC. The effect of moisture on the properties of ibuprofen tablets. Int J Pharm. 1995;118:191–7. doi: 10.1016/0378-5173(94)00354-8. [DOI] [Google Scholar]
- 19.Eriksson L, Johansson E, Kettaneh-Wold N, Wikström C, Wold S. Design of experiments: principles and applications. 3. Umeå: MKS Umetrics AB; 2008. [Google Scholar]
- 20.Teng Y, Qiu Z, Wen H. Systematical approach of formulation and process development using roller compaction. Eur J Pharm Biopharm. 2009;73:219–29. doi: 10.1016/j.ejpb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Thalberg K, Lindholm D, Axelsson A. Comparison of different flowability tests for powders for inhalation. Powder Technol. 2004;146:206–13. doi: 10.1016/j.powtec.2004.08.003. [DOI] [Google Scholar]
- 22.Cao X, Leyva N, Anderson SR, Hancock BC. Use of prediction methods to estimate true density of active pharmaceutical ingredients. Int J Pharm. 2008;355:231–7. doi: 10.1016/j.ijpharm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Hancock BC, Colvin JT, Mullarney MP, Zinchuk AV. The relative densities of pharmaceutical powders, blends, dry granulations, and immediate-release tablets. Pharm Technol. 2003;27:64–80. [Google Scholar]
- 24.Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. 5. USA: Pharmaceutical Press; 2006. [Google Scholar]
- 25.Pitt KG, Newton MJ, Stanley P. Tensile fracture of doubly-convex cylindrical discs under diametral loading. J Mater Sci. 1988;23:2723–8. doi: 10.1007/BF00547442. [DOI] [Google Scholar]
- 26.Fell JT, Newton JM. Determination of tablet strength by the diametrical compression test. J Pharm Sci. 1970;59:688–91. doi: 10.1002/jps.2600590523. [DOI] [PubMed] [Google Scholar]
- 27.Souihi N, Dumarey M, Wikström H, Tajarobi P, Fransson M, Svensson O, et al. A quality by design approach to investigate the effect of mannitol and dicalcium phosphate qualities on roll compaction. Int J Pharm. 2013;477:47–61. doi: 10.1016/j.ijpharm.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Herting MG, Kleinebudde P. Studies on the reduction of tensile strength of tablets after roll compaction/dry granulation. Eur J Pharm Biopharm. 2008;70:372–9. doi: 10.1016/j.ejpb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Prescott JK, Barnum RA. On powder flowability. Pharm Technol. 2000;24:60–85. [Google Scholar]
- 30.McKenna A, McCafferty D. Effect of particle size on the compaction mechanism and tensile strength of tablets. J Pharm Pharmacol. 1982;34:347–51. doi: 10.1111/j.2042-7158.1982.tb04727.x. [DOI] [PubMed] [Google Scholar]
- 31.Nokhodchi A, Rubinstein MH, Ford JL. The effect of particle size and viscosity grade on the compaction properties of hydroxypropylmethylcellulose 2208. Int J Pharm. 1995;126:189–97. doi: 10.1016/0378-5173(95)04122-2. [DOI] [Google Scholar]
- 32.Bacher C, Olsen PM, Bertelsen P, Sonnergaard JM. Compressibility and compactibility of granules produced by wet and dry granulation. Int J Pharm. 2008;358:69–74. doi: 10.1016/j.ijpharm.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Wikberg M, Alderborn G. Compression characteristics of granulated materials: VI. pore size distributions, assessed by mercury penetration, of compacts of two lactose granulations with different fragmentation propensities. Int J Pharm. 1992;84:191–5. doi: 10.1016/0378-5173(92)90059-B. [DOI] [Google Scholar]
- 34.Wu CY, Hung WL, Miguélez-Morán AM, Gururajan B, Seville JPK. Roller compaction of moist pharmaceutical powders. Int J Pharm. 2010;391:90–7. doi: 10.1016/j.ijpharm.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Weyenberg W, Vermeire A, Vandervoort J, Remon JP, Ludwig A. Effects of roller compaction settings on the preparation of bioadhesive granules and ocular minitablets. Eur J Pharm Biopharm. 2005;59:527–36. doi: 10.1016/j.ejpb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Juppo A. Relationship between breaking force and pore structure of lactose, glucose and mannitol tablets. Int J Pharm. 1996;127:95–102. doi: 10.1016/0378-5173(95)04203-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 134 kb)


