Abstract
Background
Direct-to-consumer advertising (DTCA) remains a controversial issue, with concerns that it leads to unnecessary and inappropriate prescribing. Whether DTCA shifts prescribing from first-line (guideline-recommended) therapy to second-line drugs has not been studied.
Objective
The purpose of this study was to determine the impact of sequential DTCA campaigns for two drugs used to treat benign prostatic hyperplasia (BPH): one newer agent, dutasteride (Avodart®), and one older first-line agent, tamsulosin (Flomax®).
Design
Interrupted time series analysis was used to assess the impact of each DTCA campaign using data on consumer “response” from Google Trends and dispensed prescriptions from IMS Health.
Participants
We analyzed data for the United States from January 2003 to December 2007.
Intervention
DTCA for dutasteride and tamsulosin commenced on July, 2005 and April, 2006, respectively.
Main Measures
Monthly Internet search volume (scaled from 0 to 100) for the advertised trade name of each drug and monthly U.S. prescription rates per 1,000 population were analyzed.
Key Results
The dutasteride campaign was associated with an increase in Internet searches for both “Avodart” (level change +31.3 %, 95 % CI: 27.2–35.4) and “Flomax” (level change +8.3 %, 95 % CI: 0.9–15.7), whereas the tamsulosin campaign was associated with increased “Flomax” searches (level change +25.3 %, 95 % CI: 18.7–31.8). The dutasteride campaign was associated with an increase in the prescription of dutasteride (trend = 0.45/month, 95 % CI: 0.33–0.56), but a larger impact was observed with tamsulosin prescriptions (trend = 0.76/month, 95 % CI: 0.02–1.50). Similarly, the tamsulosin campaign was associated with an immediate fourfold increase in the prescribing of tamsulosin (level change +5.76 units, 95 % CI: 1.79–9.72) compared to dutasteride (level change +1.47 units, 95 % CI: 0.79–2.14).
Conclusions
DTCA was associated with the utilization of drugs to treat symptomatic BPH. However, both campaigns were associated with greater increases in the use of the guideline-recommended first-line agent. DTCA campaigns may increase the overall levels of guideline-recommended treatments to a greater extent than the specific advertised agents.
Keywords: Health policy, Advertising as topic, Benign prostatic hyperplasia
Introduction
Direct-to-consumer advertising (DTCA) is the marketing and promotion of pharmaceuticals or medical services to the public through lay media. Despite the diminishing use of DTCA in recent years, pharmaceutical companies still spent $4.3 billion on such promotion in 2010.1 Opponents of DTCA argue that it leads to overdiagnosis, unnecessary and inappropriate prescribing, more adverse drug reactions, and increased healthcare costs.2–4 Proponents, on the other hand, maintain that it raises public awareness of underdiagnosed diseases and leads to appropriate increases in diagnostic testing and treatment.5 Thus far, the limited evidence—of varying methodological quality—on the effects of DTCA is inconclusive.6
Numerous studies have investigated the impact of DTCA on prescription drug use. Previous time series analyses have shown that DTCA was associated with increased volume of prescriptions for tegaserod (the only available medication for irritable bowel syndrome), antihistamines (first-line therapy for allergic rhinitis), statins (hyperlipidemia), histamine H2-receptor antagonists (acid reflux), triptans (migraine), and terbinafine (onychomycosis).6–10 In contrast, DTCA for drugs such as etanercept (for symptom refractory rheumatoid arthritis), mometasone (second-line therapy for allergic rhinitis), and clopidogrel (anti-platelet agent for acute coronary syndrome or following percutaneous intervention) have not been shown to alter prescribing rates.7,11 Overall, it appears from the existing body of evidence that when DTCA has been shown to increase prescribing volume, it typically involves greater use of medications for common and underdiagnosed diseases.
In this context, it remains unknown how the many DTCA campaigns affect competing medications. For example, a DTCA campaign that led to an increase in prescriptions for first-line therapy of an undertreated condition might be considered beneficial, whereas greater use of second-line therapy could be considered wasteful and counterproductive. To our knowledge, no prior study has investigated the effect of DTCA campaigns for drugs from different pharmaceutical classes but for the same therapeutic indication. While some research has suggested a “class effect,”12 no prior study has investigated the comparative effect of DTCA on consumer interest and utilization of first-line versus second-line therapies. During the peak period of DTCA spending, two medications for benign prostatic hyperplasia (BPH), dutasteride and tamsulosin, were sequentially marketed, providing a unique opportunity to study the relationship between two campaigns for different drugs that treat the same symptomatic disease. Thus, we investigated the effect of two sequential DTCA campaigns for dutasteride and tamsulosin on both patient interest and prescription volume.
Methods
Study Drugs
Lower urinary tract symptoms in men are most commonly attributed to BPH by treating physicians.13 In men, the prevalence of moderate to severe lower urinary tract symptoms increases with age—affecting over one-third of men over the age of 65. However, only 10 % of men with such symptoms are treated with prescription medications.14,15 As BPH is underdiagnosed and undertreated, the potential exists for large market expansion through DTCA.15 Medical therapy for BPH includes alpha 1-adrenergic antagonists (alpha-blockers), 5-alpha reductase inhibitors (5ARIs), or both in combination. In 2003, the American Urological Association published recommendations for the management of BPH in patients seeking medical treatment.16 The guidelines stated that alpha-blockers were more effective than 5ARIs in improving lower urinary tract symptoms in men with BPH. Comparatively, 5ARIs were known to lead to more sexual side effects and were only considered effective in men with clinical evidence of prostatic enlargement. Due to their efficacy and rapid effect, alpha-blockers were considered first-line therapy for men with BPH.17
Starting in 2005, two drugs prescribed for BPH were heavily marketed through national DTCA campaigns. Tamsulosin (Flomax®) is an alpha-blocker that acts by relaxing the prostatic smooth muscle to relieve bladder outlet obstruction. Compared to other alpha-blockers, tamsulosin is the most uroselective (alpha-1A) and has fewer systemic adverse effects than other drugs in its class.18,19 Dutasteride (Avodart®) is a 5ARI that blocks the conversion of testosterone to dihydrotestosterone, leading to a reduction in the size of the prostate and improving urinary function. Dutasteride acts on both 5-alpha reductase isoenzymes (types 1 and 2), resulting in a greater reduction in systemic dihydrotestosterone compared to the other available 5ARI, finasteride.20
Data Sources
Advertising Spending
To determine total DTCA expenditures for tamsulosin and dutasteride, we followed previous studies and used monthly estimates of national advertising expenditures from Kantar Media/TNS Media Intelligence for the period from January 2003 through December 2007.7,21,22
Web Search Interest
To measure the effect of the DTCA campaigns on consumer interest and response, we analyzed trends in search volumes from Google. We focused on online searches, as surveys have found that 80 % of U.S. adult Internet users sought health information online in 2005 and 2006.23,24 Google, which is the most widely used Internet search engine in the world, was used for almost half of U.S. online searches at the time that BPH advertising began.25 Google Trends charts the volume of searches for particular terms over time.26 The available data is normalized and scaled from 0 to 100, with 100 representing the month with the highest search volume during the period. As both DTCA campaigns advertised the brand names of the medicines, we obtained monthly data on searches for “Avodart” and “Flomax” in the United States from January 2004 through December 2007 (data prior to 2004 are not available through Google Trends). Results were scaled to the highest month of search volume for Flomax (April 2006). Google Trends was accessed on June 21, 2013.
Drug Utilization
We obtained monthly U.S. prescription rates from IMS Health, a leading provider of market intelligence to the pharmaceutical and health care industries, through their National Prescription Audit database.27 This database estimates national prescription activity at retail, mail-order, long-term care, and managed care outlets based on a sample of 70 % of all outlets.28,29 As both drugs are predominantly prescribed at fixed dosage strengths, we examined changes in the number of units sold. Monthly rates of units dispensed were obtained for tamsulosin and dutasteride for the periods from January 2003 through December 2007 in order to ensure a 36-month window around the start of each DTCA campaign.
Statistical Analysis
We used interrupted time series analysis, one of the strongest quasi-experimental designs, to study the impact of the two sequential DTCA campaigns on the longitudinal trends in the use of dutasteride and tamsulosin.30 We examined the number of units per 1,000 U.S. population per month for each drug to assess changes in use before and after DTCA initiation. National estimates for the number of units dispensed per 1,000 people were obtained by dividing monthly estimates by the U.S. population and multiplying by 1,000.31
Time series models were fitted to assess for changes in the level (step) and trend (slope) in the study outcomes (Internet search volume, number of units dispensed) for each drug following the start of each of the two DTCA campaigns. This method controls for existing trends in use prior to DTCA. The start dates for the two DTCA campaigns for dutasteride and tamsulosin were July 2005 and April 2006, respectively. A generalized least-squares model was used, and included autoregressive terms to control for correlation over time. Two binary variables indicating the post-DTCA periods for each drug were used to assess changes in level, and a variable indicating the number of months elapsed since DTCA had begun was used to assess changes in trend. Autocorrelation, partial autocorrelation, and inverse autocorrelation functions were assessed for model/parameter appropriateness and seasonality. Stationarity was assessed using the autocorrelation function and the augmented Dickey–Fuller test. The presence of “white noise” was assessed by examining the autocorrelation values at various lags using the Ljung-Box X2 statistic.
Results
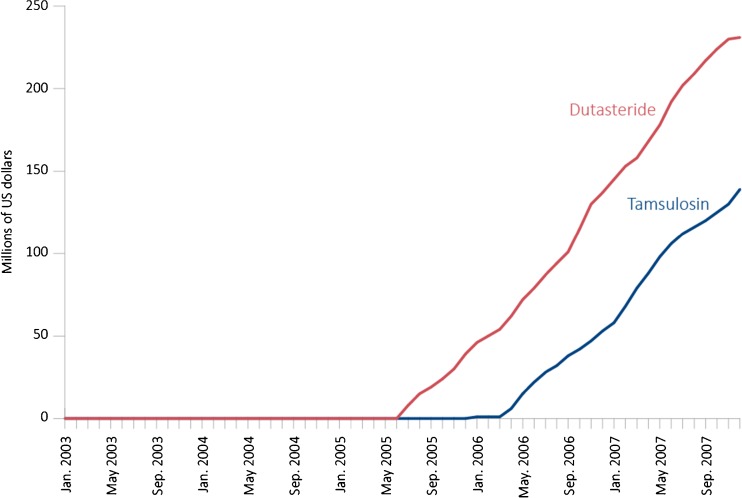
Total DTCA spending for these two competing drugs was $369.9 million over the time period studied: $231 million for dutasteride and $139 million for tamsulosin. Average monthly spending on DTCA for dutasteride and tamsulosin from the start of their campaigns through December 2007 was roughly comparable, at $6.6 million and $7.7 million per month, respectively (Fig. 1).
Figure 1.
Estimated cumulative expenditure on direct-to-consumer advertising (DTCA) for dutasteride (red) and tamsulosin (blue) from 2003 through 2007.
Web Search Interest
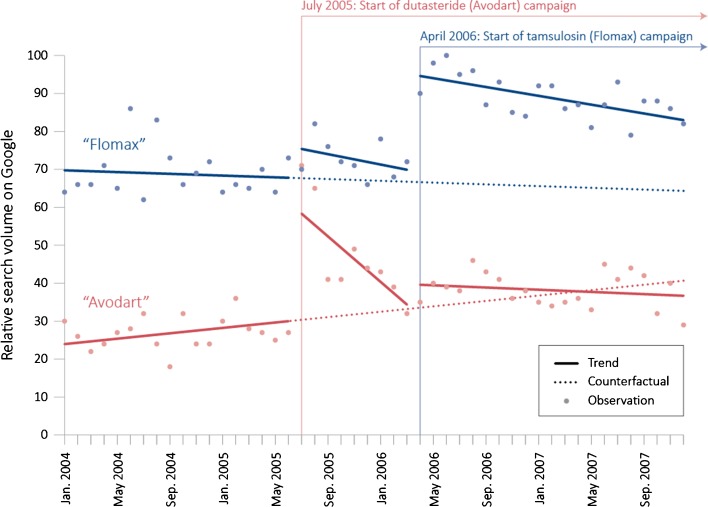
Both DTCA campaigns were associated with increased volume of Internet searches for their respective drugs (Fig. 2). The dutasteride campaign was associated with an increase in search level for the target drug “Avodart” of 31.3 % (95 % confidence interval [CI]: 27.2–35.4). This campaign was also associated with a significant, but smaller, change in the level of search activity for the competitor drug “Flomax” (+8.3 %, 95 % CI: 0.9–15.7). The second DTCA campaign, for tamsulosin, was associated with an increase in searches for the target drug “Flomax” of 25.3 % (95 % CI: 18.7–31.8). There was also a corresponding change in level of search activity for the competitor drug “Avodart” (+5.4 %, 95 % CI: 2.1–8.6); however, this likely resulted from the high spike in search volume at the start of the dutasteride campaign.
Figure 2.
Trends in Web search interest for dutasteride (red) and tamsulosin (blue) on the Google search engine from 2004 through 2007. The analysis indicated that the dutasteride campaign was associated with a statistically significant change in level of searches for both “Avodart” (+31.3 %, 95 % CI: 27.2–35.4) and “Flomax” (+8.3 %, 95 % CI: 0.9–15.7). The tamsulosin campaign was associated with a significant change in search level for “Flomax” (+25.3 %, 95 % CI: 18.7–31.8).
Use of Study Drugs
Avodart Campaign (July 2005)
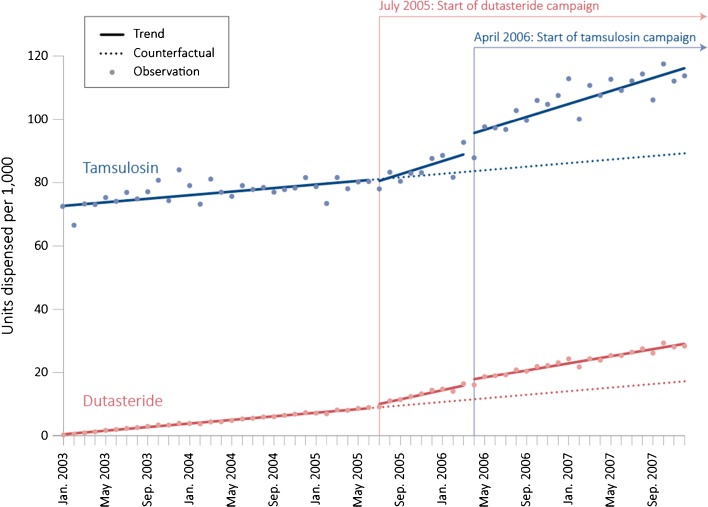
As shown in Fig. 3, prior to the initiation of the DTCA campaigns, population-level rates in units sold for both dutasteride and tamsulosin were increasing at relatively constant rates of 0.28 (95 % CI: 0.27–0.30, p < 0.001) and 0.28 (95 % CI: 0.12–0.45, p = 0.002) units per 1,000 per month, respectively. There was no statistically significant change following the start of the first DTCA campaign, for dutasteride, in the level of units dispensed for either the target drug dutasteride (0.61, 95 % CI: −0.11 to 1.32; p = 0.103) or the competitor drug tamsulosin (−1.31, 95 % CI: −5.49 to 2.88; p = 0.543). There was, however, an increase in the trend for dutasteride (0.45, 95 % CI: 0.33–0.56; p < 0.001). In addition, the campaign was associated with an almost twofold increase in trend for the competitor drug tamsulosin (0.76, 95 % CI: 0.02–1.50; p = 0.048).
Figure 3.
Number of units dispensed for dutasteride (red) and tamsulosin (blue) per 1,000 population per month in the United States from 2003 through 2007. The dutasteride campaign was associated with a greater increase in trend for tamsulosin units dispensed (0.76, 95 % CI: 0.02–1.50) than for dutasteride (0.45, 95 % CI: 0.33–0.56). The tamsulosin campaign was associated with an immediate increase in the number of tamsulosin units dispensed (5.76, 95 % CI: 1.79–9.72). Source: IMS Health National Prescription Audit™, January 2003–December 2007, IMS Health Incorporated.
Flomax Campaign (April 2006)
The start of the second DTCA campaign, for tamsulosin, was associated with an increase in the level of units dispensed for both medications. Following the start of the campaign, there was an immediate increase in the number of dispensed units of the target drug tamsulosin (5.76, 95 % CI: 1.79–9.72, p = 0.006). No change was observed in trend (−0.02, 95 % CI: −0.80 to 0.77; p = 0.969). The number of units of the competitor dutasteride also increased (1.47, 95 % CI: 0.79–2.14, P < 0.001), although there was also a statistically significant decrease in trend for dutasteride units that offset this increase after just nine months (−0.17, 95 % CI: −0.28 to −0.05, p = 0.007).
Discussion
Over the last 15 years, pharmaceutical manufacturers have spent many billions of dollars on advertising campaigns directed at consumers.1 Our study lends further credence to previous results indicating that DTCA is effective in increasing the utilization of drugs for common but underdiagnosed diseases. Further, our study suggests that the impact of DTCA campaigns is heavily influenced by the clinical context in which they occur, as we found that the advertising campaign for one drug, despite raising consumer interest in the targeted drug, had a larger impact on prescribing patterns for a competing first-line drug. These findings shed light on mechanisms along the pathway necessary for DTCA to be effective. Upon seeing an advertisement for a drug, the consumer (or patient) must be motivated enough to schedule a visit and seek out a prescription for the relevant symptoms from his or her physician. Further, once the patient is in the physician’s office, after a careful history and physical (including a digital rectal examination in this setting), the choice of medication to prescribe is at the discretion of the physician, guided by the overall clinical context as well as patient expectations and preferences. As such, an increase in prescriptions for non-advertised medications for the same disease is entirely plausible.
In our study, we found that the Avodart campaign was associated with an increase in tamsulosin (Flomax) prescriptions almost twice that of the effect observed with dutasteride (Avodart) itself, and this was unexpected. Based on Google search volumes for each drug’s trade name, both campaigns influenced consumer response, suggesting that this finding was not based on an ineffective Avodart campaign. There are at least two possible explanations for our results. The first, more plausible explanation is that upon requesting dutasteride from their doctor, patients were appropriately prescribed tamsulosin as an accepted first-line medical therapy for men with moderate to severe lower urinary tract symptoms. The second, less likely possibility is that upon researching dutasteride, patients may have explored other medications for symptomatic BPH and subsequently chosen tamsulosin due to its greater efficacy, better side effect profile, and guideline recommendation. The increase in the level of searches for “Flomax” at the beginning of the dutasteride (Avodart) campaign supports this possibility.
Implicit in claims that DTCA leads to inappropriate prescribing is the belief that the preferences and requests of the patient—or consumer—will strongly influence the medical judgment of the prescribing physician. However, clinical and professional standards oblige physicians to follow accepted practice and medical guidelines to determine the appropriateness of a medication for each patient on a case-by-case basis. Evidence thus far of DTCA leading to inappropriate prescriptions is limited and has recently been contested. For example, in a select patient population, Abel et al. recently concluded that DTCA led to an appropriate increase in aromatase inhibitor prescriptions in postmenopausal but not premenopausal women.21 In a randomized trial using standardized patients, Kravitz et al. found that both brand-specific and general antidepressant requests from patients led to increased diagnosis, prescribing, and appropriate care for depression compared to patients with similar symptoms who did not request a prescription.32
The prevalence in the U.S. of untreated men with moderate to severe lower urinary tract symptoms suggests a large consumer base of men that meet the AUA guidelines for medical therapy of symptomatic BPH. In addition, the relative increase in tamsulosin prescriptions compared to dutasteride prescriptions following both DTCA campaigns suggests that (1) awareness of disease and treatment options increased, and (2) physicians were treating men according to available guidelines that recommend alpha-blockers as initial therapy. Furthermore, medical therapy for BPH has been shown to improve quality of life, reduce the risk of clinical progression, and avert the potential postoperative morbidity and complications of surgical treatment.15,33 In aggregate, this suggests to us that issues of overdiagnosis and inappropriate treatment (the main concerns put forward by opponents of DTCA) are unlikely in the case of men with symptomatic BPH.
Limitations
Despite its strengths, there are several limitations that must be acknowledged when interpreting the results of our study. First, this was an observational study of ecologic data, and therefore we cannot definitively conclude that there was a causal relationship between the DTCA campaigns and changes in Internet search and dispensed prescription levels. However, short of a randomized trial of DTCA, which would be prohibitive from a cost perspective, interrupted time series analysis is as close to causal inference as can be drawn from a non-randomized study.30 Second, we have no individual patient data, such as indication, previous use, allergy or intolerance, or other medical history. We are also unable to ascertain whether men were using dual therapy with both drugs. Third, we assumed that individual physicians were aware of medical treatment guidelines, and whether physicians themselves were influenced by DTCA itself rather than by patient requests is not clear. Fourth, tamsulosin was approved four years before the approval of dutasteride, and may have been a more accepted and established medication at the time of the DTCA campaigns, although this would be accounted for in the baseline trends. Fifth, we cannot determine whether the results would be similar if the order of the campaigns were reversed. Finally, this study focused on a single disease involving men, and thus the results may not be more broadly generalizable.
Conclusions
DTCA can have both intended and unintended effects on the prescription rates of drugs that compete with each other but belong to the same therapeutic class. This study suggests that DTCA may be effective in increasing treatment rates for common and underdiagnosed diseases such as benign prostatic hyperplasia (BPH). However, the effect of DTCA appears to be tempered by accepted medical practice and guidelines, and whether it leads to increased diagnosis and better management or overdiagnosis and over-treatment has to be determined on a condition-by-condition basis. For men with lower urinary tract symptoms related to BPH, DTCA on the whole was associated with an increase in the use of a treatment that was—at least in terms of the drug prescribed—in accordance with medical guidelines at the time.
Acknowledgments
Sean Skeldon is supported in part by the CIHR and the Western Regional Training Centre (WRTC) for Health Services Research. Katy Kozhimannil is supported by a grant from the Building Interdisciplinary Research Careers in Women’s Health BIRCWH) program (grant number K12HD055887), with funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Office of Research on Women’s Health, and the National Institute on Aging, at the National Institutes of Health, administered by the University of Minnesota Deborah E. Powell Center for Women’s Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Sumit Majumdar holds the Endowed Chair in Patient Health Management (Faculties of Medicine & Dentistry and Pharmacy & Pharmaceutical Sciences, University of Alberta) and receives salary support as a Health Scholar of the Alberta Heritage Foundation for Medical Research and Alberta Innovates - Health Solutions. Michael Law reports grants from the Canadian Institutes of Health Research during the conduct of the study, and personal fees from Health Canada outside the submitted work.
The statements, findings, conclusions, views, and opinions contained and expressed herein are based in part on data obtained under license from the following IMS Health Incorporated information service(s): National Prescription Audit™(January 2003–December 2007) and National Sales Perspectives™(January 2003–December 2007) IMS Health Incorporated. The statements, findings, conclusions, views, and opinions are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Conflict of interest
The authors each declare that they have no conflict of interest.
Abbreviations
- DTCA
Direct-to-consumer advertising
- BPH
Benign prostatic hyperplasia
- 5ARI
5-alpha reductase inhibitor
Contributor Information
Sean C. Skeldon, Phone: 604-822-4969, Email: sean.skeldon@ubc.ca.
Katy B. Kozhimannil, Email: kbk@umn.edu.
Sumit R. Majumdar, Email: majumdar@ualberta.ca.
Michael R. Law, Email: michael.law@ubc.ca.
References
- 1.Kornfield R, Donohue J, Berndt ER, Alexander GC. Promotion of prescription drugs to consumers and providers, 2001–2010. PLoS One. 2013;8(3):e55504. doi: 10.1371/journal.pone.0055504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe SM. Direct-to-consumer advertising–education or emotion promotion? N Engl J Med. 2002;346(7):524–6. doi: 10.1056/NEJM200202143460713. [DOI] [PubMed] [Google Scholar]
- 3.Stange KC. Time to ban direct-to-consumer prescription drug marketing. Ann Fam Med. 2007;5(2):101–4. doi: 10.1370/afm.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross JS, Kravitz RL. Direct-to-Consumer Television Advertising: Time to Turn Off the Tube? J Gen Intern Med. 2013;28(7):862–4. doi: 10.1007/s11606-013-2424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmer AF. Direct-to-consumer advertising–strengthening our health care system. N Engl J Med. 2002;346(7):526–8. doi: 10.1056/NEJM200202143460714. [DOI] [PubMed] [Google Scholar]
- 6.Mintzes B. Advertising of prescription-only medicines to the public: does evidence of benefit counterbalance harm? Annu Rev Public Health. 2012;33:259–77. doi: 10.1146/annurev-publhealth-031811-124540. [DOI] [PubMed] [Google Scholar]
- 7.Law MR, Majumdar SR, Soumerai SB. Effect of illicit direct to consumer advertising on use of etanercept, mometasone, and tegaserod in Canada: controlled longitudinal study. BMJ. 2008;337(sep02 1):a1055. doi: 10.1136/bmj.a1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachry WM III, Shepherd MD, Hinich MJ, Wilson JP, Brown CM, Lawson KA. Relationship between direct-to-consumer advertising and physician diagnosing and prescribing. Am J Health Syst Pharm. 2002;59(1):42–9. doi: 10.1093/ajhp/59.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Dorn SD, Farley JF, Hansen RA, Shah ND, Sandler RS. Direct-to-consumer and physician promotion of tegaserod correlated with physician visits, diagnoses, and prescriptions. Gastroenterology. 2009;137(2):518–24. doi: 10.1053/j.gastro.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.’t Jong GW, Stricker BHC, Sturkenboom MCJM. Marketing in the lay media and prescriptions of terbinafine in primary care: Dutch cohort study. BMJ. 2004;328(7445):931. doi: 10.1136/bmj.38007.711481.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law MR, Soumerai SB, Adams AS, Majumdar SR. Costs and consequences of direct-to-consumer advertising for clopidogrel in Medicaid. Arch Intern Med. 2009;169(21):1969–74. doi: 10.1001/archinternmed.2009.320. [DOI] [PubMed] [Google Scholar]
- 12.Hansen RA, Shaheen NJ, Schommer JC. Factors influencing the shift of patients from one proton pump inhibitor to another: the effect of direct-to-consumer advertising. Clin Ther. 2005;27(9):1478–87. doi: 10.1016/j.clinthera.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Abrams P. New words for old: lower urinary tract symptoms for “prostatism”. BMJ. 1994;308(6934):929–30. doi: 10.1136/bmj.308.6934.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150(1):85–9. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 15.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166(21):2381–7. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 16.AUA Practice Guidelines Committee AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170(2 Pt 1):530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan ED. Medical management of benign prostatic hyperplasia–are two drugs better than one? N Engl J Med. 2003;349(25):2449–51. doi: 10.1056/NEJMe038154. [DOI] [PubMed] [Google Scholar]
- 18.Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171(3):1029–35. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- 19.Lowe FC. Role of the newer alpha, -adrenergic-receptor antagonists in the treatment of benign prostatic hyperplasia-related lower urinary tract symptoms. Clin Ther. 2004;26(11):1701–13. doi: 10.1016/j.clinthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(Suppl 9):S31–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Abel GA, Chen K, Taback N, Hassett MJ, Schrag D, Weeks JC. Impact of oncology-related direct-to-consumer advertising: association with appropriate and inappropriate prescriptions. Cancer. 2013;119(5):1065–72. doi: 10.1002/cncr.27814. [DOI] [PubMed] [Google Scholar]
- 22.Ad$pender™ | Kantar Media [Internet]. [cited 2013 Jul 29]; Available from: http://kantarmediana.com/intelligence/products/adspender
- 23.Fox S. Health Information Online. Washington, D.C.: 2005.
- 24.Fox S. Online Health Search 2006. Washington, D.C.: 2006.
- 25.Nielsen NetRatings Search Engine Ratings - Search Engine Watch [Internet]. [cited 2013 Jul 29]; Available from: http://searchenginewatch.com/article/2067079/Nielsen-NetRatings-Search-Engine-Ratings
- 26.Google Trends [Internet]. [cited 2013 Jul 29]; Available from: http://www.google.com/trends/explore#cmpt=q
- 27.IMS National Prescription Audit™, January 2003–December 2007, IMS Health Incorporated.
- 28.Majumdar SR, Almasi EA, Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. JAMA. 2004;292(16):1983–8. doi: 10.1001/jama.292.16.1983. [DOI] [PubMed] [Google Scholar]
- 29.Jackevicius CA, Tu JV, Ross JS, Ko DT, Carreon D, Krumholz HM. Use of fibrates in the United States and Canada. JAMA. 2011;305(12):1217–24. doi: 10.1001/jama.2011.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Census Bureau DID. Population Estimates [Internet]. [cited 2013 Jul 29]; Available from: http://www.census.gov/popest/data/intercensal/national/nat2010.html
- 32.Kravitz RL, Epstein RM, Feldman MD, et al. Influence of patients’ requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. JAMA. 2005;293(16):1995–2002. doi: 10.1001/jama.293.16.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]