Abstract
BACKGROUND
The efficacy of perineal self-acupressure in treating constipation is uncertain.
OBJECTIVE
We aimed to evaluate whether perineal self-acupressure would improve patient reports of quality of life and bowel function at 4 weeks after training.
DESIGN
A randomized, parallel group trial was conducted.
SETTING
The study took place at the UCLA Department of Medicine.
PATIENTS
One hundred adult patients who met Rome III criteria for functional constipation participated.
INTERVENTION
The control group received information about standard constipation treatment options, while the treatment group received training in perineal self-acupressure plus standard treatment options.
MEASUREMENTS
Primary outcome was the Patient Assessment of Constipation Quality of Life (PAC-QOL). Secondary outcomes included patient assessments of bowel function (as measured by a modified Bowel Function Index (BFI)), and health and well-being (as measured by the SF-12v2).
RESULTS
The mean PAC-QOL was improved by 0.76 in the treatment group and by 0.17 in the control group (treatment-effect difference, 0.59 [95 % CI, 0.37 to 0.81]; p < 0.01). The mean modified BFI was improved by 18.1 in the treatment group and by 4.2 in the control group (treatment-effect difference, 13.8 [95 % CI, 5.1 to 22.5]; p < 0.01). The mean SF-12v2 Physical Component Score was improved by 2.69 in the treatment group and reduced by 0.36 in the control group (treatment-effect difference, 3.05, [95 % CI, 0.85 to 5.25]; p < 0.01); and the mean SF-12v2 Mental Component Score was improved by 3.12 in the treatment group and improved by 0.30 in the control group (treatment-effect difference, 2.82, [95 % CI, −0.10 to 5.74]; p < 0.07).
LIMITATION
The trial was not blinded.
CONCLUSION
Among patients with constipation, perineal self-acupressure improves self-reported assessments of quality of life, bowel function, and health and well-being relative to providing standard constipation treatment options alone.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-3084-6) contains supplementary material, which is available to authorized users.
KEY WORDS: perineum, constipation, acupressure
INTRODUCTION
Constipation is characterized by the difficult or infrequent passage of stool, often accompanied by a sensation of incomplete evacuation or straining. It is one of the most common digestive complaints in the general population, affecting approximately 12 to 19 % of North Americans.1 Constipation is more prevalent among women, nonwhites, and patients over 60 years of age. It is also more prevalent among those with little daily physical activity, poor education, and low income.2,3 Patients with constipation report substantial impairment in health-related quality of life,4 higher incidence of depression, and significant loss of work productivity.4,5 Furthermore, constipation is associated with significant health care costs. U.S. hospital costs alone associated with constipation were estimated at over $4.25 billion in 2010.6 Treatment for constipation generally consists of increasing intake of dietary fiber and fluid, increasing exercise, and taking medications such as laxatives.4
Perineal massage is a technique commonly recommended to pregnant women to prevent laceration during labor and to decrease the need for an episiotomy. A randomized controlled trial has shown that perineal massage can increase the elasticity of the perineum and reduce the risk of third-degree perineal tears.7 Several non-controlled studies also suggest perineal or transvaginal pressure may aid in defecation.2,3,8–16 In addition, digitally applying perineal or transvaginal pressure has been used by patients with rectocele or descending perineum syndrome.8–10 Perineal pressure has been shown to increase rectal tone by an average of approximately 52 %.12
The objective of this study was to test whether perineal self-acupressure, which consists of a patient repeatedly applying external pressure to the perineum prior to defecation, can improve symptoms of constipation and improve health-related quality of life. While perineal self-acupressure has not been previously evaluated in a controlled setting, research suggests it might facilitate defecation by breaking up scybalous stools,13,14 relaxing the anal sphincters and puborectalis muscle,3,16,17 stimulating extrinsic parasympathetic sacral nerves,12 and compensating for rectal wall abnormalities (such as descending perineum syndrome or rectocele).8,9 See Online Appendix 1. In addition to treating constipation, perineal self-acupressure may treat hemorrhoids, which are associated with constipation and may be caused by excessive straining.2 One way to prevent the development and progression of hemorrhoids is to effectively treat constipation.17
METHODS
Participants
Eligible participants were aged 18 years or older and met Rome III criteria for functional constipation, namely, experiencing two or more of the following criteria for the last 3 months with symptom onset at least 6 months prior to diagnosis: straining during at least 25 % of defecations; lumpy or hard stools in at least 25 % of defecations; sensation of incomplete evacuation for at least 25 % of defecations; sensation of anorectal obstruction/blockage for at least 25 % of defecations; manual maneuvers to facilitate at least 25 % of defecations (e.g., digital evacuation, support of the pelvic floor); and fewer than three defecations per week. Patients were excluded if they were pregnant, if they experienced significant weight loss (more than 10 % of their usual body weight in the preceding 6 months), and if they had a history of blood mixed in stool. The perineal self-acupressure for constipation (PSAC) study protocol was approved by the institutional review board at UCLA (IRB#13-000738) and registered with ClinicalTrials.gov (Identifier: NCT01867944), and all participants provided written informed consent.
Study Design and Treatment
The study was a randomized, parallel group trial, with each eligible participant randomly assigned to either standard care (control) or standard care plus perineal self-acupressure (treatment). Potential participants were recruited from the local community and screened by phone from July 2013 to April 2014. Eligible participants who met all study criteria were enrolled on a rolling basis after they arrived at UCLA to complete the informed consent process and baseline survey instruments. Patients unable to travel to UCLA were given the option to meet with study personnel in their homes or at a public place of their choosing. With 50 patients per group and with 20 % of patients lost to follow-up, we projected 90 % power to detect a clinically meaningful change in the PAC-QOL total score of 0.5 (given a population mean of 1.85 and a SD of 0.6718).
Computer-generated randomization was not stratified, with treatment assignments made in random permuted blocks of size 2. Subjects in both groups received educational materials about constipation and conventional treatment options (e.g., increased dietary fiber intake, stool softeners, increased exercise). Subjects in the perineal self-acupressure group also received sex-specific educational material and training in perineal self-acupressure. Training consisted of 3 to 5 min of oral instruction on how to apply perineal pressure, with the aid of a plastic anatomic model displaying the perineal region. Subjects in the perineal self-acupressure group were not restricted from using standard care. All of the patient materials appear in Online Appendix 2. All participants completed an initial set of survey instruments immediately following the informed consent process. One month after completing the initial set of survey instruments, subjects in both groups were asked to return a follow-up set of survey instruments. The last participant completed follow-up in June 2014.
Outcome Measures
The primary outcome measure was patient assessment of constipation-related health quality of life by means of the Patient Assessment of Constipation Quality of Life Questionnaire (PAC-QOL). The validated PAC-QOL is composed of 28 items grouped into four subscales: physical discomfort, psychosocial discomfort, worries and concerns, and satisfaction.18 The first three subscales comprise the patient dissatisfaction index, with an overall score ranging from 0 to 96 (where higher scores correspond to worse quality of life). The satisfaction subscale includes four items that produce a combined score ranging from 0 to 16, with scores defined as poor (0–4), fairly good (5–8), good (9–12), or excellent (13–16).
Secondary measures were a modified version of the Bowel Function Index (BFI) for patient self-completion, original questions to measure change in hemorrhoid impact, the Short Form Health Survey version 2 (SF-12v2), and original questions (for the perineal self-acupressure group only) regarding use and effectiveness of the technique and associated educational materials. The modified BFI was a three-item questionnaire that measured bowel symptoms by asking patients to rate ease of defecation, feeling of incomplete bowel evacuation, and constipation on a scale from 0 to 100 (where higher scores indicate greater disease impact).19 The hemorrhoid questions asked participants whether they had experienced new episodes with hemorrhoids in the last 4 weeks, and to rate bleeding, itching, and pain associated with hemorrhoids during that time frame. The SF-12 is a validated multipurpose short survey with 12 questions, all selected from the SF-36 Health Survey.20 Physical and Mental Health Composite Scores (PCS & MCS) are computed using the scores of 12 questions and range from 0 to 100, where higher scores indicate better quality of life. Finally, the perineal self-acupressure group was asked a series of open and closed-ended questions related to their use of perineal self-acupressure, its perceived effectiveness, their desire to continue using the technique and to recommend it to others, and the effectiveness of the educational materials. All patient survey instruments appear in Online Appendix 3.
Statistical Analysis
Analyses were based on the intention-to-treat method in all randomized participants. Missing outcomes during the follow-up period were imputed using the last-observation-carried-forward method. To assess the robustness of the analytical results under alternative imputation methods, analyses were repeated on all outcomes using multiple regression imputation as well as available data only (no imputation). Results did not differ between these two methods and the last-observation-carried-forward method. Treatment effects were also estimated using Seemingly Unrelated Regression (SUR), Ordered Logit, and Instrumental Variables regressions. Potential interactions between treatment and other factors, also used to assess heterogeneity of results across subgroups, were also tested with patient fixed-effect and difference-in-difference regressions. See Online Appendix 4. All reported confidence intervals (CIs) were two-sided 95 % intervals, and tests were two-sided with a 5 % significance level. All analyses were performed with STATA version 13 (Stata Corp, College Station, Texas).
RESULTS
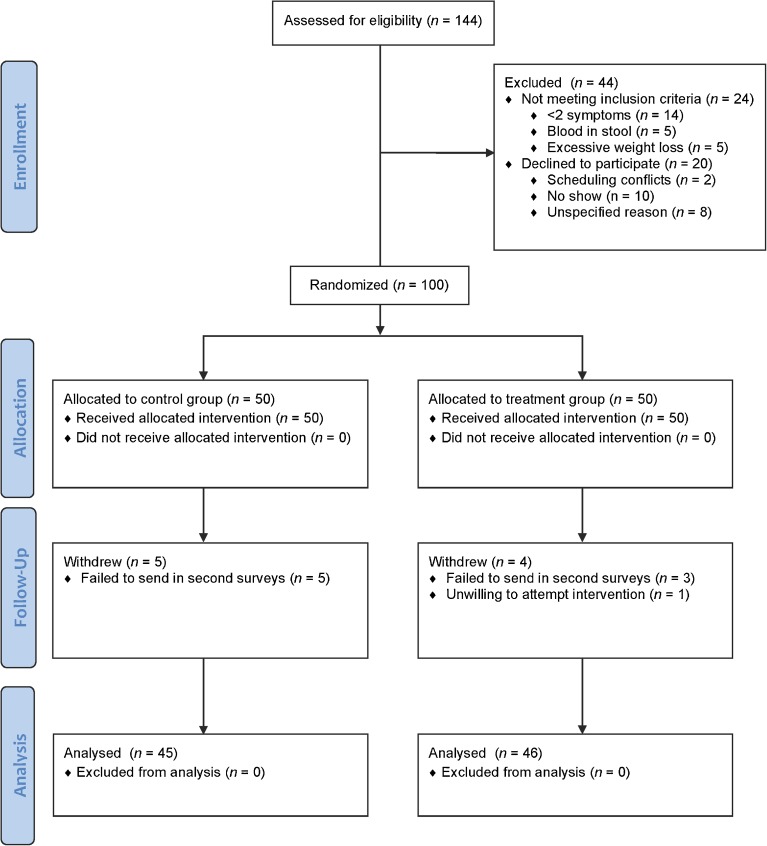
Of the 144 patients evaluated for eligibility, 44 were excluded: 24 for not meeting the inclusion criteria and 20 for declining to participate (Fig. 1). Of the 100 eligible patients that underwent randomization and completed the baseline surveys, 50 were randomly assigned to the control group and 50 were randomly assigned to the treatment group. Eight patients were lost to follow-up (five from the control group failed to return their follow-up surveys, and three from the treatment group failed to return their follow-up surveys). One patient from the treatment group declined to practice perineal self-acupressure after receiving oral instruction. A total of 91 patients (46 in treatment group and 45 in control group) reached the primary endpoint by completing a second survey 1 month after the initial intervention. There were no reported adverse events.
Figure 1.
Participant enrollment and follow-up.
The treatment and control groups were well balanced in terms of age, race, and baseline measures of primary outcomes (Table 1). There were more men in the treatment group than in the control treatment group (34.0 vs. 16.0 %; p = 0.04), and the baseline measure of the Physical Component Score of the SF-12v2 was lower for the treatment group than for the control group (46.4 vs. 50.5; p = 0.03). The other baseline variables did not differ significantly between groups.
Table 1.
Baseline Patient-Reported Outcome Data
| Baseline demographic data | Control | Treatment | p Value |
|---|---|---|---|
| (N = 50) | (N = 50) | ||
| Male, no. (%) | 8 (0.16) | 17 (0.34) | 0.04 |
| Age, mean (SD) | 44.5 (17.2) | 47.6 (18.2) | 0.39 |
| Race and ethnicity* | |||
| American Indian, no. (%) | 4 (0.08) | 4 (0.08) | 1.00 |
| Asian, no. (%) | 11 (0.22) | 7 (0.14) | 0.30 |
| Black, no. (%) | 10 (0.20) | 11 (0.22) | 0.81 |
| White, no. (%) | 32 (0.64) | 31 (0.62) | 0.62 |
| Hispanic, no. (%) | 9 (0.18) | 11 (0.22) | 0.84 |
| Primary outcomes | |||
| Patient assessment of constipation PAC-QOL (scale: 0–4) | |||
| Physical discomfort | 2.30 | 2.45 | 0.35 |
| Psychosocial discomfort | 1.43 | 1.67 | 0.15 |
| Worries and concerns | 2.15 | 2.30 | 0.36 |
| Satisfaction | 0.66 | 0.86 | 0.13 |
| All | 2.17 | 2.29 | 0.38 |
| Secondary outcomes | |||
| Bowel function index score | 68.20 | 68.95 | 0.83 |
| Hemorrhoid score | 0.59 | 0.82 | 0.16 |
| SF-12v2 | |||
| Physical component score | 50.49 | 46.39 | 0.03 |
| Mental component score | 41.44 | 41.60 | 0.93 |
* Race totals do not add up to the total in each group because nine patients marked more than one race
Improved Patient Assessment of Constipation Quality of Life
The mean patient-reported quality-of-life measures differed significantly between the treatment and control groups (Table 2). The mean overall PAC-QOL improved by 0.76 units in the perineal-pressure treatment group and by 0.17 units in the control group 4 weeks after randomization (treatment-effect difference, 0.59 [95 % CI, 0.37 to 0.81]; p < 0.01). The treatment-effect improvements for each of the PAC subgroups were also estimated to be statistically significant. The mean Physical Discomfort Score improved by 0.84 units in the treatment group and by 0.23 units in the control group (treatment-effect difference, 0.61 [95 % CI, 0.34 to 0.88]; p < 0.01). The mean Psychosocial Discomfort Score improved by 0.63 units in the treatment group and by 0.15 units in the control group (treatment-effect difference, 0.48 [95 % CI, 0.22 to 0.74]; p < 0.01). The mean Worries and Concerns Score improved by 0.82 units in the treatment group and by 0.14 units in the control group (treatment-effect difference, 0.68 [95 % CI, 0.40 to 0.95]; p < 0.01). The mean Satisfaction Score improved by 0.77 units in the treatment group and by 0.21 units in the control group (treatment-effect difference, 0.56 [95 % CI, 0.22 to 0.89]; p < 0.01). The PAC-QOL survey is comprised of 28 individual questions; 23 of these questions were estimated to have statistically significant (p < 0.05) treatment-effect improvements. See Online Appendix 4.
Table 2.
Treatment Effects for Perineal Self-Acupressure
| Mean change from baseline | Tr. effect | (95 % Confidence interval) | p value | |||
|---|---|---|---|---|---|---|
| Variable | Control | Treatment | Low | High | ||
| Treatment effects on physical and psychological outcomes after 4 weeks* | ||||||
| Primary outcomes | ||||||
| Patient assessment of constipation | ||||||
| Physical discomfort | −0.23 | −0.84 | −0.61 | −0.88 | −0.34 | < 0.001 |
| Psychosocial discomfort | −0.15 | −0.63 | −0.48 | −0.74 | −0.22 | 0.001 |
| Worries and concerns | −0.14 | −0.82 | −0.68 | −0.95 | −0.40 | < 0.001 |
| Satisfaction | 0.21 | 0.77 | 0.56 | 0.22 | 0.89 | 0.001 |
| All | −0.17 | −0.76 | −0.59 | −0.81 | −0.37 | < 0.001 |
| Secondary outcomes | ||||||
| SF-12v2 | ||||||
| Physical component score | −0.36 | 2.69 | 3.05 | 0.85 | 5.25 | 0.008 |
| Mental component score | 0.30 | 3.12 | 2.82 | −0.10 | 5.74 | 0.061 |
| Modified bowel function index | −4.2 | −18.1 | −13.8 | −22.5 | −5.1 | 0.002 |
| Hemorrhoid symptom score | −0.03 | −0.43 | −0.40 | −0.63 | −0.17 | 0.001 |
* Values are the mean change from the baseline for the treatment and control groups using a Last Observation Carried Forward (LOCF) method for dealing with the nine missing values. All data included. ITT analysis. p values based on heteroskedastic robust standard errors
Patient-Reported Secondary Outcomes
The mean estimated treatment effects of the two subcomponents of the SF-12 indicate improved health and wellbeing, but only the effect for the Physical Component Score was significant at the 5 % level. The mean Physical Component Score of SF-12v2 improved by 2.69 units in the treatment group and declined by 0.36 units in the control group (treatment-effect difference, 3.05 [95 % CI, 0.85 to 5.25]; p < 0.01). The Mental Component Score improved by 3.12 units in the treatment group and by 0.30 in the control group (treatment-effect difference, 2.82 [95 % CI, −0.10 to 5.74]; p = 0.061).
The modified BFI and the Hemorrhoid Symptom indexes both exhibited statistically significant improvement in patient reports. The mean modified BFI improved by 18.1 units in the treatment group and by 4.2 units in the control group (treatment-effect difference, 13.8 [95 % CI, 5.1 to 22.5]; p < 0.01). The mean Hemorrhoid Symptom Index score improved by 0.43 in the treatment group and by 0.03 in the control group (treatment-effect difference, 0.40 [95 % CI 0.17 to 0.63]; p < 0.01). The estimated treatment effect for pain associated with a hemorrhoid was a statistically significant improvement (treatment-effect difference, 0.68 [95 % CI 0.35 to 1.02]; p < 0.01).
A multivariate regression separately controlling for all the demographic and baseline variables in Table 1 confirmed that the treatment effects reported in Table 2 remain robustly significant. See Online Appendix 4. Moreover, analyses of male-only and female-only subsamples were estimated as statistically significant on 13 of the 18 primary and secondary outcomes. A test for heterogeneous treatment effects found that patients who reported worse baseline hemorrhoid-associated symptoms (of bleeding, itching and pain) were estimated to have significantly higher treatment effects. See Online Appendix 4.
In addition, patients in the treatment group reported substantial satisfaction with perineal self-acupressure (in response to questions only asked to the treatment group). After conservatively assuming the most dissatisfied response to the four treatment group members who were lost to follow-up, 88 % of the treatment group reported using the technique, and the mean frequency of all 50 members was estimated to be 3.6 times per week. Of the treatment group, 72 % reported that the perineal pressure technique helped them to “break up, soften, or pass [their] stools.” Fifty-four percent reported that the technique helped them to “avoid having a hemorrhoid or lessened the impact of an existing hemorrhoid.” Seventy two percent reported that the technique helped them to “avoid or better manage the effects of constipation.” Eighty-two percent of the treatment group patients indicated that they would continue to use the technique, and 72 % indicated that they would recommend the technique to family and friends. See Online Appendix 4.
DISCUSSION
This is the first randomized trial with sufficient statistical power to test the effect of perineal self-acupressure on patient-reported outcomes. The PSAC trial has produced evidence of perineal self-acupressure treatment effects for PAC-QOL and modified BFI measures that are statistically significant and clinically meaningful. The estimated PAC-QOL improvement of 0.59 exceeds the 0.5 standard deviation (SD) standard that has been used to define a clinically meaningful difference with respect to interventions for constipation.21 The estimated treatment-effect improvement on the modified BFI of 13.8 exceeds both the 0.5 SD standard and the 12-point standard for changes that “are likely to be related to clinically meaningful changes in patient’s perception of their bowel habits” [18, p. 382]. The modified BFI treatment effect also exceeds the standard error of measurement (SEM).22 Our SEM estimate of 5.93 further supports the conclusion that the estimated treatment-effect improvement on the modified BFI is clinically meaningful.18
Our estimated treatment-effect improvements are generally larger and more statistically significant using the alternative multiple imputation, completed case, SUR and IV analyses. See Online Appendix 4.
This study suggests that clinicians should consider incorporating education in perineal self-acupressure as a first-line treatment for constipation, along with conventional interventions such as increased exercise and dietary fiber intake. As a non-invasive, non-pharmacological treatment intervention for constipation, perineal self-acupressure likely carries a lower risk for side effects and complications than commonly used medications such as stool softeners, fiber supplements, stimulants, laxatives, and lubricants. In addition, perineal self-acupressure may help to control treatment costs because it only requires a brief, initial period of training. Furthermore, not all patients respond favorably to existing treatment options, and perineal self-acupressure may represent an effective alternative to conventional treatment options. Finally, as this study has shown, perineal self-acupressure also benefits health-related quality of life.
The PSAC trial, however, has important limitations. The sample size is modest, as fewer than 100 patients completed the study. Despite careful randomization, the trial was not well balanced with regard to patients’ gender or baseline SF-12 Physical Component Score. Most importantly, the PSAC trial, like all behavioral intervention trials, was not blinded. Patients in the treatment group knew that they were performing perineal self-acupressure, and the researchers knew which patients received training. While all patients received education with respect to standard care, it is possible that patients in the treatment group, surmising that they were receiving a non-standard training, responded more positively than patients in the control group because of a placebo-like effect (possibly combined with a Hawthorne observer effect).
As with all trials, there are also questions of whether the treatment effects are generalizable to other conditions and patient populations. For example, it is unclear whether perineal self-acupressure may be used to prevent constipation—an application that may merit future exploration. Likewise, it is uncertain how perineal self-acupressure would impact patients with a history of hemorrhoids or active hemorrhoids. The PSAC trial eligibility was dependent on meeting Rome III criteria for functional constipation. While patients with poorer baseline scores on the Hemorrhoid Symptom Index had statistically higher treatment effects, only 19 % of the PSAC trial subjects (N = 10 in treatment group, nine in control group) reported at baseline having experienced new episodes with hemorrhoids in the last 4 weeks. Thus, further testing is required before making any confident inferences about the impact of perineal self-acupressure on patients with historic or active hemorrhoid problems. Similarly, it is uncertain whether perineal self-acupressure would aid patients with rectal wall abnormalities (such as rectocoele or descending perineum syndrome)8,9 or impaired rectal tone.11
Additionally, it is uncertain whether similar pressure techniques would produce analogous improvements to patient-reported outcome. Some female patients have reported “applying transvaginal pressure [to] aid fragmentation and expulsion” [14, p. 661]. Applying rostral pressure to the perineal area below the coccyx might also be used to fragment hardened stools. An intervention that trained patients in alternative methods of applying pressure might secure higher patient compliance by letting patients choose the form of pressure that they prefer.
CONCLUSIONS
A four-week intervention of perineal self-acupressure in addition to standard care was more effective than standard care alone at improving constipation-related quality of life, bowel function and health, and well-being among patients with constipation. Clinicians should consider incorporating education in perineal self-acupressure as a treatment for constipation, along with conventional interventions such as increased exercise and dietary fiber intake.
Electronic supplementary material
(DOCX 2721 kb)
Acknowledgements
Dr. Abbott had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors would like to thank research assistants Katherine Diep, Lance Powell, and Samantha Phung for their excellent work. The authors would also like to thank Jake Richardson, Sidney Bogardus, and Wen Boynton for their insightful comments. The study was funded with support from the Annenberg Foundation, the Gerald Oppenheimer Family Foundation, the Panda Foundation, and Yale University. The sponsors played no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Funding
The study was funded with support from the Annenberg Foundation, the Gerald Oppenheimer Family Foundation, the Panda Foundation, and Yale University.
Footnotes
Trial Registration: ClinicalTrials.gov: NCT01867944.
REFERENCES
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–9. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology. 1990;98(2):380–6. doi: 10.1016/0016-5085(90)90828-o. [DOI] [PubMed] [Google Scholar]
- 3.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349(14):1360–8. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 4.Sun SX, Dibonaventura M, Purayidathil FW, Wagner JS, Dabbous O, Mody R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sci. 2011;56(9):2688–95. doi: 10.1007/s10620-011-1639-5. [DOI] [PubMed] [Google Scholar]
- 5.Koloski NA, Jones M, Wai R, Gill RS, Byles J, Talley NJ. Impact of persistent constipation on health-related quality of life and mortality in older community-dwelling women. Am J Gastroenterol. 2013;108(7):1152–8. doi: 10.1038/ajg.2013.137. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Mikami S, LeClair J, et al. Inpatient burden of constipation in the United States: an analysis of national trends in the United States from 1997 to 2010. Am J Gastroenterol. 2014;109(2):250–6. doi: 10.1038/ajg.2013.423. [DOI] [PubMed] [Google Scholar]
- 7.Stamp G, Kruzins G, Crowther C. Perineal massage in labour and prevention of perineal trauma: randomised controlled trial. BMJ. 2001;322(7297):1277–80. doi: 10.1136/bmj.322.7297.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rath-Harvey D, Pemberton JH. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94(1):126–30. doi: 10.1111/j.1572-0241.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarles JC, Arnaud A, Selezneff I, Olivier S. Endorectal repair of rectocele. Int J Colorectal Dis. 1989;4(3):167–71. doi: 10.1007/BF01649696. [DOI] [PubMed] [Google Scholar]
- 10.Parker MC, Phillips RK. Repair of rectocoele using Marlex mesh. Ann R Coll Surg Engl. 1993;75(3):193–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Shafik A, Ahmed I, El-Sibai O. Effect of perineal compression on the rectal tone: a study of the mechanism of action. Dis Colon Rectum. 2003;46(10):16–70. doi: 10.1007/s10350-004-6751-6. [DOI] [PubMed] [Google Scholar]
- 12.Gosselink M, Schouten W. The perineorectal reflex in health and obstructed defecation. Dis Colon Rectum. 2002;45(3):370–6. doi: 10.1007/s10350-004-6185-1. [DOI] [PubMed] [Google Scholar]
- 13.Prather C, Ortiz-Camacho C. Evaluation and treatment of constipation and fecal impaction in adults. Mayo Clin Proc. 1998;73(9):881–6. doi: 10.4065/73.9.881. [DOI] [PubMed] [Google Scholar]
- 14.Wrenn K. Fecal impaction. N Engl J Med. 1989;321(10):658–62. doi: 10.1056/NEJM198909073211007. [DOI] [PubMed] [Google Scholar]
- 15.Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: ‘idiopathic slow transit constipation. Gut. 1986;27(1):41–8. doi: 10.1136/gut.27.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halligan S, Bartram CI. Is digitation associated with proctographic abnormality? Int J Colorectal Dis. 1996;11(4):167–71. doi: 10.1007/s003840050036. [DOI] [PubMed] [Google Scholar]
- 17.Alonso-Coello P, Guyatt G, Heels-Ansdell D, et al. Laxatives for the treatment of hemorrhoids. Cochrane Database Syst Rev. 2005;19(4):CD004649. doi: 10.1002/14651858.CD004649.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rentz AM, Yu R, Muller-Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12(4):371–83. doi: 10.3111/13696990903430481. [DOI] [PubMed] [Google Scholar]
- 19.Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW. Defecography in normal volunteers: Results and implications. Gut. 1989;30(12):1737–49. doi: 10.1136/gut.30.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandek B, Ware JE, Aaronson NK, et al. Cross-Validation of item selection and score for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J Clin Epidemiol. 1998;51(11):1171–8. doi: 10.1016/S0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 21.Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40(5):540–51. doi: 10.1080/00365520510012208. [DOI] [PubMed] [Google Scholar]
- 22.Wyrwich KW, Tierney WM, Wolinsky FD. Using the standard error of measurement to identify important changes on the Asthma Quality of Life Questionnaire. Qual Life Res. 2002;11(1):1–7. doi: 10.1023/A:1014485627744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 2721 kb)