Abstract
Introduction
Acepromazine is a phenothiazine that is used exclusively in veterinary medicine for multiple purposes. Human overdoses are rarely reported and toxicokinetic data has never been reported. We present a case of intentional acepromazine overdose resulting in central nervous system and cardiovascular toxicity with confirmatory toxicokinetic data.
Case Report
A 54-year-old woman intentionally ingested 950 mg of her dog’s acepromazine. Within 3 h of ingestion, she developed central nervous system and respiratory depression along with hypotension requiring non-invasive ventilation and vasopressors. Clinical toxicity resolved over the following 8 h. Serial plasma acepromazine levels were determined using gas chromatography/mass spectrometry. The initial acepromazine level (1-h post-ingestion) was 63 ng/ml. Follow-up levels at 8-, 10.5-, and 13.5-h post-ingestion were 8.9 ng/ml, 7.6 ng/ml, and 6.3 ng/ml, respectively.
Discussion
Human acepromazine toxicity is rarely reported but results in clinical toxicity (central nervous system depression, respiratory depression, hypotension) are similar to other phenothiazines. Compared to other phenothiazines, it appears to have a short elimination half-life that may account for the brief duration of clinical toxicity with relatively rapid improvement. No significant human cardiac toxicity has been reported. Treatment is supportive.
Conclusion
This case highlights the unique toxicity of acepromazine in demonstrating rapid improvement of severe toxicity within 8 h consistent with a short elimination half-life.
Keywords: Acepromazine, Veterinary medication, Phenothiazine
Introduction
Acepromazine is a phenothiazine derivative that was introduced in the 1950s for the treatment of schizophrenia [1]. Its use in humans was quickly abandoned due to side effects and lack of efficacy [1]; however, it remains popular in veterinary medicine. In both small and large animals, it is used for motion sickness, sedation, and pre-operative anxiolysis [2–4]. A combination product of acepromazine and etorphine, a synthetic opioid with potency of 1,000–80,000× morphine, is commercially available and used primarily in large animals [5]. Although it has been available for several decades, cases of isolated human acepromazine exposure and toxicity are rarely described and toxicokinetic data has not been reported. We report a case of intentional acepromazine overdose resulting in central nervous system (CNS) and cardiovascular toxicities with confirmatory toxicokinetic data.
Case Report
A 54-year-old woman presented to a local emergency department 1 h after intentionally ingesting 950 mg total of her dog’s acepromazine tablets. She denied other coingestants. Her past medical history was significant for depression, anxiety, and hypothyroidism. She denied current medication use. She drank alcohol occasionally but denied illicit drug use. Vitals signs on arrival were the following: temperature 99 F, HR 100 beats/min, BP 110/70 mmHg, RR 20 breaths/min, and oxygen saturation 100 % on room air. Physical examination demonstrated that she was alert and answering questions appropriately with 4-mm reactive pupils and a non-focal neurologic exam. Activated charcoal was administered. Laboratory studies were unremarkable including complete blood count, basic metabolic profile, and liver function tests. Serum acetaminophen, salicylate, and ethanol were undetectable. An immunoassay urine drug screen was negative. An electrocardiogram (ECG) demonstrated a heart rate of 104 beats/min, QRS of 80 msec, and QTc of 421 msec. Over the next 2 h, the patient developed increasing somnolence, tachycardia, and hypotension. The heart rate ranged from 100–130/min and the systolic BP fell to 70 mmHg. Arterial blood gas demonstrated a mild respiratory acidosis (pH 7.25, pCO2 50 mmHg, HCO3 21 meq/l). She was given 3 l of normal saline without improvement in her blood pressure. Bilevel positive pressure ventilation (bipap) and a norepinephrine drip (6 mcg/min) were instituted, and she was admitted to the intensive care unit. She slowly improved, and the bipap and norepinephrine were weaned and discontinued after 8 h. Repeat laboratory studies and ECGs remained normal, and the patient was transferred to a psychiatric facility the following day. Serial plasma acepromazine levels were determined using gas chromatography/mass spectrometry. The initial acepromazine level (1-h post-ingestion) was 63 ng/ml. Follow-up levels at 8, 10.5, and 13.5 h post-ingestion were 8.9 ng/ml, 7.6 ng/ml, and 6.3 ng/ml, respectively. These levels demonstrated first-order kinetics when plotted using linear regression on a semi-log graph versus time. This resulted in an elimination half-life of 2.95 h.
Discussion
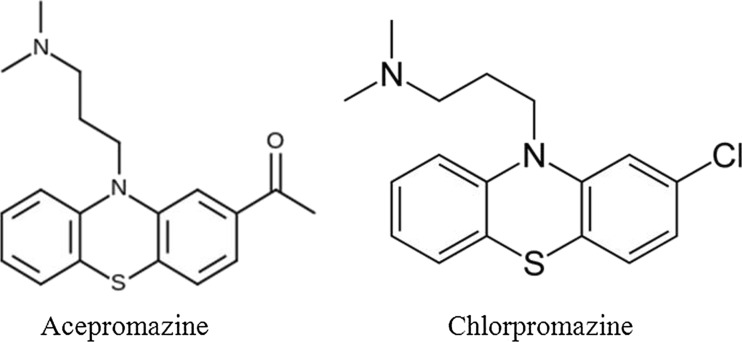
Acepromazine is a phenothiazine that shares structural similarity to chlorpromazine (Fig. 1). It is marketed for veterinary use and there are no approved human uses. It is formulated for both oral (5, 10, 25 mg tablets) and parenteral (10 mg/ml) uses. The typical oral dose in animals is 0.5–2.2 mg/kg, whereas the recommended parenteral dose is 0.5–1.1 mg/kg. Human toxicity has resulted from both unintentional and intentional exposures. There are also rare reports of it being used as an agent for drug-facilitated sexual assault.
Fig. 1.
Structures of acepromazine and chlorpromazine
Pharmacologically, acepromazine is similar to other phenothiazines. It acts as an antagonist at D1-4 dopaminergic and 5-HT1,2 receptors. It also antagonizes histamine, muscarinic acetylcholine, and alpha-1 receptors. In animals, it is thought to be highly lipophilic with high protein binding and a large volume of distribution [6]. The toxic amount of acepromazine in humans has not been established, and dosing estimates in overdose cases are not well reported [7–13]. In symptomatic cases of isolated oral acepromazine exposure, the ingested dose ranged from 75–1,250 mg [10, 11]. The toxicokinetics of acepromazine in humans has not been described; however, previous analysis has identified 2-(1-hydroxyethyl)promazine as a major metabolite [14]. No whole blood or plasma acepromazine levels have been reported in previous overdose cases (excluding fatalities). The peak acepromazine level in our case was 63 ng/ml with an elimination half-life of 2.95 h.
Clinically, cases of overdose manifest toxicity similar in appearance to other phenothiazines. Our case is similar to previous published cases that support the rapid development of CNS depression as the most prominent finding following both oral and parenteral exposures [8–11, 13]. However, in several of these cases, other medications likely contributed to CNS depression (etorphine, flunixin) [8, 9]. A unique finding with acepromazine is that the CNS depression resolves more quickly than what is typically observed with other cases of phenothiazine toxicity. Our case had resolution of her CNS and respiratory depression with 8 h of her exposure. In other cases of isolated acepromazine overdose, CNS depression has resolved within 6–12 h [10, 11]. The quick elimination half-life observed could explain a shorter duration of toxicity.
Sinus tachycardia is the most common cardiovascular manifestation of toxicity [8, 10, 11]. Hypotension, presumably from the peripheral alpha adrenergic blockade, has also been reported [8]. No cases of arrhythmia, QRS, or QTc prolongation have been reported.
Treatment of toxicity focuses on providing supportive care. Given the risk of progressive CNS depression, any potential benefit of decontamination must be weighed against the risk of aspiration. Close monitoring of the airway and mental status is prudent. Endotracheal intubation may be necessary in cases of profound CNS or respiratory depression. Alternatively, as in our case, non-invasive ventilation may be employed in those cases of respiratory depression if airway reflexes remain intact. Cardiac monitoring is necessary, and treatment of hypotension should initially focus on fluid resuscitation with initiation of vasopressors in refractory cases. An ECG should be performed to assess the QRS and QT intervals.
Routine laboratory studies are often performed to rule out other etiologies or complications. Acepromazine levels (whole blood or plasma) may be sent to a reference laboratory for exposure confirmation. Post-mortem analysis has confirmed the presence of acepromazine in several suicide cases [7, 8, 14]. Blood levels in these cases ranged from 600–2,400 ng/ml [7, 15]. Post-mortem drug levels must be interpreted with caution as in vitro metabolism of the parent compound occurs and may result in erroneously low or absent acepromazine concentrations [14]. Identification of 2-(1-hydroxyethyl)promazine, the major metabolite, may be beneficial in clarifying those cases in which there are negative acepromazine levels [13, 14]. Based on its pharmacokinetics, post-mortem drug redistribution likely occurs with acepromazine [7, 16]. Hair analysis, using liquid chromatography-electrospray-tandem mass spectrometry, identified acepromazine up to 1.5 months post-exposure in a case of drug-facilitated sexual assault [13].
Conclusion
Acepromazine is a phenothiazine derivative that like many veterinary medications is capable of causing significant human toxicity. Toxicity is associated with CNS depression, respiratory depression, and hypotension. We present the first report of acepromazine toxicokinetics and highlight issues associated with laboratory and forensic analyses. Health-care providers should be aware of the toxicity associated with acepromazine as well as that the toxicokinetics suggests a short elimination half-life that is consistent with a shorter duration of clinical toxicity than is observed with many other phenothiazines.
Acknowledgments
Conflict of Interest
The authors have no conflicts of interest, and no funding was obtained for preparation of this manuscript.
References
- 1.Collard JF, Maggs R. Clinical trial of acepromazine maleate in chronic schizophrenia. BMJ. 1958;1(5085):1452–1454. doi: 10.1136/bmj.1.5085.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans AL, Fahlman A, Ericsson G, Haga HA, Arnemo JM. Physiological evaluation of free-ranging moose (Alces alces) immobilized with etorphine-xylazine-acepromazine in Northern Sweden. Acta Vet Scand. 2012;54:77. doi: 10.1186/1751-0147-54-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grint NJ, Alderson B, Dugdale AH. A comparison of acepromazine-buprenorphine and medetomidine-buprenorphine for preanesthetic medication of dogs. J Am Vet Med Assoc. 2010;237(12):1431–1437. doi: 10.2460/javma.237.12.1431. [DOI] [PubMed] [Google Scholar]
- 4.Driessen B, Zarucco L, Kalir B, Bertolotti L. Contemporary use of acepromazine in the anesthetic management of male horses and ponies: a retrospective study and opinion poll. Equine Vet J. 2011;43(1):88–98. doi: 10.1111/j.2042-3306.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- 5.Blane GF, Boura AL, Fitzgerald AE, Lister RE. Actions of etorphine hydrochloride, (M99): a potent morphine-like agent. Br J Pharmacol Chemother. 1967;30:11–22. doi: 10.1111/j.1476-5381.1967.tb02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballard S, Shults T, Kownacki AA, Blake JW, Tobin T. The pharmacokinetics, pharmacological responses and behavioral effects of acepromazine in the horse. J Vet Pharmacol Ther. 1982;5:21–31. doi: 10.1111/j.1365-2885.1982.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 7.Stowell LI. Suicide with the veterinary drug acepromazine. J Anal Toxicol. 1998;22:166–168. doi: 10.1093/jat/22.2.166. [DOI] [PubMed] [Google Scholar]
- 8.Sterken J, Troubleyn J, Gasthuys F, et al. Intentional overdose of Large Animal Immobilon. Eur J Emerg Med. 2004;11:298–301. doi: 10.1097/00063110-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Kamali MF, Wilson AC, Acquisto NM, Spillane L, Schneider SM. Acute encephalopathy with concurrent respiratory and metabolic disturbances in first known parenteral human administration of flunixin meglumine and acepromazine maleate. J Emerg Med. 2013;45:206–209. doi: 10.1016/j.jemermed.2012.11.088. [DOI] [PubMed] [Google Scholar]
- 10.Berns SD, Wright JL. Pediatric acepromazine poisoning: the importance of child-resistant packaging for veterinary drugs. Am J Emerg Med. 1993;11:247–248. doi: 10.1016/0735-6757(93)90137-Z. [DOI] [PubMed] [Google Scholar]
- 11.Clutton RE. Attempted suicide with acepromazine maleate: a case report. Vet Hum Toxicol. 1985;27:391. [PubMed] [Google Scholar]
- 12.Bryant SM, Mycyk MB. Human exposures to pet prescription medications. Vet Hum Toxicol. 2002;44:218. [PubMed] [Google Scholar]
- 13.Gaulier JM, Sauvage FL, Pauthier H, et al. Identification of acepromazine in hair: an illustration of the difficulties encountered in investigating drug-facilitated crimes. J Forensic Sci. 2008;53:755–759. doi: 10.1111/j.1556-4029.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- 14.Elliott SP, Hale KA. A previously unidentified acepromazine metabolite in humans: implications for the measurement of acepromazine in blood. J Anal Toxicol. 1999;23:367–371. doi: 10.1093/jat/23.5.367. [DOI] [PubMed] [Google Scholar]
- 15.Tracqui A, Kintz P, Mangin P. A fatality involving two unusual compounds—zolpidem and acepromazine. Am J Forensic Med Pathol. 1993;14:309–312. doi: 10.1097/00000433-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Leikin JB, Watson WA. Post-mortem toxicology: what the dead can and cannot tell us. J Toxicol Clin Toxicol. 2003;41:47–56. doi: 10.1081/CLT-120018270. [DOI] [PubMed] [Google Scholar]