Abstract
Context
Massive naproxen overdose is not commonly reported. Severe metabolic acidosis and seizure have been described, but the use of renal replacement therapy has not been studied in the context of overdose.
Case Details
A 28-year-old man ingested 70 g of naproxen along with an unknown amount of alcohol in a suicidal attempt. On examination in the emergency department 90 min later, he was drowsy but had normal vital signs apart from sinus tachycardia. Serum naproxen level 90 min after ingestion was 1,580 mg/L (therapeutic range 25–75 mg/L). He developed metabolic acidosis requiring renal replacement therapy using sustained low efficiency dialysis (SLED) and continuous venovenous hemofiltration (CVVH) and had recurrent seizure activity requiring intubation within 4 h from ingestion. He recovered after 48 h.
Discussion
Massive naproxen overdose can present with serious toxicity including seizures, altered mental status, and metabolic acidosis.
Conclusion
Hemodialysis and renal replacement therapy may correct the acid base disturbance and provide support in cases of renal impairment in context of naproxen overdose, but further studies are needed to determine the extraction of naproxen.
Keywords: Naproxen, Seizure, Metabolic acidosis, Hemodialysis
Introduction
Naproxen is a propionic acid type of nonsteroidal anti-inflammatory agent with analgesic, antipyretic, and anti-inflammatory properties. Despite being FDA approved in 1994 for over-the-counter use, naproxen is still not commonly reported among overdoses compared to other analgesics [1]. Naproxen is extensively protein bound to plasma proteins, primarily to albumin and to smaller degree globulins. Protein binding is 99.6 % with plasma concentrations between 23 and 40 mg/L, but as naproxen concentrations increase, protein binding declines, resulting in a higher fraction of unbound drug [2] allowing for possible removal by renal replacement therapy in massive poisonings accompanied by high levels. There are no previous reports on the effect of renal replacement therapy on naproxen levels. We report the first case of massive naproxen overdose (70 g) suffering serious toxicity with documented serial serum levels.
Case Report
A 28-year-old male with a past history of depression, ethanol abuse, and a previous suicidal attempt was brought to the emergency department (ED) by EMS following acute ingestion of approximately 320 tablets of naproxen 220 mg (70.4 g) with an unknown quantity of alcohol 90 min before arrival. He was given a single dose of activated charcoal by the paramedics in the ambulance. Upon arrival in the ED, the patient’s blood pressure was 117/57 mmHg, heart rate was 118/min, temperature was 37.1 °C, respiratory rate was 16/min, and pulse oximetry saturation (SPO2) was 99 % on room air. His initial exam was notable only for drowsiness and lethargy with a Glasgow Coma Score (GCS) of 13/15. His skin was warm and dry. Pupils were small and reactive to light. Laboratory studies showed hemoglobin 16.4 g/dL, hematocrit 45 %, white blood cell count 10,400/μL, and platelets 186,000/μL. Metabolic panel revealed sodium 141 mmol/L, potassium 3.5 mmol/L, chloride 102 mmol/L, bicarbonate 19 mmol/L, BUN 13 mg/dL, creatinine 0.81 mg/dL, and glucose 205 mg/dL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 150 and 224 IU/L, respectively. Total bilirubin was 3.4 mg/dL, alkaline phosphatase was 89 IU/L, albumin was 5.1 g/dL, and INR was 0.9. Ethanol was 166 mg/dL. Acetaminophen and aspirin were undetectable. Urine drug screening was negative for amphetamines, barbiturates, benzodiazepines, cocaine, methadone, phencyclidine (PCP), cannabinoids, and opiates. Admission serum naproxen concentration was 1,580 mg/L. Initial chest x-ray was normal. Electrocardiogram (ECG) showed sinus tachycardia with normal intervals.
One hour after ED arrival, the patient had a generalized tonic-clonic seizure, which responded to lorazepam. After a second seizure, he was intubated endotracheally and sedated with midazolam and propofol and loaded with intravenous phenytoin on the advice of a neurologist. A computed tomographic (CT) scan of his head was negative. An electroencephalogram (EEG) showed generalized slowing consistent with encephalopathy and sedation.
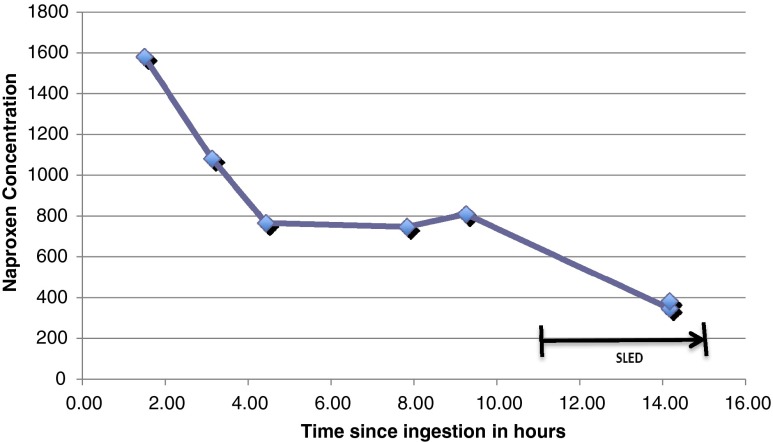
He was treated with IV N-acetylcysteine because of the possibility of an unrecognized delayed-presentation acetaminophen overdose with a standard 21 h IV course. He was admitted to the intensive care unit (ICU), and his blood gases performed 4 h post admission showed pH 7.14, PCO2 32, PO2 224, HCO3 10 mmol/L, and lactate 16.7 mmol/L. He developed hypotension 6 h post admission and was started on a norepinephrine drip. The nephrologist and the intensivist decided on renal replacement therapy as the patient became anuric with progressive acidosis 5 h post admission, and his creatinine increased to 1.72 mg/dL despite aggressive fluid resuscitation. He was treated with sustained low efficiency dialysis (SLED) for 4 h followed by continuous venovenous hemofiltration (CVVH) for 60 h. Naproxen levels were measured in serial serum samples using liquid chromatography tandem mass spectrometry (Agilent 1200 LC system and ABSciex 3200 QTRAP®), as depicted in Fig 1. Pre- and post-cartridge (“arterial” and “venous”) samples were taken, but unfortunately, these were obtained near the end of the procedure, and at that time, the levels were essentially equal (pre-cartridge 347 mg/L, post-cartridge 383 mg/L; precision +/−15 %), indicting no significant extraction by the procedure, probably because of extensive protein binding at these lower levels. Repeated lactate levels trended down to 8.6 mmol/L and then 2.3 mmol/L. His creatine kinase (CK) level peaked the next day at 10,000 U/L. Table 1 Summarizes selected laboratory results. His acidosis improved and he was extubated by day 3 of admission, and his creatinine normalized to 0.94 mg/dL. His urinalysis and urine microscopy were normal other than trace protein and ketones. Renal ultrasound was also normal. Transaminase levels fell to AST 139 IU/L and ALT 144 IU/L and creatinine to 0.74 mg/dL. He was cleared to the psychiatry service after 7 days.
Fig. 1.
Serial naproxen serum concentration
Table 1.
Summary of laboratory results
| Time since ingestion | Lactate (mmol/L) | HCO3 (mmol/L) | CPK (U/L) | Creatinine (mg/dL) |
|---|---|---|---|---|
| 3–4 h | Not done (ND) | 19 | 279 | 0.81 |
| 6 h | 16.7 | ND | ND | ND |
| 8 h | 11.3 | ND | ND | ND |
| 9 h | 8.6 | 15 | ND | 1.72 |
| 16 h | 2.3 | 24 | 3,797 | 1.44 |
| 24 h | ND | 26 | 7,427 | 1.55 |
| 30 h | ND | 27 | 10,171 | 1.33 |
| 50 h | ND | 30 | 4,928 | 1.07 |
| On discharge | 0.9 | 26 | ND | 0.74 |
Discussion
This case describes a patient with massive naproxen ingestion whose peak naproxen serum concentration of 1,580 mg/L at 90 min is, to our knowledge, the highest ever reported. One previous case report of naproxen overdose (25 g) described only minor symptoms of nausea and stomach upset with a serum level of 414 mg/L [3]. Although seizure activity is rarely documented in nonsteroidal anti-inflammatory drug (NSAID) overdose, it has been reported after massive overdose of propionic acid derivates [4] including naproxen and ibuprofen. In one report, a 15-year-old girl ingested 12.5 g of naproxen and presented with abdominal pain then developed altered mental status. Her initial chemistry panel revealed a high anion gap acidosis. She had a seizure within 1 h after presentation and continued to have seizures after intubation. She recovered within 24 h. Peak measured serum naproxen level was1,290 mg/L (note: the authors reported the results in units of mg%, but they were probably actually mg/L, based on their reported therapeutic range) [5]. The exact mechanism for the seizure activity reported in patients following NSAID overdose is unknown; however, in animals, there is evidence that COX inhibitors have excitatory effects on selected areas of the hippocampus that are associated with seizures [6, 7]. In a rat model, mefenamic acid and meclofenamic acid, both prostaglandin synthetase inhibitors, have been shown to produce high voltage activity in the EEG with concurrent dose-related increases in excitation, myoclonus, and convulsions [6]. Triphasic T waves in the EEG are strongly associated with metabolic encephalopathies of various types, especially hepatic encephalopathy and have also been reported in acute encephalopathy due to naproxen intoxication [8]. The patient in our case did not have these waves in his EEG.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been reported to cause liver injury, and mixed cholestasis and hepatitis patterns have been reported with naproxen [9]. Ibuprofen has been reported to cause hepatitis as well as fulminant liver failure requiring transplant [10, 11]. Pancreatitis has also been reported after naproxen overdose and with therapeutic use, but our patient’s amylase was normal [12, 13]. The transaminitis noted on presentation was treated empirically with IV N-acetylcysteine as a prudent step in case of unreported prior acetaminophen ingestion. It could be argued that his transaminases were alcohol-induced as he was known to have chronic alcohol abuse. Alcoholic cirrhosis has been found to diminish the intrinsic clearance of naproxen, by affecting both conjugation and demethylation [14].
Renal toxicity has been commonly reported with NSAID overdoses [15]. The NSAID-mediated reduction in prostaglandins can cause prerenal azotemia as well as acute tubular necrosis from vasoconstriction of the afferent renal arterioles and reduction of the GFR. Acute kidney injury may occur within hours after a large dose of a NSAID. Our patient experienced anuria and an increase in his creatinine despite aggressive fluid resuscitation, which suggests it was more likely acute tubular necrosis. This was followed by polyuria for a short period the next day; this latter finding might have been due to the extra volume of fluid he received or due to post acute tubular necrosis effect; polyuria has been reported previously in an ibuprofen overdose [16]. Other reported adverse renal effects reported in chronic NSAID use include tubulointerstitial nephritis, minimal change disease, membranous nephropathy, and papillary necrosis [17]. Acute papillary necrosis causing bilateral ureteral obstruction has been reported after acute therapeutic use of ibuprofen [18].
There is little data on hemodialysis of naproxen and other NSAIDs in the setting of overdose, and NSAIDs are generally not considered amenable to hemodialysis due to their extensive protein binding. One report examined the effect of hemodialysis on plasma naproxen concentrations in end-stage renal disease patients receiving a therapeutic (500 mg) dose of naproxen; it found that naproxen was not cleared by dialysis, probably because of extensive protein binding at therapeutic concentrations [19]. Similar studies were done after therapeutic doses of ibuprofen in uremic patients undergoing routine hemodialysis, with recovery of only a small fraction of the ingested dose of ibuprofen (less than 4 %) [20]. However, these studies do not reflect the situation in acute overdose where the blood level of drug is very high, protein binding is saturated, and the dialysis extraction ratio would be expected to be much higher.
To the best of the authors’ knowledge, this case report is the first to describe serial levels of naproxen after overdose and during renal replacement therapy. Unfortunately, the only matched “arterial/venous” samples we were able to obtain during renal replacement therapy were collected 14 h after ingestion and 3.5 h into the 4-h SLED procedure. At this time, naproxen serum levels had already declined to 347 mg/L when protein binding was expected to have returned to its normal high percentage. As a result, the extraction ratio (and therefore clearance) for naproxen at that time was virtually zero. Thus, we cannot draw any meaningful conclusions about the efficacy of SLED or CVVH for acute naproxen overdose.
In this patient, serum naproxen levels dropped rapidly from 2 to 4 h after ingestion. Serum naproxen levels then plateaued from 4 to 10 h after ingestion (Fig 1). This could have been an artifact caused by continued absorption from a large tablet mass in the gastrointestinal tract. Given this possibility, gastrointestinal decontamination with whole bowel irrigation and additional doses of activated charcoal might have helped prevent continued absorption and should be considered in massive overdoses. It is also possible that once the drug distributed into tissues it began a slower process of elimination, which at the relatively high plateau levels was saturated resulting in zero-order kinetics. A human volunteer study showed that the volume of distribution increases as the free unbound naproxen level increases [21]. Unfortunately, we were unable to measure free naproxen levels and measured the total naproxen levels instead. Naproxen glucoronidation has been shown to exhibit biphasic kinetics [22]. The subsequent more rapid fall in levels could be due to restoration of first-order kinetics at lower levels or enhanced elimination by the SLED procedure or both.
Our patient presented with a high anion gap metabolic acidosis before his first seizure. He also developed hypotension 6 h post admission requiring inotropic support. Metabolic acidosis and shock have been reported in massive ibuprofen overdose [23, 24]. Naproxen is a weak acid with a pKa 4.15 [25]. The most plausible explanation for our patient’s early acidosis was the large amount of ingested naproxen as well as its metabolites, considering they are also weak acids. Hypotension has also been postulated to be due the effect of prostaglandins inhibition in blood vessels, but the mechanism remains unclear [24]. An elevated lactate level was measured 4 h after admission and 3 h after the last seizure, which could be due to the high naproxen concentration, in addition to the hypotension and poor hepatic clearance of lactate. Lactic acidosis has been reported before in massive ibuprofen overdose including fatal cases [16, 26]. Although naproxen is a weak acid, there is no evidence suggesting that urinary alkalinization can enhance elimination, possibly due to its high protein binding [25].
Conclusion
Massive naproxen overdose can present with serious toxicity including seizures, altered mental status, metabolic acidosis, and renal failure. Hemodialysis and CVVH are of uncertain benefit in removing naproxen but may correct the acid base disturbance and provide support in cases of renal impairment. Further studies are needed to determine the hemodialysis clearance of naproxen after acute overdose.
References
- 1.Todd PA, Clissold SP. Naproxen. A reappraisal of its pharmacology, and therapeutic use in rheumatic diseases and pain states. Drugs. 1990;40(1):91–137. doi: 10.2165/00003495-199040010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Runkel RA, Forchielli E, Sevelius H, Chaplin M, Segre E. Nonlinear plasma level response to high doses of naproxen. Clin Pharmacol Ther. 1974;15(3):261–266. doi: 10.1002/cpt1974153261. [DOI] [PubMed] [Google Scholar]
- 3.Fredell EW, Strand LJ. Naproxen Overdose JAMA. 1977;238(9):938. doi: 10.1001/jama.238.9.938b. [DOI] [PubMed] [Google Scholar]
- 4.Smolinske SC, Hall AH, Vandenberg SA, Spoerke DG, McBride PV. Toxic effects of nonsteroidal anti-inflammatory drugs in overdose. An overview of recent evidence on clinical effects and dose–response relationships. Drug Saf. 1990;5(4):252–274. doi: 10.2165/00002018-199005040-00003. [DOI] [PubMed] [Google Scholar]
- 5.Martinez R, Smith DW, Frankel LR. Severe metabolic acidosis after acute naproxen sodium ingestion. Ann Emerg Med. 1989;18(10):1102–1104. doi: 10.1016/S0196-0644(89)80939-4. [DOI] [PubMed] [Google Scholar]
- 6.Wallenstein MC. Differential effects of prostaglandin synthetase inhibitors on EEG in rat. Eur J pharmacol. 1985;111(2):201–209. doi: 10.1016/0014-2999(85)90757-5. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Chung JI, Lee SH, Jung YS, Moon CH, Baik EJ. Involvement of endogenous prostaglandin F2alpha on kainic acid-induced seizure activity through FP receptor: the mechanism of proconvulsant effects of COX-2 inhibitors. Brain Res. 2008;1193:153–161. doi: 10.1016/j.brainres.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Bortone E, et al. Triphasic waves associated with acute naproxen overdose: a case report. Clin Electroencephalogr. 1998;29(3):142–145. doi: 10.1177/155005949802900307. [DOI] [PubMed] [Google Scholar]
- 9.Ali S, Pimentel JD, Ma C. Naproxen-induced liver injury. Hepatobiliary Pancreat Dis Int. 2011;10(5):552–556. doi: 10.1016/S1499-3872(11)60093-3. [DOI] [PubMed] [Google Scholar]
- 10.Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232(2):133–138. doi: 10.1111/j.1365-2796.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Rahier J, Geubel AP, Lerut J, Horsmans Y. Subfulminant hepatitis requiring liver transplantation following ibuprofen overdose. Liver. 2000;20(1):93–94. doi: 10.1034/j.1600-0676.2000.020001093.x. [DOI] [PubMed] [Google Scholar]
- 12.Aygencel G, Akbuga B, Keles A. Acute pancreatitis following naproxen intake. Eur J Emerg Med. 2006;13(6):372. doi: 10.1097/01.mej.0000224428.51623.b2. [DOI] [PubMed] [Google Scholar]
- 13.Castiella A, Lopez P, Bujanda L, Arenas JI. Possible association of acute pancreatitis with naproxen. J Clin Gastroenterol. 1995;21(3):258. doi: 10.1097/00004836-199510000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Williams RL, Upton RA, Cello JP, et al. Naproxen disposition in patients with alcoholic cirrhosis. Eur J Clin Pharmacol. 1984;27:291–296. doi: 10.1007/BF00542162. [DOI] [PubMed] [Google Scholar]
- 15.Kulling PE, Backman EA, Skagius AS, Beckman EA. Renal impairment after acute diclofenac, naproxen, and sulindac overdoses. J Toxicol Clin Toxicol. 1995;33(2):173–177. doi: 10.3109/15563659509000469. [DOI] [PubMed] [Google Scholar]
- 16.Levine M, Khurana A, Ruha AM. Polyuria, acidosis, and coma following massive ibuprofen ingestion. J Med Toxicol. 2010;6(3):315–317. doi: 10.1007/s13181-010-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol. 1993;33:435–465. doi: 10.1146/annurev.pa.33.040193.002251. [DOI] [PubMed] [Google Scholar]
- 18.Broadis E, et al. Bilateral ureteric obstruction secondary to renal papillary necrosis. Pediatr Surg Int. 2010;26(8):867–869. doi: 10.1007/s00383-010-2608-3. [DOI] [PubMed] [Google Scholar]
- 19.Weber SS, Troutman WG, Trujeque L. Effect of hemodialysis on plasma naproxen concentration. Am J Hosp Pharm. 1979;36(11):1567–1569. [PubMed] [Google Scholar]
- 20.Senekjian HO, Lee CS, Kuo TH, Au DS, Krothapalli R. Absorption and disposition of ibuprofen in hemodialyzed uremic patients. Eur J Rheumatol Inflamm. 1983;6(2):155–162. [PubMed] [Google Scholar]
- 21.Niazi SK, Alam SM, Ahmad SI. Dose dependent pharmacokinetics of naproxen in man. Biopharm Drug Dispos. 1996;17(4):355–361. doi: 10.1002/(SICI)1099-081X(199605)17:4<355::AID-BDD960>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Bowalgaha K, Elliot DJ, Mackenzie PI, Knights KM, Swedmark S, Miners JO. S-naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): role of UGT2B7 in the elimination of naproxen. Br J Clin Pharmacol. 2005;60(4):423–433. doi: 10.1111/j.1365-2125.2005.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuckerman GB, Uy CC. Shock, metabolic acidosis, and coma following ibuprofen overdose in a child. Ann Pharmacother. 1995;29(9):869–871. doi: 10.1177/106002809502900908. [DOI] [PubMed] [Google Scholar]
- 24.Marciniak KE, Thomas IH, Brogan TV, Roberts JS, Czaja A, Mazor SS. Massive ibuprofen overdose requiring extracorporeal membrane oxygenation for cardiovascular support. Pediatr Crit Care Med. 2007;8(2):180–182. doi: 10.1097/01.PCC.0000257036.26436.42. [DOI] [PubMed] [Google Scholar]
- 25.Davies N, Anderson K. Clinical pharmacokinetics of naproxen. Clin Pharmacokinet. 1997;32(4):268–293. doi: 10.2165/00003088-199732040-00002. [DOI] [PubMed] [Google Scholar]
- 26.Holubek W, Stolbach A, Nurok S, et al. A report of two deaths from massive ibuprofen ingestion. J Med Toxicol. 2007;3:52–55. doi: 10.1007/BF03160908. [DOI] [PMC free article] [PubMed] [Google Scholar]