Abstract
Introduction
Toxic leukoencephalopathy is a possible but rare complication of chronic cocaine abuse. The role of adulterants, mainly levamisole, is still debated.
Case Report
We describe an atypical case of fatal leukoencephalopathy mimicking Susac syndrome in a 22-year-old man who was chronically abusing cannabis and cocaine. Exposure to levamisole as adulterant to cocaine was proven by hair analysis. Despite cessation of exposure to cocaine and aggressive immunosuppressive therapy, the patient remained in a minimally conscious state until death.
Discussion
Susac syndrome is a rare entity, and its etiology is not yet fully elucidated. The toxic etiologies have been poorly investigated to date. Further observations are required to determine if cocaine and/or adulterants might play a significant role.
Keywords: Susac syndrome, Cocaine, Levamisole
Introduction
Despite the wide abuse of cocaine, toxic leukoencephalopathy remains still a relatively rare complication [1, 2]. The possible synergistic role of adulterants is frequently debated, and among them, levamisole is an agent that has been associated with leukoencephalopathy [3–6]. Among inflammatory brain disorders, Susac syndrome is a rare disease featured by the triad of encephalopathy, branch retinal arteries occlusions, and hearing loss [7]. To date, the pathogenesis of this syndrome is unknown and toxic etiologies have been poorly investigated [8]. We herein report a case of devastating leukoencephalopathy with typical radiological pattern of Susac syndrome, with a rapid deterioration of consciousness. The patient was a long-standing cocaine abuser in whom levamisole exposure was demonstrated by hair analysis.
Case Presentation
A 22-year-old man, with a long history of cannabis (>10 years) and cocaine (>5 years) abuse, was admitted with headache, ataxia, and right-sided paresthesias of several days duration. He had no relevant medical history and was HIV-negative. He was working as a roofer and had no recognized occupational exposures. Cerebrospinal fluid (CSF) analysis revealed a hyperproteinorrachia at 2.49 g/l with low cellularity (15 cells/mm3, mainly lymphocytes) and no CSF-specific IgG oligoclonal bands; the IgG index was not available. Serological testing was negative for antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), and anticardiolipine antibodies. Complement was normal, as was the activity of antithrombin III, protein C and S. Comprehensive screening for infectious diseases was negative. The initial brain magnetic resonance imaging (MRI) was obtained on hospital day (HD) 1, 1 week after symptoms onset, and was repeated on HD 25 and 32 (Figs. 1, 2, and 3). A diffusely severe multiple sclerosis (MS)-like leukoencephalopathy was observed with numerous small foci of T2-weighted hypersignal intensity throughout the supra- and infratentorial white matter (Fig. 3). Multiple lesions were present within the corpus callosum (i.e., on the midline) raising a strong suspicion for Susac syndrome; they progressively evolved to necrotico-cystic lesions (Figs. 1 and 2). Only a faint contrast enhancement was observed within a few lesions, thereby decreasing the probability of an acute disseminated encephalomyelitis (ADEM). Despite heavy steroid pulse therapy, the neurological status rapidly worsened, with altered consciousness, mutism, and quadriparesia. The follow-up MR examination revealed a progression of the T2-weighted images (Fig. 3). Occlusion of some branch retinal arteries was noted at fundoscopy performed by a neuro-ophthalmologist. Brainstem auditory evoked potentials were normal. Despite maximal immunosuppressive therapy (repeated steroids, cyclophosphamide), the patient remained in a minimally conscious state. He deteriorated with spasticity and dysautonomic symptoms. He died 3 months later from septic complications.
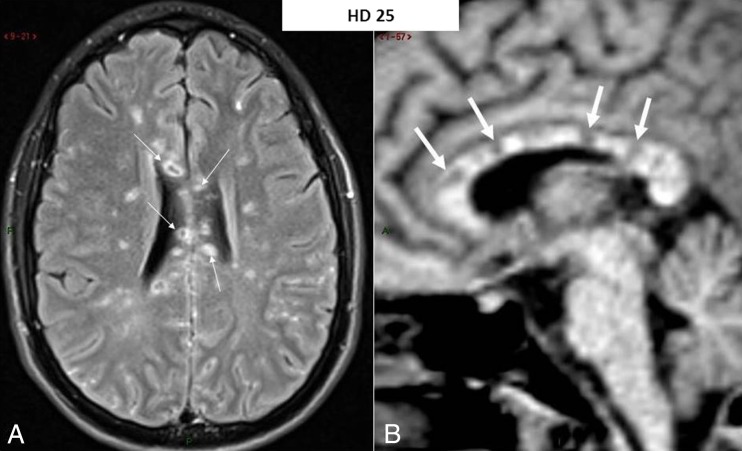
Fig. 1.
MR imaging 4 weeks after admission featuring a Susac syndrome pattern. a Axial-transverse fluid-attenuated inversion recovery (FLAIR) image through the body of the corpus callosum: hyperintense foci are seen throughout the white matter of both cerebral hemispheres together with prominent involvement of the corpus callosum with necrotico-cystic lesions (arrows). This latter location is almost pathognomonic for Susac syndrome. b Mid-sagittal pre-contrast T1-weighted image (poor image quality because of the “scout-view” image for slice location prescription) confirming the presence of multiple hypointense foci within the corpus callosum (arrows) corresponding to the necrotico-cystic lesions seen on a
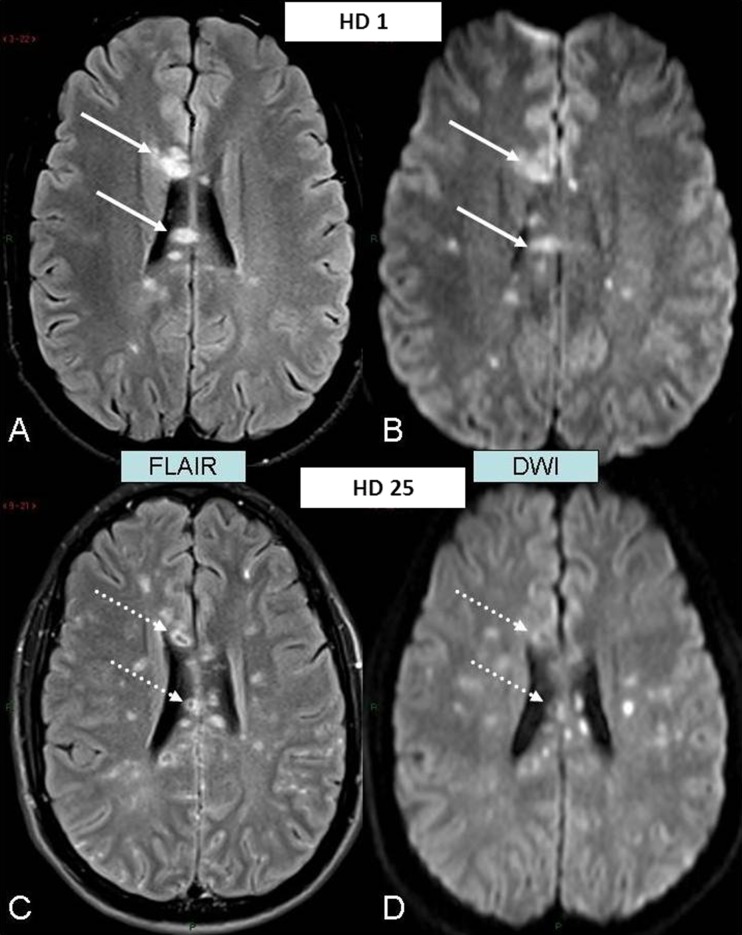
Fig. 2.
Evolution of the lesions of the corpus callosum to necrosis. a–b Admission MR examination. c–d Follow-up MR examination almost 4 weeks later. a Axial-transverse FLAIR image through the body of the corpus callosum showing prominent lesions appearing as homogeneous foci of hypersignal intensity (arrows). b Axial-transverse diffusion-weighted image (DWI) in similar slice location as a showing homogeneous hyperintensity of the lesions (arrows) due to restricted diffusivity within them with decreased apparent diffusion coefficient (ADC) values (ADC map not shown). c Axial-transverse FLAIR image in similar slice location as a 4 weeks later showing hypointense necrotic core of the lesions (dotted arrows). d DWI in similar slice location as b 4 weeks later confirming dramatic increase in free-water diffusivity within hypointense necrotic core (dotted arrows)
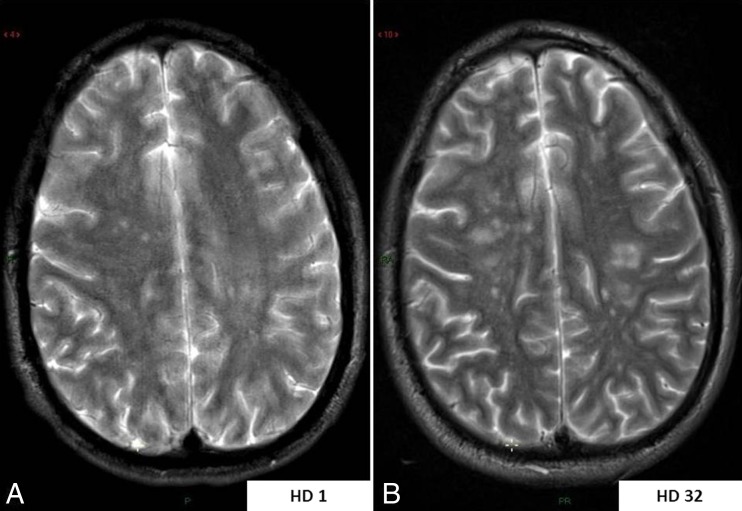
Fig. 3.
MR monitoring of hemispheric white matter lesion load throughout the full disease course. a Axial-transverse T2-weighted image through centrum semi-ovale at initial examination showing only a few aspecific hyperintense white matter lesions within both cerebral hemispheres. b Axial-transverse T2-weighted image in similar slice location at the end of the disease course showing a significant increase in number and size of the white matter lesions
In order to explore possible toxic etiologies, hair sampling was done from hair specimens collected 2 months after he was initially hospitalized. There was no potential for further drug use over this time. Hair samples were cut in three segments of 1 cm starting from the root. The analysis was performed using an ultra-performing liquid chromatography tandem mass spectrometry operating in positive electrospray mode (Quattro Premier, Waters, Milford, MA, US). Analysis included investigation for cocaine, levamisole, opioids, and several amphetamines. Except for cocaine (C), benzoylecgonine (B), and levamisole (L), no other compounds were detected (Table 1). Quantification was possible with use of deuterated analogues for cocaine and benzoylecgonine and 6-acetylmorphine-D6 for levamisole.
Table 1.
Results of hair analysis
| Hair segments (from the root) (cm) | Cocaine (pg/mg) | Benzoylecgonine (pg/mg) | Levamisole (pg/mg) |
|---|---|---|---|
| 0–1 | 49 | <30 | <30 |
| 1–2 | 541 | 192 | 138 |
| 2–3 | 2,271 | 801 | 572 |
Discussion
Multifocal inflammatory leukoencephalopathy (MIL) is a well-described complication of monotherapy by levamisole, and the hypothesis that adulteration of cocaine by levamisole could enhance brain inflammatory lesions has been repeatedly debated [3–6]. A recent review of 203 cases of complications reported from levamisole-adulterated cocaine failed to retrieve any subject with leukoencephalopathy [9]. MIL is characterized by multiple foci of demyelination appearing hyperintense on the T2-weighted images and exhibiting a variable degree of enhancement on the T1-weighted images after contrast agent (CA) perfusion due to focal breakdown of the brain-blood barrier. The usual differential diagnosis in the condition mainly includes multiple sclerosis (MS) and ADEM. The MIL lesions typically are typically prominent in the centrum ovale, peri-lateral ventricles, and basal ganglia bilaterally [5]. However, brain stem and cerebellar areas, as well as subcortical white matter areas, can also be involved [6]. Mild pleiocytosis has been described as well as hyperproteinorrachia [6, 10]. Contrary to our case, clinical and radiologic features have been reported to improve with cessation of levamisole exposure [10].
In the very few reports of cocaine-associated leukoencephalopathy, the presence of levamisole has been suspected but never demonstrated [1–4]. One originality of the present observation is that a significant exposure to levamisole was proven by hair testing. The cocaine and levamisole hair concentrations, as well as the cocaine/levamisole ratio (3.9 in segment 2 and 4.0 in segment 3), were in accordance with previous data [11]. Although cocaine, benzoylecgonine, and levamisole concentrations in segment 3 were approximately four times higher than in segment 2, the cocaine/levamisole ratio in these two segments was comparable, suggesting a common exposure.
Obviously, this does not demonstrate a causal link of levamisole exposure to the clinical/radiological features. Another significant limitation was the impossibility to determine whether the patient had been or not exposed to other compounds or adulterants in the frame of his long-standing drug abuse.
In the present observation, the radiological course seemed more consistent with a Susac, or at least Susac-like, syndrome, rather than MIL. Susac syndrome is a recently described and rare neurological entity characterized by the clinical triad of encephalopathy, branch retinal artery occlusions, and hearing loss [7]. At MR investigation, lesions typically involve the midline areas of the corpus callosum, with a relative sparing of the more lateral ones (Table 2). In contrast, in two recent papers analyzing clinical and MR features of levamisole-induced MIL, Yan et al. failed to demonstrate the presence of callosal lesions in the 15 patients of their series [5] and Xu et al. sorted only 1/16 patient with such lesions [6].
Table 2.
Usual brain MRI findings according to different etiologies [2, 5, 12]. The differential diagnosis should be made mainly with lesions seen in multiple sclerosis (MS) and acute disseminated encephalomyelitis (ADEM)
| Levamisole-associated MIL | Cocaine leukoencephalopathy | Susac syndrome |
|---|---|---|
| Hyperintense T2-weighted images with contrast enhancement predominating in the bilateral centrum ovale, peri-lateral ventricles and basal ganglia | Hyperintense T2-weighted images, usually without contrast enhancement | T2-weighted images: multifocal small hyperintense foci with contrast enhancement in the white and gray matter (up to 70 %) |
| Round, mass like foci, also irregular patchy foci | Diffuse bihemispheric white matter lesions, often with preservation of U fibers, consistent with confluent vacuolar degeneration | Typical lesions of the corpus callosum, with linear defects in the central fibers and “snowball-like” lesions |
| Leptomeninges involvement (30 %) |
Susac syndrome is a presumptively auto-immune retinocochleocerebral microangiopathy that is speculated to be triggered by an unknown antigen, with a female/male ratio > 3:1. Anti-endothelial cell antibodies have been detected in a subset of a small series of patients with Susac syndrome (7/20) [12]. The role of other etiologic agents/processes, such as viral infections or idiopathic vasospasm, is still debated. Toxic causes have been poorly investigated to date. The cerebrospinal fluid examination usually shows mild lymphocytic pleiocytosis and an elevated total protein level, suggestive of blood-brain barrier dysfunction. CSF-specific oligoclonal bands are typically missing [13].
In our patient, Susac syndrome was incomplete since hearing loss could never be demonstrated and atypical since consciousness rapidly worsened despite aggressive immunosuppressive therapy. Only one lethal case of Susac syndrome with severe encephalopathy and mutism has previously been reported [14].
Finally, the only faint enhancement of a few subsets of lesions on post-contrast T1-weighted images needs to be highlighted as a speculative clue for a toxic condition rather than an inflammatory one.
Conclusion
The limitations of this single observation have to be acknowledged. Our patient had retinal and MRI features strongly suggesting Susac syndrome, while other features were atypical, such as fulminant disease course and lack of auditory findings. Hair analysis confirmed exposure to cocaine and levamisole in this previously healthy patient. Causality from one or both or even other agents remains speculative at this time. Nevertheless, physicians should consider toxic exposure in patients with MIL or Susac syndrome.
Acknowledgments
Funding
None.
Conflict of Interest
No conflict to declare.
References
- 1.González-Duarte A, Williams R. Cocaine-induced recurrent leukoencephalopathy. Neuroradiol J. 2013;26(5):560–2. doi: 10.1177/197140091302600503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco F, Iacovelli E, Tinelli E, Lepre C, Pauri F. Recurrent leukoencephalopathy in a cocaine abuser. Neurotoxicology. 2011;32(4):410–2. doi: 10.1016/j.neuro.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Blanc PD, Chin C, Lynch KL. Multifocal inflammatory leukoencephalopathy associated with cocaine-abuse: is levamisole responsible? Clin Toxicol (Phila) 2012;50(6):534–5. doi: 10.3109/15563650.2012.692794. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman RS, Larocque A. Response to “Multifocal inflammatory leukoencephalopathy associated with cocaine-abuse: is levamisole responsible?”. Clin Toxicol (Phila) 2012;50(6):536. doi: 10.3109/15563650.2012.692795. [DOI] [PubMed] [Google Scholar]
- 5.Yan R, Wu Q, Ren J, Cui H, Zhai K, Zhai Z, et al. Clinical features and magnetic resonance imaging analysis of 15 cases of demyelinating leukoencephalopathy induced by levamisole. Exp Ther Med. 2013;6(1):71–4. doi: 10.3892/etm.2013.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu N, Zhou W, Li S, Zhou G, Zhang N, Liang J. Clinical and MRI characteristics of levamisole-induced leukoencephalopathy in 16 patients. J Neuroimaging. 2009;19(4):326–31. doi: 10.1111/j.1552-6569.2008.00344.x. [DOI] [PubMed] [Google Scholar]
- 7.Susac JO. Susac’s syndrome: the triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44(4):591–3. doi: 10.1212/WNL.44.4.591. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Carrasco M, Mendoza-Pino C, Cervera R. Diagnosis and classification of Susac syndrome. Autoimmun Rev. 2014;13(4–5):347–50. doi: 10.1016/j.autrev.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Larocque A, Hoffman RS. Levamisole in cocaine: unexpected news from an old acquaintance. Clin Toxicol (Phila) 2012;50(4):231–41. doi: 10.3109/15563650.2012.665455. [DOI] [PubMed] [Google Scholar]
- 10.Wu VC, Huang JW, Lien HC, Hsieh ST, Liu HM, Yang CC, et al. Levamisole-induced multifocal inflammatory leukoencephalopathy: clinical characteristics, outcome, and impact of treatment in 31 patients. Medicine. 2006;85(4):203–13. doi: 10.1097/01.md.0000230250.95281.60. [DOI] [PubMed] [Google Scholar]
- 11.Nalesso A, Viel G, Terranova C, Vogliardi S, Stocchero G, Favretto D, Davide Ferrara S (2011) LC-HRMS method for determination of levamisole in hair: application to real samples. Abstracts of the SOFT-TIAFT meeting, San Francisco, September 25–30, 2011. http://www.tiaft.org/socialmediauploads/2011_soft_tiaft.pdf. Accessed 24 June 2014.
- 12.Jarius S, Kleffner I, Dörr JM, Sastre-Garriga J, Illes Z, Eggenberger E, et al. Clinical, paraclinical and serological findings in Susac syndrome: an international multicenter study. J Neuroinflammation. 2014;11:46. doi: 10.1186/1742-2094-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateen FJ, Zubkov AY, Muralidharan R, Fugate JE, Rodriguez FJ, Winters JL, et al. Susac syndrome: clinical characteristics and treatment in 29 new cases. Eur J Neurol. 2012;19(6):800–11. doi: 10.1111/j.1468-1331.2011.03627.x. [DOI] [PubMed] [Google Scholar]
- 14.Saux A, Niango G, Charif M, Morales R, Mura F, Bonafe A, et al. Susac’s syndrome, a rare, potentially severe or lethal neurological disease. J Neurol Sci. 2010;297(1–2):71–3. doi: 10.1016/j.jns.2010.07.020. [DOI] [PubMed] [Google Scholar]