Abstract
Purpose
Three different techniques of low-dose-rate seed implantation for prostate cancer have been used since its use started in our hospital. The purpose of this study was to compare the results of the three different techniques.
Material and methods
The data of 305 prostate cancer patients who underwent low-dose-rate seed implantation were retrospectively analyzed. Pre-plan technique (n = 27), intraoperative pre-plan technique (n = 86), and interactive plan technique (n = 192) were tried in chronological order. The prescribed dose was set at 145 Gy.
Results
Median follow-up was 66 months (range: 12-94 months). The 5-year biochemical control rate was 95.5% (pre-plan group: 100%, intraoperative pre-plan group: 90.7%, interactive plan group: 97.0%; p = 0.08). Dosimetric parameters were generally increased from the pre-plan group to the interactive group. The differences in some dosimetric parameters between the planning phase and the CT analysis were significantly reduced with the interactive plan compared to the other techniques. The interactive plan showed a significant reduction of the seed migration rate compared to the two other groups. Acute genitourinary toxicity, acute gastrointestinal toxicity, frequency, and urinary retention increased gradually from the pre-plan period to the interactive plan period.
Conclusions
There was no significant difference in biochemical control among the three groups. Dose-volume parameters were increased from the pre-plan technique to the interactive plan technique. However, this may not necessarily be due to technical improvements, since dose escalation was started during the same period. Lower seed migration rates and the smaller differences between the planning phase and CT analysis with the interactive plan technique suggest the superiority of this technique to the two other techniques.
Keywords: interactive plan, intraoperative pre-plan, pre-plan, low-dose-rate, prostate cancer, seed implantation
Purpose
Seed implantation for localized prostate cancer is one of the most important techniques that has been imported from foreign countries into Japan in this decade. This technique has spread rapidly throughout Japan and is now one of the standard treatments for localized prostate cancer. When this technique began to be used in Japan in 2004, there were several techniques already established in the United States and European countries [1, 2], and it was necessary to identify the best technique for Japanese patients and physicians.
To date, we have tried three different techniques including the “pre-plan technique”, the “intraoperative pre-plan technique”, and the “interactive plan technique” in our hospital. Of these three techniques, we selected the interactive plan technique as the best for our patients, and over 1,000 patients have been treated with this technique. The purpose of this study was to analyze the results of the three different techniques, and to find which technique could lead to higher D90 or V100, or less toxicity, or lower seed migration rates than others.
Material and methods
Patients
Data of 305 consecutive Japanese prostate cancer patients who underwent low-dose-rate seed implantation between May 2004 and August 2007 were analyzed retrospectively. The patients’ characteristics are shown in Table 1. Although there was no clear reason, the pre-plan group included a slightly higher number of intermediate-risk patients with T2b or a Gleason score of 4 + 3 (p < 0.01). Patients with clinical stage T1c or T2a who also had a prostate-specific antigen (PSA) level ≤ 10 ng/ml and a biopsy Gleason score (GS) of 2-6, have been defined as a low-risk group. Conversely, patients with clinical stage T2c or a PSA level > 20 ng/ml or a biopsy GS of ≥ 8 have been defined as a high-risk group. The remaining patients have been defined as an intermediate-risk group. All patients in this study were low-risk or intermediate-risk patients.
Table 1.
Patients’ characteristics
| Factors | Pre-plan group | Intraoperative pre-plan group | Interactive plan group | p |
|---|---|---|---|---|
| n | 27 | 86 | 192 | |
| Age (years) | 69 (5.5) | 68 (6.3) | 68 (6.0) | ns |
| T stage | ||||
| 1c | 17 (63.0%) | 78 (90.7%) | 156 (81.3%) | < 0.001 |
| 2a | 4 (14.8%) | 8 (9.3%) | 35 (18.2%) | |
| 2b | 6 (22.2%) | 0 (0.0%) | 1 (0.5%) | |
| 2c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Gleason score | ||||
| ≤ 3 + 3 | 17 (63.0%) | 63 (73.3%) | 97 (50.5%) | < 0.01 |
| 3 + 4 | 5 (18.5%) | 15 (17.4%) | 70 (36.5%) | |
| 4 + 3 | 5 (18.5%) | 8 (9.3%) | 25 (13.0%) | |
| Initial prostate-specific antigen (ng/ml) | 8.03 (4.48) | 6.49 (2.24) | 6.45 (2.16) | ns |
| Hormonal therapy | 9 (33.3%) | 28 (32.6%) | 56 (29.2%) | ns |
Values are given as means (standard deviation or percentage) or numbers.
Clinical staging was decided based on the results of digital rectal examinations and bone scintigraphy. Computed tomography (CT) and/or magnetic resonance imaging (MRI) of pelvis were also used to determine the T stage. Basically, hormonal manipulation was performed for three months in patients with a large prostate gland (≥ 40 cm3) before implantation. Gonadotropin-releasing hormone agonist with or without androgen blockade was used for hormonal manipulation. Some patients, however, received hormonal therapy before admission to our hospital.
Pre-plan technique period
In the first 27 patients (treated from May 2004 to October 2004), dosimetry was planned based on ultrasound (US) performed 4 weeks before implantation. Ultrasound images were acquired from transrectal ultrasonography in the extended lithotomy position. All dosimetry was planned using Interplant 3.2 software (CMS, St. Louis, MO, USA) with acquired US images as pre-planning. The prescribed dose to the prostate with a 3- to 5-mm margin was set at 145 Gy. Needle positions were symmetrical, and seeds were generally placed 5-mm apart from each other.
The procedure was performed in the extended lithotomy position. Seeds were placed one by one transperineally through needles attached to a Mick applicator (Mick Radio-Nuclear Instrument, Mount Vernon, NY, USA) under transrectal ultrasonography. Stranded seed was not used. All patients were hospitalized the day before seed implantation and discharged 2 days after implantation.
Intraoperative pre-plan technique period
In the next 86 patients (treated from October 2004 to October 2005), dosimetry was planned intraoperatively based on US performed just before implantation in the anesthetized patient. Using this technique, the problem of prostate volume change during the waiting time between pre-plan and operation could be resolved. Since dose escalation was started in this period, seeds were sequentially placed with no space if needed. The other procedures were the same as in the previous period.
Interactive plan technique period
In the remaining 192 patients (treated from October 2005 to August 2007), an interactive plan technique [3] was used. During this period, the prostate image was acquired after needle insertion. Therefore, prostate swelling and deformation were included in planning images. If needed, dosimetry was modified based on real-time dose calculation during the operation. The other procedures were the same as in the previous period.
Follow-up
Serum PSA levels were monitored every 3 months for the first year, and every 3-6 months thereafter. Biochemical failure was defined according to the Phoenix definition [4]. Urinary and rectal morbidities were assessed using the Radiation Therapy Oncology Group (RTOG) scale [5] and National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3. All patients received α-blockers just after seed implantation to relieve urinary symptoms.
Dosimetric analysis
Post-implantation dosimetric analysis was performed using CT scans performed 30 days after implantation according to the recommendations of the American Brachytherapy Society [6]. Dose-volume histograms (DVHs) were calculated for each patient. Instead of actual urethral contouring, the surrogate urethra assumed to be at the geometric center of the prostate was contoured on the same slices as the prostate. The rectal wall was contoured, including the sphincter muscle, on the same slices as the prostate contouring.
Statistics
One-way analysis of variance or the Kruskal-Wallis test was used to compare the parameters of the three groups. The log-rank test was used to compare survival among the three groups. Stat Mate version 4.01 statistical software (ATMS, Tokyo, Japan) was used for data analysis. Differences were regarded as significant at the p < 0.05 level.
Results
Biochemical control
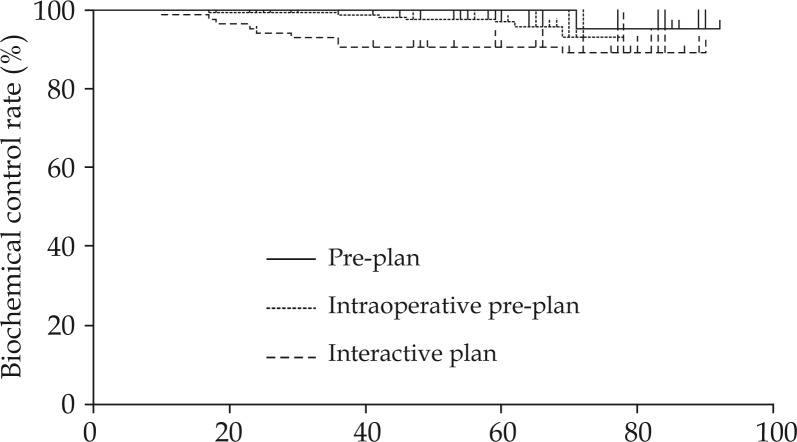
Median follow-up was 66 months (range: 12-94 months). The 5-year biochemical control rate was 95.5% (pre-plan group: 100%, intraoperative pre-plan group: 90.7%, interactive plan group: 97.0%; p = 0.08; Fig. 1). The 5-year clinical non-evidence of disease rate was 98.9% (pre-plan group: 100%, intraoperative pre-plan group: 97.7%, interactive plan group: 99.4%). Two patients died of lung cancer, and the 5-year overall survival rate was 99.6%. There were no significant differences among the three groups.
Fig. 1.
Biochemical control rates of the pre-plan, intraoperative pre-plan, and interactive plan groups
Planning phase
Table 2 shows comparisons of the three techniques in the planning phase. Interestingly, the numbers of seeds, needles, and total activity were increased when the technique was switched from pre-plan to intraoperative pre-plan (p < 0.01), and decreased when switched from intraoperative pre-plan to interactive plan (p < 0.01). Prostate volume was not different among the three groups. Dosimetric parameters, such as dose to 90% of prostate volume (D90), were generally increased from the pre-plan group to the interactive group. However, rectal volume receiving at least 150% of prescription dose (RV150) was significantly reduced in the interactive plan group compared to the other two groups. The dose non-homogeneity ratio (DNR), which is the volume of 150% dose divided by the volume of the 100% dose, increased from pre-plan to interactive plan (p < 0.001).
Table 2.
Planning phase
| Factors | Pre-plan group | Intraoperative pre-plan group | Interactive plan group | p |
|---|---|---|---|---|
| n | 27 | 86 | 192 | |
| Number of seeds | 84.1 (16.54) | 97.5 (14.3) | 89.4 (15.4) | < 0.01 |
| Number of needles | 21.4 (4.14) | 30.6 (4.63) | 21.6 (3.2) | < 0.001 |
| Total activity (MBq) | 1057.7 (208.09) | 1226.7 (180.31) | 1146.3 (194.0) | < 0.01 |
| Prostate volume (ml) | ||||
| US | 29.66 (11.5) | 32.4 (8.9) | 32.9 (9.3) | ns |
| 1-month CT | 29.08 (12.54) | 27.0 (8.0) | 28.4 (8.7) | ns |
| US plan | ||||
| D90 (Gy) | 168.2 (11.13) | 184.0 (11.08) | 192.7 (9.4) | < 0.01 |
| V100 (%) | 97.4 (1.87) | 99.6 (0.49) | 99.5 (0.6) | < 0.001 |
| V150 (%) | 54.2 (12.40) | 62.5 (13.40) | 71.8 (9.4) | < 0.001 |
| RV100 (ml) | 0.47 (0.45) | 0.53 (0.37) | 0.7 (0.4) | < 0.01 |
| RV150 (ml) | 0.04 (0.08) | 0.02 (0.03) | 0.0 (0.1) | < 0.05 |
| UD90 (Gy) | 149.6 (17.9) | 168.4 (12.2) | 171.8 (20.4) | < 0.001 |
| UD30 (Gy) | 183.5 (15.7) | 188.5 (10.8) | 200.0 (9.5) | < 0.001 |
| UD10 (Gy) | 189.7 (14.90) | 194.4 (10.26) | 208.3 (11.0) | < 0.001 |
| DNR | 0.56 (0.13) | 0.63 (0.13) | 0.72 (0.09) | < 0.001 |
Values are given as means (standard deviation) or numbers.
D90 – dose to 90% of prostate volume; V100 – prostate volume receiving at least 100% of prescription dose; V150 – prostate volume receiving at least 150% of prescription dose; RV100 – rectal volume receiving at least 100% of prescription dose; RV150 – rectal volume receiving at least 150% of prescription dose; UD90 – dose to 90% of urethral volume; UD30 – dose to 30% of urethral volume; UD10 – dose to 10% of urethral volume; US – ultrasound; DNR – dose non-homogeneity ratio
Computed tomography analysis
Table 3 shows comparisons of the three techniques on the 1-month CT analysis. Dosimetric parameters such as D90 (except RV150) increased from the pre-plan group to the interactive group. Dose non-homogeneity ratio also increased from the pre-plan group to the interactive group. Seed migration rates were not different between the pre-plan group and the intraoperative pre-plan group. However, the interactive plan group showed a significantly decreased seed migration rate compared to the other two groups (p < 0.001).
Table 3.
Computed tomography at 1 month
| Factors | Pre-plan group | Intraoperative pre-plan group | Interactive plan group | p |
|---|---|---|---|---|
| n | 27 | 86 | 192 | |
| D90 (Gy) | 145.0 (33.9) | 169.4 (23.2) | 179.1 (21.8) | < 0.001 |
| V100 (%) | 87.9 (11.9) | 95.7 (5.7) | 97.0 (3.3) | < 0.001 |
| V150 (%) | 51.7 (16.0) | 60.1 (15.4) | 68.5 (13.9) | < 0.01 |
| RV100 (ml) | 0.8 (0.7) | 1.16 (1.0) | 0.8 (0.7) | < 0.05 |
| RV150 (ml) | 0.1 (0.2) | 0.15 (0.2) | 0.1 (0.2) | ns |
| UD90 (Gy) | 133.3 (42.1) | 155.1 (25.4) | 161.5 (28.7) | < 0.01 |
| UD30 (Gy) | 194.0 (29.5) | 192.6 (20.7) | 218.5 (26.1) | < 0.001 |
| UD10 (Gy) | 209.7 (36.4) | 218.3 (31.5) | 234.6 (31.9) | < 0.001 |
| DNR | 0.58 (0.13) | 0.62 (0.14) | 0.70 (0.13) | < 0.01 |
| Patients with migration (1 month) | ||||
| Total | 19 (70.4%) | 60 (69.8%) | 77 (40.1%) | < 0.001 |
| Lung | 14 (51.9%) | 47 (54.7%) | 42 (21.9%) | < 0.001 |
| Abdomino-pelvis | 14 (51.9%) | 41 (47.7%) | 41 (21.4%) | < 0.001 |
Values are given as means (standard deviation or percentage) or numbers.
Abbreviations are the same as in Table 2.
Differences between the planning phase and computed tomography analysis
Table 4 shows the differences in the DVH parameters between the planning phase and the 1-month CT analysis. When each corresponding parameter was compared, D90, prostate volume receiving at least 100% of the prescription dose (V100), prostate volume receiving at least 150% of the prescription dose (V150), and dose to 90% of urethral volume (UD90) tended to decrease from the planning phase to the 1-month CT analysis. Meanwhile, rectal volume receiving at least 100% of the prescription dose (RV100) and RV150 tended to be stable. Dose to 30% of urethral volume (UD30) and dose to 10% of urethral volume (UD10) tended to increase from the planning phase to the 1-month CT analysis.
Table 4.
Differences between the planning phase and the 1-month computed tomography analysis
| Factors | Pre-plan group | Intraoperative pre-plan group | Interactive plan group | p |
|---|---|---|---|---|
| n | 27 | 86 | 192 | |
| D90 (Gy) | −23.2 (32.0) | −14.8 (24.2) | −12.6 (20.4) | ns |
| V100 (%) | −9.5 (11.4) | −4.0 (5.7) | −2.4 (3.3) | < 0.001 |
| V150 (%) | −2.5 (17.0) | −0.2 (21.8) | −2.0 (14.7) | ns |
| RV100 (ml) | 0.3 (0.7) | 0.60 (0.9) | −0.4 (1.1) | < 0.01 |
| RV150 (ml) | 0.1 (0.2) | 0.11 (0.2) | 0.0 (0.2) | < 0.05 |
| UD90 (Gy) | −16.3 (40.1) | −13.7 (25.6) | −8.7 (31.6) | ns |
| UD30 (Gy) | 10.5 (26.6) | 14.2 (27.5) | 19.9 (26.1) | ns |
| UD10 (Gy) | 20.0 (31.6) | 20.5 (39.4) | 29.6 (32.5) | ns |
Values are given as means (standard deviation) or numbers.
Abbreviations are the same as in Table 2.
The amount of the decrease for V100 was significantly reduced from pre-plan to intraoperative pre-plan (p < 0.05), and it tended to be reduced from intraoperative pre-plan to interactive plan, but the difference was not significant. The amounts of the increases for RV100 and RV150 did not show significant differences between pre-plan and intraoperative pre-plan, but they showed a significant reduction from intraoperative pre-plan to interactive plan. These results suggest that the interactive plan technique helps achieve ideal dosimetry with a small difference between the planning phase and CT analysis compared to the other techniques.
Toxicity
Table 5 shows comparisons of toxicities among the three techniques. Acute morbidity was defined as morbidity occurring ≤ 12 months after implantation, while late morbidity was defined as morbidity occurring > 12 months after implantation. Acute genitourinary toxicity, acute gastrointestinal toxicity, frequency, and urinary retention increased gradually from the pre-plan period to the interactive plan period. The other toxicities were not different among the three groups.
Table 5.
Crude toxicity rates
| Factors | Pre-plan group | Intraoperative pre-plan group | Interactive plan group | p |
|---|---|---|---|---|
| n | 27 | 86 | 192 | |
| RTOG GU acute | ||||
| Grade 1 | 19 (70%) | 56 (65%) | 119 (62%) | < 0.01 |
| Grade 2 | 1 (4%) | 12 (14%) | 50 (26%) | |
| Grade 3 | 0 (0%) | 4 (5%) | 10 (5%) | |
| RTOG GU late | ||||
| Grade 1 | 13 (48%) | 53 (62%) | 108 (56%) | ns |
| Grade 2 | 1 (4%) | 2 (2%) | 18 (9%) | |
| Grade 3 | 0 (0%) | 3 (3%) | 3 (2%) | |
| RTOG GI acute | ||||
| Grade 1 | 2 (7%) | 8 (9%) | 44 (23%) | < 0.05 |
| Grade 2 | 1 (4%) | 1 (1%) | 2 (1%) | |
| RTOG GI late | ||||
| Grade 1 | 6 (22%) | 18 (21%) | 46 (24%) | ns |
| Grade 2 | 0 (0%) | 1 (1%) | 2 (1%) | |
| Grade 3 | 0 (0%) | 0 (0%) | 1 (1%) | |
| Micturition pain | ||||
| Grade 1 | 10 (37%) | 37 (43%) | 83 (43%) | ns |
| Grade 2 | 0 (0%) | 3 (3%) | 8 (4%) | |
| Proctitis | ||||
| Grade 1 | 6 (22%) | 8 (9%) | 34 (18%) | ns |
| Grade 2 | 1 (4%) | 0 (0%) | 1 (1%) | |
| Incontinence (stool) | ||||
| Grade 1 | 1 (4%) | 0 (0%) | 1 (1%) | ns |
| Grade 2 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Diarrhea | ||||
| Grade 1 | 1 (4%) | 6 (7%) | 5 (3%) | ns |
| Grade 2 | 0 (0%) | 1 (1%) | 0 (0%) | |
| Rectal bleeding | ||||
| Grade 1 | 3 (11%) | 19 (22%) | 47 (24%) | ns |
| Grade 2 | 0 (0%) | 1 (1%) | 3 (2%) | |
| Frequency | ||||
| Grade 1 | 19 (70%) | 55 (64%) | 99 (52%) | < 0.01 |
| Grade 2 | 1 (4%) | 15 (17%) | 58 (30%) | |
| Grade 3 | 0 (0%) | 0 (0%) | 6 (3%) | |
| Incontinence (urine) | ||||
| Grade 1 | 0 (0%) | 5 (6%) | 3 (2%) | ns |
| Grade 2 | 1 (4%) | 1 (1%) | 4 (2%) | |
| Urinary retention | ||||
| Grade 1 | 10 (37%) | 50 (58%) | 137 (71%) | < 0.01 |
| Grade 2 | 1 (4%) | 2 (2%) | 10 (5%) | |
| Grade 3 | 0 (0%) | 4 (5%) | 7 (4%) | |
| Hematuria | ||||
| Grade 1 | 4 (15%) | 11 (13%) | 29 (15%) | ns |
| Grade 2 | 1 (4%) | 0 (0%) | 5 (3%) | |
| Stricture | ||||
| Grade 1 | 0 (0%) | 1 (1%) | 1 (1%) | ns |
| Grade 2 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Grade 3 | 0 (0%) | 1 (1%) | 0 (0%) | |
Values are given as means (standard deviation) or numbers.
RTOG – Radiation Therapy Oncology Group; GU – genitourinary; GI – gastrointestinal
Discussion
The present results showed a favorable biochemical control rate for Japanese prostate cancer patients, although more biochemical failures will occur when the follow-up data are updated. The detailed analysis of biochemical control and its predictive factors were reported in our previous paper [7]. This study focused on the differences among the three techniques.
Regarding biochemical control, Shah et al. reported the superiority of the intraoperative pre-plan technique to the pre-plan technique [8]. Their 4-year biochemical control rates were 96% and 82% for the intraoperative pre-plan and pre-plan groups, respectively. Meanwhile, the present results showed a lower biochemical control rate for the intraoperative pre-plan group compared to the other groups, although the difference was not significant (p = 0.08). Additional multivariate analysis including initial PSA, Gleason score (these parameters were identified as significant variables in our previous paper), and the type of planning techniques was also performed. It also did not show that type of planning technique was significant (data not shown). Therefore, it seems that some confounding factors caused relatively low biochemical control of the intraoperative pre-plan group in this study. In addition, our follow-up time was too short to draw a conclusion regarding biochemical control. Because pre-plan group had lower D90 value compared to the other groups (Table 3), longer follow-up might reveal its lower biochemical control compared to the other groups.
Regarding DVH parameters, the dose of the planning phase was directly correlated to that of the 1-month CT analysis in the present study. The increased DVH parameters from pre-plan to interactive plan represented our dose-escalation policy that started in the intraoperative pre-plan period. The landmark paper of Stock et al. that reported dose dependency of biochemical failure [9] strongly encouraged our dose-escalation policy. As shown in Table 2, D90 increased gradually from the pre-plan group to the interactive plan group, because it has been suggested that a higher dose leads to better bio-chemical control. The upper limit was set at about 200 Gy. Therefore, the improvement of DVH parameters may not necessarily be due to technical improvements, although our technical improvements must have had some effects. Several studies reported the superiority of intra-operative pre-plan to pre-plan [10, 11], and the superiority of interactive plan to pre-plan [12, 13]. However, these studies were retrospective analyses and may have had some biases, such as dose-escalation policy, as in the present study.
Meanwhile, as the present study demonstrated, toxicity was increased with dose escalation, especially urinary frequency and urinary retention. However, only acute toxicity showed a significant difference but late toxicity and grade 3 toxicities were at acceptable levels. Matzkin et al. reported increased urinary toxicity from pre-plan to intraoperative pre-plan [14]. They reported a higher International Prostate Symptom Score (IPSS) in the intra-operative pre-plan group than in the pre-plan group. However, they also reported increased DVH parameters such as D90 and urethral V150, as in the present study, and their increased toxicity may not have come from technical changes but increased prostate or urethral doses. Keyes et al. reported number of needles as one of predictive factors of acute urinary toxicity [15]. Although our number of needles was temporarily increased during intraoperative pre-plan, it decreased during interactive plan technique (Table 2). In the same period, acute urinary toxicity was steadily increased (Table 5). Therefore, the number of needles did not seem to have a significant effect on urinary toxicity at least in our study population.
Regarding seed migration, the present study results suggest that the interactive plan technique can reduce the seed migration rate. Stone et al. already reported that the interactive technique can reduce the seed migration rate [16], and the present study confirmed their report. Meanwhile, stranded-seed or linked-seed were suggested to have a strong impact on seed migration rate. Our recent publication reveals that stranded-seed or linked-seed can reduce seed migration rate significantly without compromising dosimetric parameters [17].
The learning curve of our brachytherapy team was one of the important factors related to our dosimetric results. Lower seed migration rates and the smaller DVH differences between the planning phase and CT analysis may suggest some technical improvements of our brachytherapy team. The personal learning curve of each team member was another important factor. Our brachytherapy team, however, consisted of at least 4 urologists and 3 radiation oncologists during the study period, and it was difficult to conduct separate analyses in our database.
In our planning phase, the numbers of seeds and needles and total activity were increased when the technique was switched from pre-plan to intraoperative pre-plan, and decreased when switched from intraoperative pre-plan to interactive plan (Table 2). Our dose escalation policy might have increased these numbers, and then our technical improvements, including efficient seed positioning, might have decreased these numbers.
Conclusions
In conclusion, the present study showed that DVH parameters improved from the pre-plan technique to the interactive plan technique. However, this improvement may not necessarily be due to technical improvements, because dose escalation was started during the same period. Lower seed migration rates and the smaller DVH differences between the planning phase and the CT analysis in the interactive plan period suggest the superiority of the interactive plan to the two other techniques.
Disclosure
This work was supported by JSPS KAKENHI Grant Number 24791334.
H. Ishiyama, T. Satoh and K. Hayakawa received honoraria for lecture fees from Medicon Co., Ltd. and Nihon Medi-Physics Co., Ltd.
References
- 1.Aronowitz JN, Rivard MJ. The phylogeny of permanent prostate brachytherapy. J Contemp Brachytherapy. 2013;5:89–92. doi: 10.5114/jcb.2013.35562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skowronek J. Low-dose-rate or high-dose-rate brachytherapy in treatment of prostate cancer – between options. J Contemp Brachytherapy. 2013;5:33–41. doi: 10.5114/jcb.2013.34342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stock RG, Stone NN, Wesson MF, et al. A modified technique allowing interactive ultrasound-guided three-dimensional transperineal prostate implantation. Int J Radiat Oncol Biol Phys. 1995;32:219–225. doi: 10.1016/0360-3016(95)00521-Y. [DOI] [PubMed] [Google Scholar]
- 4.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the rtog-astro phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 6.Nag S, Beyer D, Friedland J, et al. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;44:789–799. doi: 10.1016/s0360-3016(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 7.Sekiguchi A, Ishiyama H, Satoh T, et al. 125iodine monotherapy for Japanese men with low- and intermediate-risk prostate cancer: Outcomes after 5 years of follow-up. J Rad Res. 2014;55:328–333. doi: 10.1093/jrr/rrt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah JN, Wuu CS, Katz AE, et al. Improved biochemical control and clinical disease-free survival with intraoperative versus preoperative preplanning for transperineal interstitial permanent prostate brachytherapy. Cancer J. 2006;12:289–297. doi: 10.1097/00130404-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Stock RG, Stone NN, Cesaretti JA, et al. Biologically effective dose values for prostate brachytherapy: Effects on PSA failure and posttreatment biopsy results. Int J Radiat Oncol Biol Phys. 2006;64:527–533. doi: 10.1016/j.ijrobp.2005.07.981. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson DA, Lee EJ, Ciezki JP, et al. Dosimetric comparison of pre-planned and or-planned prostate seed brachytherapy. Int J Radiat Oncol Biol Phys. 2000;48:1241–1244. doi: 10.1016/s0360-3016(00)00734-3. [DOI] [PubMed] [Google Scholar]
- 11.Matzkin H, Kaver I, Bramante-Schreiber L, et al. Comparison between two iodine-125 brachytherapy implant techniques: Pre-planning and intra-operative by various dosimetry quality indicators. Radiother Oncol. 2003;68:289–294. doi: 10.1016/s0167-8140(03)00242-1. [DOI] [PubMed] [Google Scholar]
- 12.Shanahan TG, Nanavati PJ, Mueller PW, et al. A comparison of permanent prostate brachytherapy techniques: preplan vs. hybrid interactive planning with postimplant analysis. Int J Radiat Oncol Biol Phys. 2002;53:490–496. doi: 10.1016/s0360-3016(02)02757-8. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky MJ, Yamada Y, Cohen G, et al. Postimplantation dosimetric analysis of permanent transperineal prostate implantation: improved dose distributions with an intraoperative computer-optimized conformal planning technique. Int J Radiat Oncol Biol Phys. 2000;48:601–608. doi: 10.1016/s0360-3016(00)00655-6. [DOI] [PubMed] [Google Scholar]
- 14.Matzkin H, Kaver I, Stenger A, et al. Iodine-125 brachytherapy for localized prostate cancer and urinary morbidity: a prospective comparison of two seed implant methods-preplanning and intraoperative planning. Urology. 2003;62:497–502. doi: 10.1016/s0090-4295(03)00407-2. [DOI] [PubMed] [Google Scholar]
- 15.Keyes M, Miller S, Moravan V, et al. Predictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: long-term outcome in 712 consecutive patients. Int J Radiat Oncol Biol Phys. 2009;73:1023–1032. doi: 10.1016/j.ijrobp.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Stone NN, Stock RG. Reduction of pulmonary migration of permanent interstitial sources in patients undergoing prostate brachytherapy. Urology. 2005;66:119–123. doi: 10.1016/j.urology.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Ishiyama H, Satoh T, Kawakami S, et al. A prospective quasi-randomized comparison of intraoperatively built custom-linked seeds versus loose seeds for prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2014;90:134–139. doi: 10.1016/j.ijrobp.2014.05.009. [DOI] [PubMed] [Google Scholar]