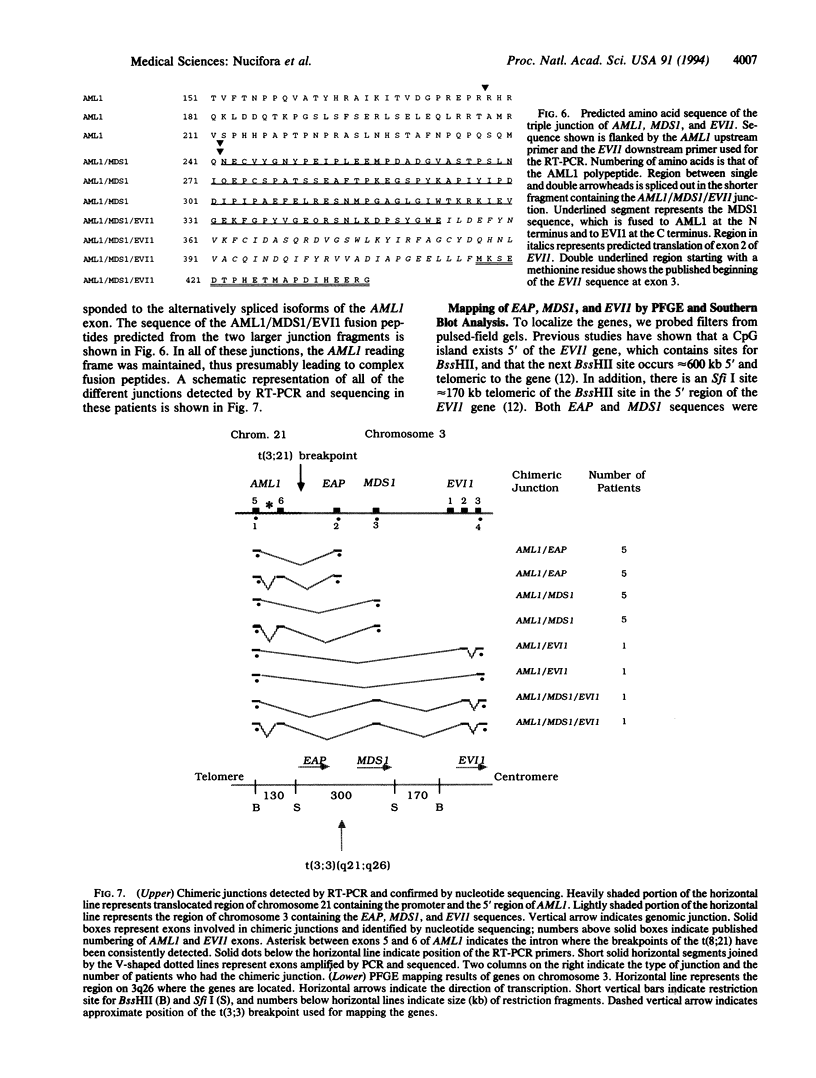
Abstract
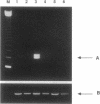
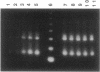
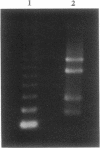
Two genes have been implicated in leukemias of patients with abnormalities of chromosome 3, band q26: EVI1, which can be activated over long distances by chromosomal rearrangements involving 3q26, and EAP, a ribosomal gene that fuses with AML1 in a therapy-related myelodysplasia patient with a t(3;21)(q26.2;q22). AML1 was identified by its involvement in the t(8;21)(q22;q22) of acute myeloid leukemia. Here we report the consistent identification of fusion transcripts between AML1 and EAP or between AML1 and previously unidentified sequences that we named MDS1 (MDS-associated sequences) in the leukemic cells of four patients with therapy-related myelodysplasia/acute myeloid leukemia and in one patient with chronic myelogenous leukemia in blast crisis, all of whom had a t(3;21). In addition, we have identified a third chimeric transcript, AML1/EVI1, in one of the therapy-related acute myeloid leukemia patients. Pulsed-field gel electrophoresis established the order of the genes as EAP, the most telomeric, and EVI1, the most centromeric, gene. The results indicate that translocations could involve multiple genes and affect gene expression over long distances.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae S. C., Yamaguchi-Iwai Y., Ogawa E., Maruyama M., Inuzuka M., Kagoshima H., Shigesada K., Satake M., Ito Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993 Mar;8(3):809–814. [PubMed] [Google Scholar]
- Bartholomew C., Ihle J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991 Apr;11(4):1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter M. A., Neilly M. E., Le Beau M. M., Pearson M. G., Rowley J. D. Rearrangements of chromosome 3 involving bands 3q21 and 3q26 are associated with normal or elevated platelet counts in acute nonlymphocytic leukemia. Blood. 1985 Dec;66(6):1362–1370. [PubMed] [Google Scholar]
- Daga A., Tighe J. E., Calabi F. Leukaemia/Drosophila homology. Nature. 1992 Apr 9;356(6369):484–484. doi: 10.1038/356484b0. [DOI] [PubMed] [Google Scholar]
- Erickson P., Gao J., Chang K. S., Look T., Whisenant E., Raimondi S., Lasher R., Trujillo J., Rowley J., Drabkin H. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992 Oct 1;80(7):1825–1831. [PubMed] [Google Scholar]
- Gerber M. J., Drabkin H. A., Firnhaber C., Miller Y. E., Scoggin C. H., Smith D. I. Regional localization of chromosome 3-specific DNA fragments by using a hybrid cell deletion mapping panel. Am J Hum Genet. 1988 Oct;43(4):442–451. [PMC free article] [PubMed] [Google Scholar]
- Henglein B., Synovzik H., Groitl P., Bornkamm G. W., Hartl P., Lipp M. Three breakpoints of variant t(2;8) translocations in Burkitt's lymphoma cells fall within a region 140 kilobases distal from c-myc. Mol Cell Biol. 1989 May;9(5):2105–2113. doi: 10.1128/mcb.9.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani K., Ogawa S., Tanaka T., Miyoshi H., Kurokawa M., Mano H., Yazaki Y., Ohki M., Hirai H. Generation of the AML1-EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic crisis in chronic myelocytic leukemia. EMBO J. 1994 Feb 1;13(3):504–510. doi: 10.1002/j.1460-2075.1994.tb06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Shimizu K., Kozu T., Maseki N., Kaneko Y., Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parganas E., Douglass E. C., Ihle J. N. Unique expression of the human Evi-1 gene in an endometrial carcinoma cell line: sequence of cDNAs and structure of alternatively spliced transcripts. Oncogene. 1990 Jul;5(7):963–971. [PubMed] [Google Scholar]
- Morishita K., Parganas E., William C. L., Whittaker M. H., Drabkin H., Oval J., Taetle R., Valentine M. B., Ihle J. N. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300-400 kilobases on chromosome band 3q26. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3937–3941. doi: 10.1073/pnas.89.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Nucifora G., Begy C. R., Erickson P., Drabkin H. A., Rowley J. D. The 3;21 translocation in myelodysplasia results in a fusion transcript between the AML1 gene and the gene for EAP, a highly conserved protein associated with the Epstein-Barr virus small RNA EBER 1. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7784–7788. doi: 10.1073/pnas.90.16.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Birn D. J., Erickson P., Gao J., LeBeau M. M., Drabkin H. A., Rowley J. D. Detection of DNA rearrangements in the AML1 and ETO loci and of an AML1/ETO fusion mRNA in patients with t(8;21) acute myeloid leukemia. Blood. 1993 Feb 15;81(4):883–888. [PubMed] [Google Scholar]
- Nucifora G., Birn D. J., Espinosa R., 3rd, Erickson P., LeBeau M. M., Roulston D., McKeithan T. W., Drabkin H., Rowley J. D. Involvement of the AML1 gene in the t(3;21) in therapy-related leukemia and in chronic myeloid leukemia in blast crisis. Blood. 1993 May 15;81(10):2728–2734. [PubMed] [Google Scholar]
- Rubin C. M., Larson R. A., Anastasi J., Winter J. N., Thangavelu M., Vardiman J. W., Rowley J. D., Le Beau M. M. t(3;21)(q26;q22): a recurring chromosomal abnormality in therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 1990 Dec 15;76(12):2594–2598. [PubMed] [Google Scholar]
- Rubin C. M., Larson R. A., Bitter M. A., Carrino J. J., Le Beau M. M., Diaz M. O., Rowley J. D. Association of a chromosomal 3;21 translocation with the blast phase of chronic myelogenous leukemia. Blood. 1987 Nov;70(5):1338–1342. [PubMed] [Google Scholar]
- Toczyski D. P., Steitz J. A. EAP, a highly conserved cellular protein associated with Epstein-Barr virus small RNAs (EBERs). EMBO J. 1991 Feb;10(2):459–466. doi: 10.1002/j.1460-2075.1991.tb07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczyski D. P., Steitz J. A. The cellular RNA-binding protein EAP recognizes a conserved stem-loop in the Epstein-Barr virus small RNA EBER 1. Mol Cell Biol. 1993 Jan;13(1):703–710. doi: 10.1128/mcb.13.1.703. [DOI] [PMC free article] [PubMed] [Google Scholar]