Most oncologists encountered drug shortages in the year before our survey, but experiences with shortages varied with practice structure. Further research is needed to quantitatively assess the impact of drug shortages on patients.
Abstract
Purpose:
There have been numerous reports of shortages of injectable drugs for cancer in the last decade. We assessed physician experiences with drug shortages in a population-based cohort of medical oncologists caring for patients with lung or colorectal cancer.
Methods:
We surveyed medical oncologists caring for patients with lung or colorectal cancer in the Cancer Care Outcomes Research and Surveillance Consortium from 2012 to 2013 (participation rate, 53%). Oncologists reported experiences with shortages of leucovorin, fluorouracil, dexamethasone, cyanocobalamin, paclitaxel, cisplatin, and etoposide in the prior year and whether they had used a less-effective alternative because of a shortage. We used multivariable logistic regression to assess for associations between physician or practice characteristics and encountering shortages.
Results:
Among 330 respondents, 74% reported experiences with a shortage of at least one drug in our survey, and 28% reported using a less-effective alternative because of a shortage. Although physician demographic characteristics did not predict reports of drug shortages, practice characteristics did. Veterans Affairs (VA) oncologists were less likely to report experiencing any shortage than oncologists in single-specialty group practice (odds ratio [OR], 0.4; 95% CI, 0.2 to 0.9). The reported use of a less effective alternative to any drug was also less common among VA oncologists (OR, 0.3; 95% CI, 0.1 to 0.9) and oncologists affiliated with health maintenance organizations (OR, 0.4; 95% CI, 0.2 to 0.9) compared with physicians in single-specialty groups.
Conclusion:
Most oncologists encountered drug shortages in the year before our survey, but experiences with shortages varied with practice structure. Further research is needed to quantitatively assess the impact of drug shortages on patients and evaluate various strategies for managing them.
Introduction
The number of drug shortages in the United States has increased significantly over the past decade.1 Injectable generic drugs, particularly medications for cancer, have been especially affected.2–7 There have been shortages of numerous drugs that play important roles in treating adult and pediatric cancers, including leucovorin, bleomycin, cisplatin, liposomal doxorubicin, etoposide, vinblastine, vincristine, paclitaxel, cytarabine, and methotrexate.2,3,6,8 Substitutions of alternative medications for unavailable drugs have been associated with increased cost of care,3,9–11 emergence of a so-called gray market for generic medications sold with a significant markup,3,7,9,12 increased medication errors,13,14 impaired clinical trial accrual,13 and negative impact on outcome in childhood Hodgkin disease.15
Several factors have contributed to drug shortages, including consolidation of generic drug production among a small number of manufacturers12; low profit potential from generic drug production, attributed in part to 2003 Medicare reimbursement changes for injectable drugs3,12; manufacturing and distribution issues16; and increased demand for generic medications.4 The shortage problem was particularly severe from 2011 to 2012, and President Obama issued an executive order calling for early reporting of drug shortages to the US Food and Drug Administration and examination of price gouging problems.9 Nevertheless, although the number of new shortages has decreased somewhat, existing shortages have persisted.17–19
Relatively few studies have assessed health professionals' experiences with drug shortages. A 2011 multi-institutional survey of hospital-based oncology pharmacists identified high rates of reported treatment changes, increased costs, and effects on clinical trial accrual; 22% of pharmacists reported experiences with shortage-related therapeutic changes leading to near-miss or actual medication errors at their institutions, including errors in dosing conversions and wrong drug concentrations, some of which resulted in incorrect drug administration to patients.13 A 2013 survey of hospital oncology pharmacists found that 99% had experienced a shortage of at least one injectable oncology drug in the last year, which had resulted in adverse safety events and caused providers to change medications, adjust doses, delay treatments, and prioritize some patients over others for receipt of available drugs.19 Two reports have summarized experiences of American Society of Clinical Oncology (ASCO) members with drug shortages. Among 214 ASCO members surveyed from 2012 to 2013 (response rate, 55%), 83% reported shortages, and many physicians reported needing to delay treatment initiation for some patients or substitute expensive brand-name drugs for generic medications.20 The other report of 462 ASCO members surveyed in April 2013 found that the rate of reported ongoing drug shortages had decreased from the prior year, with 59% of respondents reporting ongoing shortages.21 However, these surveys included only ASCO members and did not assess experiences with drug shortages across different practice settings.
We surveyed oncologists caring for patients enrolled in the CanCORS (Cancer Outcomes Research and Surveillance Consortium) study from 2012 to 2013 about experiences with drug shortages. The CanCORS study is a population- and health-system–based study of approximately 10,000 patients diagnosed with lung or colorectal cancer from 2003 to 2005 and their care providers; CanCORS patients have been shown to be representative of patients diagnosed with these cancers in the United States.22 Oncologists who had cared for these patients were surveyed regarding whether they had encountered shortages of drugs used in treating lung and colorectal cancers in their practices and whether they had substituted equally or less effective drugs as a result of these shortages. We also assessed the association between oncologist practice structure and experiences with these shortages.
Methods
Study Design and Participants
The CanCORS study was an observational study designed to evaluate cancer care and outcomes among patients with lung or colorectal cancer newly diagnosed from 2003 to 2005 and living in one of five geographic regions (northern California, Los Angeles County, North Carolina, Iowa, or Alabama) or receiving care in one of five health maintenance organizations (HMOs) or one of 15 Veterans Affairs (VA) sites.23 Physicians caring for CanCORS patients were identified from a baseline patient survey, a follow-up survey 1 year after diagnosis, a follow-up survey in 2012, and medical records; physicians were initially surveyed from 2006 to 2008. A follow-up survey of CanCORS medical oncologists only was performed from summer 2012 to fall 2013; this provided the data for our analysis. We surveyed these oncologists by mail (with Internet option); attempts to increase participation among nonrespondents included subsequent mail and telephone contacts (Appendix, online only). The American Association of Public Opinion Research24 response rate was 46.4%, and the participation rate among physicians for whom we had valid contact information was 52.9%, yielding a total of 357 respondents (Appendix, online only). We excluded 14 who reported not caring for any patients with lung or colorectal cancer in the last year and 13 physicians who did not answer any of the questions about shortages of specific drugs, for a final analytic cohort of 330 medical oncologists.
Questionnaire
Oncologists were asked about experiences with shortages of leucovorin, fluorouracil, dexamethasone, cyanocobalamin, paclitaxel, cisplatin, and etoposide, chosen for their roles as chemotherapeutics or supportive medications for patients with lung cancer and/or patients with colorectal cancer (Data Supplement). For each drug, oncologists could respond that over the last 12 months: “I have needed to use an equally effective alternative,” “I have needed to use a less effective alternative,” “I have not been affected by the shortage,” and “I do not use this drug.” Physicians describing the use of an equally or less effective alternative were asked to describe the number of patients affected by each circumstance. Respondents were also asked to provide free-text descriptions of any experiences they had with changes in treatment or delays resulting from drug shortages; these descriptions included experiences with shortages besides those specifically assessed by the survey.
Physicians also reported their practice structure (categorized as solo practice, single-specialty group practice, multispecialty group practice, HMO affiliated, or VA site); whether they cared for patients with lung cancer, patients with colorectal cancer, or both those with lung cancer and those with colorectal cancer; and age, sex, and teaching role. We also documented the date the survey was returned.
Statistical Analysis
We assessed associations between physician and practice characteristics and reports of experience with (1) any drug shortage or (2) having to use a less effective alternative to any given medication using χ2 tests for unadjusted analyses and multivariable logistic regression for adjusted analyses. The regression models included all variables as described above, categorized as in Table 1. Multiple imputation was used to impute missing values for independent variables (including two physicians with missing age data, one missing sex, and one missing teaching role; Table 1)25; we did not use imputed data for the dependent variables concerning drug shortages. A two-sided P value of less than .05 was considered statistically significant. Analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC) and STATA software (version 13; STATA, College Station, TX).
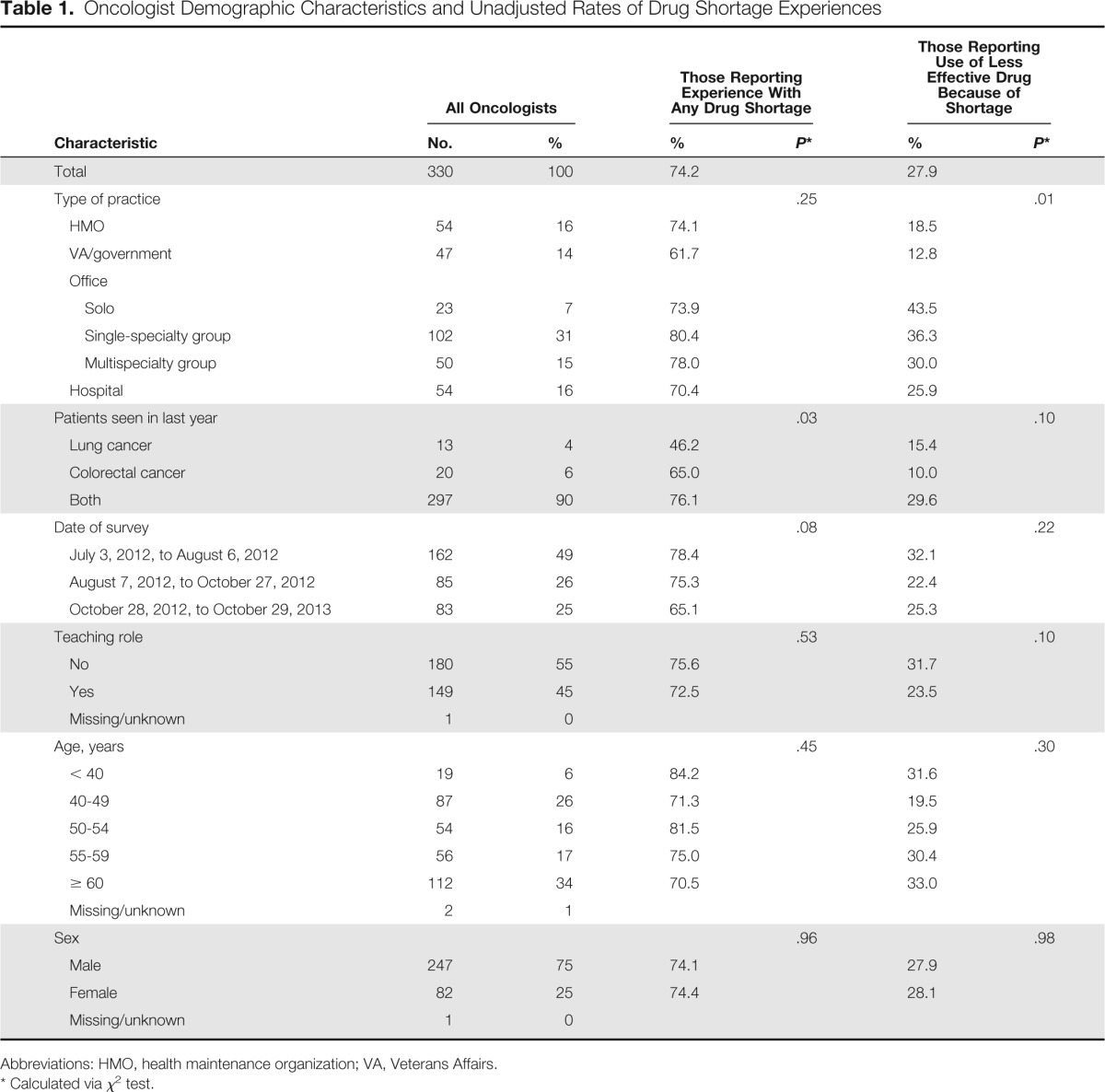
Table 1.
Oncologist Demographic Characteristics and Unadjusted Rates of Drug Shortage Experiences
| Characteristic | All Oncologists |
Those Reporting Experience With Any Drug Shortage |
Those Reporting Use of Less Effective Drug Because of Shortage |
|||
|---|---|---|---|---|---|---|
| No. | % | % | P* | % | P* | |
| Total | 330 | 100 | 74.2 | 27.9 | ||
| Type of practice | .25 | .01 | ||||
| HMO | 54 | 16 | 74.1 | 18.5 | ||
| VA/government | 47 | 14 | 61.7 | 12.8 | ||
| Office | ||||||
| Solo | 23 | 7 | 73.9 | 43.5 | ||
| Single-specialty group | 102 | 31 | 80.4 | 36.3 | ||
| Multispecialty group | 50 | 15 | 78.0 | 30.0 | ||
| Hospital | 54 | 16 | 70.4 | 25.9 | ||
| Patients seen in last year | .03 | .10 | ||||
| Lung cancer | 13 | 4 | 46.2 | 15.4 | ||
| Colorectal cancer | 20 | 6 | 65.0 | 10.0 | ||
| Both | 297 | 90 | 76.1 | 29.6 | ||
| Date of survey | .08 | .22 | ||||
| July 3, 2012, to August 6, 2012 | 162 | 49 | 78.4 | 32.1 | ||
| August 7, 2012, to October 27, 2012 | 85 | 26 | 75.3 | 22.4 | ||
| October 28, 2012, to October 29, 2013 | 83 | 25 | 65.1 | 25.3 | ||
| Teaching role | .53 | .10 | ||||
| No | 180 | 55 | 75.6 | 31.7 | ||
| Yes | 149 | 45 | 72.5 | 23.5 | ||
| Missing/unknown | 1 | 0 | ||||
| Age, years | .45 | .30 | ||||
| < 40 | 19 | 6 | 84.2 | 31.6 | ||
| 40-49 | 87 | 26 | 71.3 | 19.5 | ||
| 50-54 | 54 | 16 | 81.5 | 25.9 | ||
| 55-59 | 56 | 17 | 75.0 | 30.4 | ||
| ≥ 60 | 112 | 34 | 70.5 | 33.0 | ||
| Missing/unknown | 2 | 1 | ||||
| Sex | .96 | .98 | ||||
| Male | 247 | 75 | 74.1 | 27.9 | ||
| Female | 82 | 25 | 74.4 | 28.1 | ||
| Missing/unknown | 1 | 0 | ||||
Abbreviations: HMO, health maintenance organization; VA, Veterans Affairs.
Calculated via χ2 test.
Results
Among responding oncologists, 90% had cared for both patients with lung cancer and patients with colorectal cancer in the last year; 4% cared for patients with lung but not colorectal cancer, and 6% cared for patients with colorectal but not lung cancer (Table 1). Approximately one third (31%) worked in a single-specialty office group practice, 7% in solo practice, 15% in a multispecialty office practice, 16% in a hospital practice, 16% in an HMO setting, and 14% in a VA or government setting.
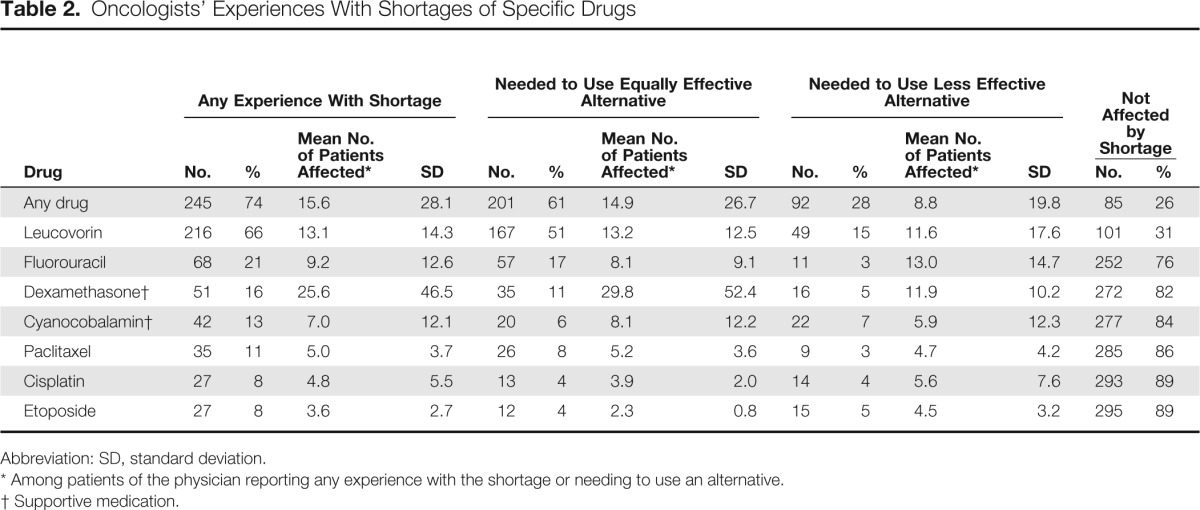
Overall, 74% of oncologist respondents reported experience with a shortage of at least one of the specific chemotherapy or supportive medications in our survey in the last year (Table 2). Leucovorin shortages were reported by 66% of oncologists; fluorouracil, by 21%; dexamethasone, by 16%; cyanocobalamin, by 13%; paclitaxel, by 11%; cisplatin, by 8%; and etoposide, by 8%. Although 61% reported having to use an equally effective alternative to at least one of these particular drugs, 28% of respondents reported having to use a less effective alternative. Among oncologists describing experience with at least one shortage, the mean (± standard deviation) reported number of patients affected was 16 (± 28); among oncologists describing the need to use a less effective alternative to at least one drug of choice, the mean reported affected was nine (± 20).
Table 2.
Oncologists' Experiences With Shortages of Specific Drugs
| Drug | Any Experience With Shortage |
Needed to Use Equally Effective Alternative |
Needed to Use Less Effective Alternative |
Not Affected by Shortage |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean No. of Patients Affected* | SD | No. | % | Mean No. of Patients Affected* | SD | No. | % | Mean No. of Patients Affected* | SD | No. | % | |
| Any drug | 245 | 74 | 15.6 | 28.1 | 201 | 61 | 14.9 | 26.7 | 92 | 28 | 8.8 | 19.8 | 85 | 26 |
| Leucovorin | 216 | 66 | 13.1 | 14.3 | 167 | 51 | 13.2 | 12.5 | 49 | 15 | 11.6 | 17.6 | 101 | 31 |
| Fluorouracil | 68 | 21 | 9.2 | 12.6 | 57 | 17 | 8.1 | 9.1 | 11 | 3 | 13.0 | 14.7 | 252 | 76 |
| Dexamethasone† | 51 | 16 | 25.6 | 46.5 | 35 | 11 | 29.8 | 52.4 | 16 | 5 | 11.9 | 10.2 | 272 | 82 |
| Cyanocobalamin† | 42 | 13 | 7.0 | 12.1 | 20 | 6 | 8.1 | 12.2 | 22 | 7 | 5.9 | 12.3 | 277 | 84 |
| Paclitaxel | 35 | 11 | 5.0 | 3.7 | 26 | 8 | 5.2 | 3.6 | 9 | 3 | 4.7 | 4.2 | 285 | 86 |
| Cisplatin | 27 | 8 | 4.8 | 5.5 | 13 | 4 | 3.9 | 2.0 | 14 | 4 | 5.6 | 7.6 | 293 | 89 |
| Etoposide | 27 | 8 | 3.6 | 2.7 | 12 | 4 | 2.3 | 0.8 | 15 | 5 | 4.5 | 3.2 | 295 | 89 |
Abbreviation: SD, standard deviation.
Among patients of the physician reporting any experience with the shortage or needing to use an alternative.
Supportive medication.
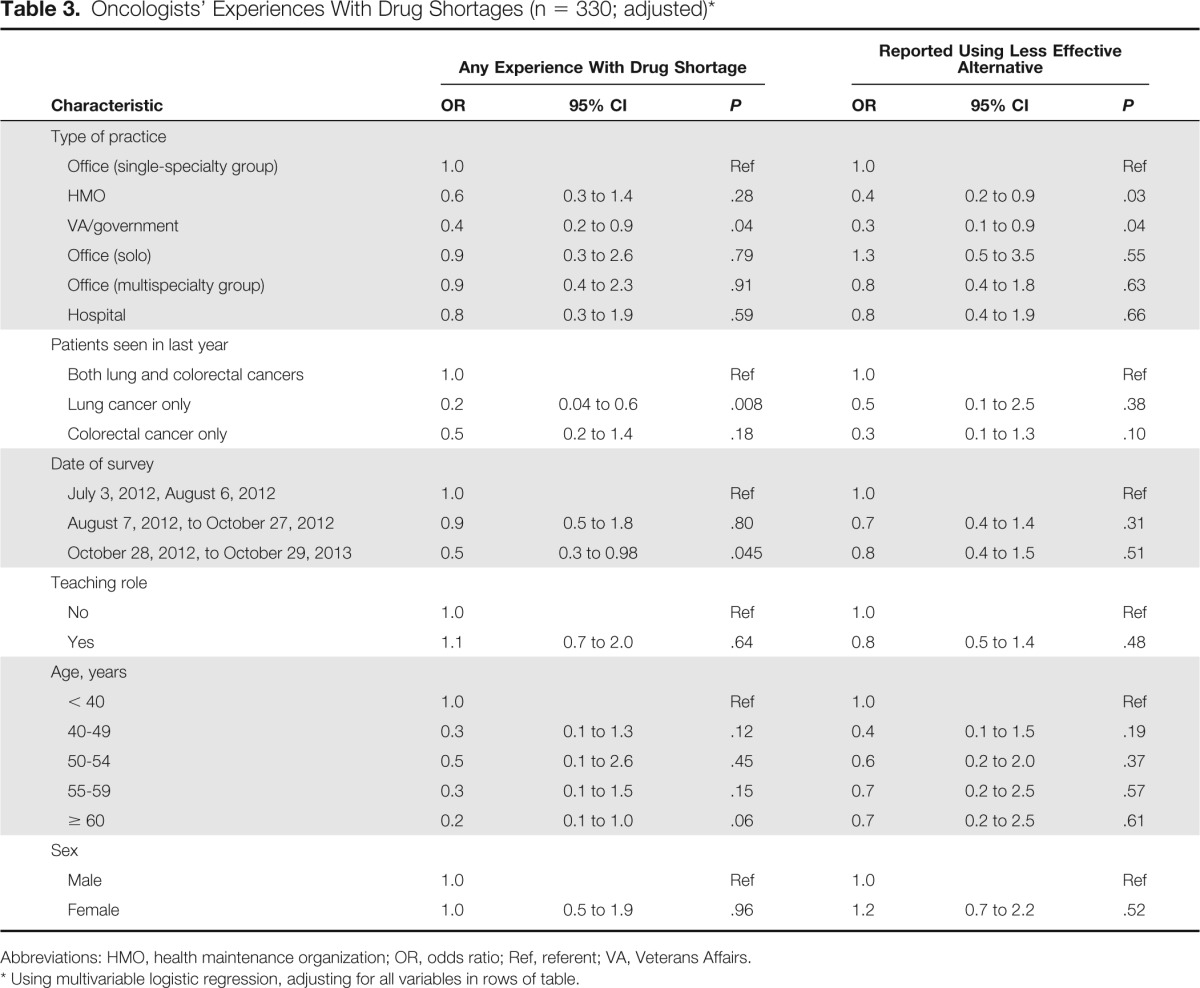
Compared with oncologists in single-specialty office practices, VA oncologists were less likely to report experiencing a shortage of any of the drugs in our survey after adjustment for other physician characteristics (odds ratio [OR], 0.4; 95% CI, 0.2 to 0.9; P = .04; Table 3). Practitioners who cared for patients with lung but not colon cancer in the last year were also less likely to experience a shortage (OR, 0.2; 95% CI, 0.04 to 0.6; P = .008) than those caring for both patients with lung cancer and patients with colorectal cancer. In addition, those completing the survey after October 2012 versus in July or early August 2012 were less likely to report any experience with a shortage in the last year (OR, 0.5; 95% CI, 0.3 to 0.98). However, there was no significant difference according to survey receipt date in reports of having to use a less effective therapy because of drug shortages. Compared with single-specialty group practitioners, oncologists practicing in an HMO (OR, 0.4; 95% CI, 0.2 to 0.9; P = .03) or a VA site (OR, 0.3; 95% CI, 0.1 to 0.9; P = .04) were less likely to report having to substitute a less effective alternative.
Table 3.
Oncologists' Experiences With Drug Shortages (n = 330; adjusted)*
| Characteristic | Any Experience With Drug Shortage |
Reported Using Less Effective Alternative |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Type of practice | ||||||
| Office (single-specialty group) | 1.0 | Ref | 1.0 | Ref | ||
| HMO | 0.6 | 0.3 to 1.4 | .28 | 0.4 | 0.2 to 0.9 | .03 |
| VA/government | 0.4 | 0.2 to 0.9 | .04 | 0.3 | 0.1 to 0.9 | .04 |
| Office (solo) | 0.9 | 0.3 to 2.6 | .79 | 1.3 | 0.5 to 3.5 | .55 |
| Office (multispecialty group) | 0.9 | 0.4 to 2.3 | .91 | 0.8 | 0.4 to 1.8 | .63 |
| Hospital | 0.8 | 0.3 to 1.9 | .59 | 0.8 | 0.4 to 1.9 | .66 |
| Patients seen in last year | ||||||
| Both lung and colorectal cancers | 1.0 | Ref | 1.0 | Ref | ||
| Lung cancer only | 0.2 | 0.04 to 0.6 | .008 | 0.5 | 0.1 to 2.5 | .38 |
| Colorectal cancer only | 0.5 | 0.2 to 1.4 | .18 | 0.3 | 0.1 to 1.3 | .10 |
| Date of survey | ||||||
| July 3, 2012, August 6, 2012 | 1.0 | Ref | 1.0 | Ref | ||
| August 7, 2012, to October 27, 2012 | 0.9 | 0.5 to 1.8 | .80 | 0.7 | 0.4 to 1.4 | .31 |
| October 28, 2012, to October 29, 2013 | 0.5 | 0.3 to 0.98 | .045 | 0.8 | 0.4 to 1.5 | .51 |
| Teaching role | ||||||
| No | 1.0 | Ref | 1.0 | Ref | ||
| Yes | 1.1 | 0.7 to 2.0 | .64 | 0.8 | 0.5 to 1.4 | .48 |
| Age, years | ||||||
| < 40 | 1.0 | Ref | 1.0 | Ref | ||
| 40-49 | 0.3 | 0.1 to 1.3 | .12 | 0.4 | 0.1 to 1.5 | .19 |
| 50-54 | 0.5 | 0.1 to 2.6 | .45 | 0.6 | 0.2 to 2.0 | .37 |
| 55-59 | 0.3 | 0.1 to 1.5 | .15 | 0.7 | 0.2 to 2.5 | .57 |
| ≥ 60 | 0.2 | 0.1 to 1.0 | .06 | 0.7 | 0.2 to 2.5 | .61 |
| Sex | ||||||
| Male | 1.0 | Ref | 1.0 | Ref | ||
| Female | 1.0 | 0.5 to 1.9 | .96 | 1.2 | 0.7 to 2.2 | .52 |
Abbreviations: HMO, health maintenance organization; OR, odds ratio; Ref, referent; VA, Veterans Affairs.
Using multivariable logistic regression, adjusting for all variables in rows of table.
In open-ended questioning about drug shortage experiences, 22% of respondents also reported encountering a drug shortage (including but not limited to chemotherapy drugs) in addition to those about which we specifically asked. The most common drugs mentioned were doxorubicin or liposomal doxorubicin, reported by 62 physicians (19%). Including the drugs about which we specifically asked and all free-text reports of shortages, a total of 79% of respondents reported being affected by a shortage of at least one cancer-related medication. Two oncologists described how their practices had to conserve drugs in shortage by reserving standard therapy for curable patients and resorting to possibly less effective alternatives in patients with incurable disease. One oncologist described how his or her large health care organization was able to manage drug shortages effectively but with significant extra work required. Four oncologists described how drug shortages interfered with their ability to successfully treat patients on clinical trial protocols. Twelve oncologists specifically described experiencing delays in patient treatment or other adverse effects on patients, and three additional oncologists described shortages that they believed contributed to patient deaths.
Discussion
We identified frequent experiences with drug shortages among oncologists caring for patients with lung cancer and/or patients with colorectal cancer, consistent with prior studies of oncologists.20,21 The most commonly reported drug in shortage was leucovorin, which is an important component of chemotherapy for colorectal cancer. This reflects the practice patterns of our cohort of physicians, most of whom cared for both patients with lung cancer and patients with colorectal cancer; indeed, the impact of the leucovorin shortage likely explains why the few oncologists who did not see patients with colorectal cancer were less likely to experience shortages overall. We also identified differences in experiences with shortages according to practice structure; oncologists affiliated with managed care or governmental organizations were not as likely to report having to use a less effective alternative to a drug in shortage, and VA oncologists were less likely to experience any drug shortage compared with physicians in single-specialty group practices.
These findings underscore the scope of the recent cancer drug shortage problem and may inform strategies for ameliorating it in the future. One possible explanation for our findings is that larger practices and integrated health systems with a centralized formulary may be better able to withstand disruptions in injectable drug supply, possibly because they can formulate organized institutional policies for responding to shortages. They may also have greater leverage with pharmaceutical partners and thus be more able to insulate themselves from short-term disruptions in the drug market. As the clinical practice of medical oncology continues to shift from small practices to hospitals, this may enable more concerted institutional policies designed to mitigate the impact on patients as well as further study of the effects of these policies on optimization of the response to future shortages.
Although data are limited on the impact of drug shortages on the care of patients with cancer, at least one prior analysis15 reported impaired survival in patients with cancer treated with nonstandard therapy. We found that 28% of oncologists reported substituting less effective alternatives for indicated drugs, and some such shortages may have adversely affected patient outcomes, as reported anecdotally by some oncologists in our study. Indeed, some of the drugs we assessed are components of regimens used in curative-intent therapy, such as fluorouracil and leucovorin for patients with colorectal cancer26,27 or cisplatin for those with non–small-cell lung cancer.28 We did not have patient-level data in this analysis to directly assess outcomes, but some of the drugs we assessed do not have known equally effective substitutes for particular clinical settings. For example, in medically inoperable stage IIIA squamous cell lung cancer, potentially curative combination chemotherapy and radiation therapy could be compromised by shortages of cisplatin, because treatment with this drug is recommended over alternative regimens containing carboplatin.28
Our study also highlights the fact that drug shortages may affect supportive medications used in cancer treatment in addition to chemotherapy. We found that approximately 16% of oncologists experienced shortages of dexamethasone, and approximately 13% experienced shortages of cyanocobalamin. Although there may be reasonable alternatives to dexamethasone for the management or prevention of hypersensitivity reactions, drug-associated rash, and chemotherapy-associated nausea and vomiting, there are no accepted alternatives to injectable B12 to reduce the toxicity associated with pemetrexed.29 More work is needed to determine whether shortages of supportive medications affect the experience of patients with cancer regarding adverse effects or quality of life.
Although our oncologist survey was not able to directly assess the financial impact of drug shortages, it is likely that shortages described by oncologists were associated with increased costs to patients and payers. For example, the most commonly reported drug shortage in our study was of leucovorin. Although an equally effective substitute for this drug exists,30–33 the increased cost associated with levoleucovorin (approximately 40× more expensive than leucovorin)3 was likely substantial and may have contributed to increased so-called financial toxicity34 to patients.
Finally, we observed slightly lower rates of drug shortages among physicians responding to our survey after October 2012, possibly because of decreases in rates of new drug shortages, although this was not associated with a decrease in the use of a less effective alternative over time. It is possible that this temporal trend reflects an impact of the 2012 US Food and Drug Administration Safety and Innovation Act, which included several measures intended to decrease the frequency of drug shortages and mitigate their impact, including strengthening reporting requirements and allowing expedited review of applications and inspections that might alleviate a shortage.17,18,35
Strengths of our analysis included our study sample of oncologists caring for a representative population of patients with lung or colorectal cancer in the United States22 and the collection of practice structure information, which enabled assessment of the differential impacts stemming from drug shortages. An important limitation (as in prior survey research into this issue) was our reliance on subjective reports from providers of their experience with drug shortages and number of patients affected. Because this was a survey of oncologists, we also did not have data available for direct assessment of effects on patients. Additionally, the 53% participation rate in our survey may limit the generalizability of our results, although we found no significant differences in basic demographic characteristics between responders and nonresponders (Appendix Table A1, online only). Finally, we asked oncologists to describe whether they had to use equally versus less effective alternatives to drugs in shortage, but this distinction may not always have been clinically obvious for individual patients or for specific therapeutic agents.
In conclusion, we found that nearly three fourths of oncologists surveyed from 2012 to 2013 who cared for patients with lung cancer and/or patients with colorectal cancer had experienced shortages of at least one of seven specific drugs commonly used in the treatment of these malignancies. More than one quarter reported having to prescribe a less effective drug as an alternative to one of these agents. Experiences with shortages were somewhat less common among oncologists working in integrated health systems. Ultimately, continued and concerted efforts to decrease the scope of the drug shortage problem in cancer care and evaluation of its impact on patient outcomes will be required.
Supplementary Material
Appendix
CanCORS II Medical Oncologist Survey Development and Administration
Survey Development
The CanCORS (Cancer Outcomes Research and Surveillance Consortium) II physician survey was designed to collect information from medical oncologists about their use of molecular biomarkers and biologic therapies as well as survivorship care. The survey also collected information about practice characteristics and financial arrangements and physicians' demographics. When possible, items were taken (or adapted) from previously developed instruments, including the CanCORS I physician survey. When items were not available from prior surveys, new questions were developed.
The survey also included two experiments where vignettes were modified in different versions of the survey instrument. The first randomly assigned respondents to receive different versions of the vignette in which a question asking about Lynch syndrome testing varied by the family history of the patient (strong family history of colon cancer, modest family history [eg, uncle who had colon cancer at age 62 years], and no family history of colon cancer). These scenarios roughly followed the Amsterdam recommendations for screening for Lynch syndrome and also addressed recommendations that suggest that all individuals age < 60 years should be screened. The second experiment asked about epidermal growth factor receptor testing for lung cancer and varied the race/ethnicity of the patient in question (white v Asian v black). EGFR mutations are quite prevalent in Asian women and less so in whites versus blacks. Some believe that mutation rates in blacks are much lower than in whites, but more recent data suggest that they are probably similar.
The survey instrument underwent cognitive testing by survey development staff at Westat (Rockville, MD), followed by revisions to the instrument to improve clarity and decrease the length. Questions were dropped if they were difficult to answer, were not likely to provide data with variation, or were of lower priority. A Web-based version of the survey was also developed. The full survey instrument is available at https://www.cancors.org/public/servlets/open/home/home.cmd.
Cohort
The target population included medical oncologists who have cared for patient participants in CanCORS. The CanCORS patient cohort is a population-based cohort of individuals diagnosed with lung or colorectal cancer from 2003 to 2005 at one of the participating sites (eight counties in northern California, Los Angeles County, state of Iowa, state of Alabama, 22 counties in central/eastern North Carolina, five integrated delivery systems, and 10 Veterans Affairs medical centers). CanCORS participants are similar to patients diagnosed with cancer in the United States as a whole.22
In the original baseline patient survey, we identified physicians who played important roles in each patient's care, including physicians who discussed and/or provided surgery, chemotherapy, or radiation therapy; providers important in referring patients to surgeons (patients with lung cancer only); chemotherapy providers or radiation therapy providers; and providers most likely to know if the patient had a symptom. We focused this second physician survey on chemotherapy providers or physicians reported as filling other roles but who self-identified as medical oncologists in the CanCORS I physician survey. We also surveyed additional medical oncologists who were identified in the CanCORS I or CanCORS II medical record abstractions and any medical oncologists identified in the CanCORS II survey of patients with advanced cancer, conducted from 2010 to 2011. Physicians were ineligible if they were deceased, no longer in practice, or not medical oncologists.
Survey Administration Procedures
In late June through August 2012, physicians were mailed a self-administered questionnaire. The questionnaire was accompanied by a cover letter cosigned by the director of the National Cancer Institute and the medical director of the American Cancer Society. Surveys were mailed by first-class mail with a stamped, preaddressed return envelope. Physicians were also given the option of responding to the survey via a secure Web site, after logging in with a username and password. Each survey was coded with a unique identifier to be used to link providers with patients and for follow-up of nonresponders. Each mailing also contained a $50 check incentive.
Three weeks after the initial mailing, another copy of the survey and cover letter was sent by first-class mail to all nonresponders. Approximately 2 weeks later, a research assistant placed telephone calls to the offices of nonresponding physicians to verify that the survey had been received, encourage physicians to complete and return it, and offer to mail or fax a replacement questionnaire. Research assistants also verified the specialty of nonresponding physicians. Up to four attempts were made to reach each nonresponding physician. From April through May 2013, a third mailing of the survey and cover letter was sent to nonresponding physicians with an additional $50 check.
Data Entry
For Web-based survey responses (10% of responses), data were entered directly into the statistical coordinating center database from the Web survey instrument. For paper survey responses, surveys were first reviewed for legibility before data entry; data were then entered by experienced staff at the statistical coordinating center into a Web version of the instrument specifically designed for data entry.
Response Rates
We calculated American Association for Public Opinion Research (AAPOR) response rates. The AAPOR definition of response rate matches our absolute response rate. The AAPOR suggests a calculation for the cooperation rate (No. responded/[No. responded + No. refused + No. died]; http://www.aapor.org/uploads/Standard_Definitions_04_08_Final.pdf). The absolute response rate was defined as the response rate among all physicians not known to be ineligible. The absolute response rate for the survey was 46.4%. The AAPOR cooperation rate, which only accounts for active refusals, was 82.5%; the AAPOR refusal rate was 9.8%.
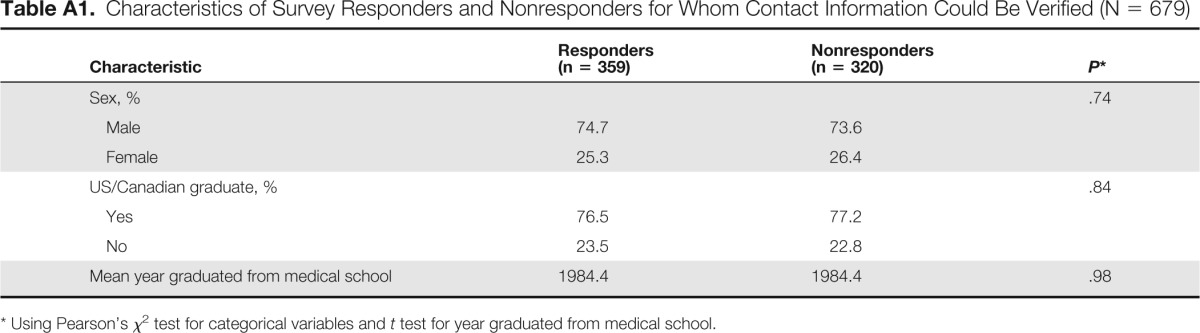
We also calculated a participation rate, defined as the response rate among eligible physicians for whom we were able to identify valid contact information. This participation rate was 52.9%. Participation rates by physician characteristics are listed in Appendix Table A1 (characteristics of physicians for whom we could not verify contact information were not available).
Table A1.
Characteristics of Survey Responders and Nonresponders for Whom Contact Information Could Be Verified (N = 679)
| Characteristic | Responders (n = 359) | Nonresponders (n = 320) | P* |
|---|---|---|---|
| Sex, % | .74 | ||
| Male | 74.7 | 73.6 | |
| Female | 25.3 | 26.4 | |
| US/Canadian graduate, % | .84 | ||
| Yes | 76.5 | 77.2 | |
| No | 23.5 | 22.8 | |
| Mean year graduated from medical school | 1984.4 | 1984.4 | .98 |
Using Pearson's χ2 test for categorical variables and t test for year graduated from medical school.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Stacy W. Gray, Benjamin Kim, Katherine L. Kahn, Maryse Roudier, Nancy L. Keating
Provision of study materials or patients: Katherine L. Kahn, Nancy L. Keating
Collection and assembly of data: Stacy W. Gray, Benjamin Kim, Katherine L. Kahn, David Haggstrom, Maryse Roudier, Nancy L. Keating
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Oncologists' Experiences With Drug Shortages
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Kenneth L. Kehl
No relationship to disclose
Stacy W. Gray
No relationship to disclose
Benjamin Kim
No relationship to disclose
Katherine L. Kahn
No relationship to disclose
David Haggstrom
No relationship to disclose
Maryse Roudier
No relationship to disclose
Nancy L. Keating
No relationship to disclose
References
- 1.US Food and Drug Administration. Editor's choice: StatBite drug shortages, 2001-2010. J Natl Cancer Inst. 2011;103:915. doi: 10.1093/jnci/djr236. [DOI] [PubMed] [Google Scholar]
- 2.Klobuchar SA. Shortages of cancer drugs in the USA. Lancet Oncol. 2011;12:313. doi: 10.1016/S1470-2045(11)70087-0. [DOI] [PubMed] [Google Scholar]
- 3.Gatesman ML, Smith TJ. The shortage of essential chemotherapy drugs in the United States. N Engl J Med. 2011;365:1653–1655. doi: 10.1056/NEJMp1109772. [DOI] [PubMed] [Google Scholar]
- 4.Jenks S. Efforts underway to curb drug shortages. J Natl Cancer Inst. 2011;103:914–915. doi: 10.1093/jnci/djr234. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PE. Drug shortages: Impact and strategies. J Natl Compr Canc Netw. 2011;9:815–819. doi: 10.6004/jnccn.2011.0070. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser J. Medicine: Shortages of cancer drugs put patients, trials at risk. Science. 2011;332:523. doi: 10.1126/science.332.6029.523. [DOI] [PubMed] [Google Scholar]
- 7.Printz C. Medication shortages threaten cancer care: The oncology community and the FDA tackle ongoing drug shortage problem. Cancer. 2012;118:289–291. doi: 10.1002/cncr.27386. [DOI] [PubMed] [Google Scholar]
- 8.Link MP, Hagerty K, Kantarjian HM. Chemotherapy drug shortages in the United States: Genesis and potential solutions. J Clin Oncol. 2012;30:692–694. doi: 10.1200/JCO.2011.41.0936. [DOI] [PubMed] [Google Scholar]
- 9.Roehr B. Obama takes action on drugs shortages. BMJ. 2011;343:d7158. doi: 10.1136/bmj.d7158. [DOI] [PubMed] [Google Scholar]
- 10.Havrilesky LJ, Garfield CF, Barnett JC, et al. Economic impact of paclitaxel shortage in patients with newly diagnosed ovarian cancer. Gynecol Oncol. 2012;125:631–634. doi: 10.1016/j.ygyno.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Becker DJ, Talwar S, Levy BP, et al. Impact of oncology drug shortages on patient therapy: Unplanned treatment changes. J Oncol Pract. 2013;9:e122–e128. doi: 10.1200/JOP.2012.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabner BA. Drug shortages: A critical challenge for the generic-drug market. N Engl J Med. 2011;365:2147–2149. doi: 10.1056/NEJMp1112633. [DOI] [PubMed] [Google Scholar]
- 13.McBride A, Holle LM, Westendorf C, et al. National survey on the effect of oncology drug shortages on cancer care. Am J Health Syst Pharm. 2013;70:609–617. doi: 10.2146/ajhp120563. [DOI] [PubMed] [Google Scholar]
- 14.McKeever AE, Bloch JR, Bratic A. Drug shortages and the burden of access to care: A critical issue affecting patients with cancer. Clin J Oncol Nurs. 2013;17:490–495. doi: 10.1188/13.CJON.490-495. [DOI] [PubMed] [Google Scholar]
- 15.Metzger ML, Billett A, Link MP. The impact of drug shortages on children with cancer: The example of mechlorethamine. N Engl J Med. 2012;367:2461–2463. doi: 10.1056/NEJMp1212468. [DOI] [PubMed] [Google Scholar]
- 16.Kweder SL, Dill S. Drug shortages: The cycle of quantity and quality. Clin Pharmacol Ther. 2013;93:245–251. doi: 10.1038/clpt.2012.235. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg P. Drug shortages reach all-time high: Ben Venue exit will make problem worse. www.cancerletter.com/downloads/20131011/download.
- 18.US Government Accountability Office. Drug shortages: Public health threat continues, despite efforts to help ensure product availability. www.gao.gov/products/GAO-14-194.
- 19.Goldsack JC, Reilly C, Bush C, et al. Impact of shortages of injectable oncology drugs on patient care. Am J Health Syst Pharm. 2014;71:571–578. doi: 10.2146/ajhp130569. [DOI] [PubMed] [Google Scholar]
- 20.Gogineni K, Shuman KL, Emanuel EJ. Survey of oncologists about shortages of cancer drugs. N Engl J Med. 2013;369:2463–2464. doi: 10.1056/NEJMc1307379. [DOI] [PubMed] [Google Scholar]
- 21.ASCO Connection. ASCO continues to survey members on drug-shortage impact. https://connection.asco.org/Magazine/Article/ID/3635/ASCO-Continues-to-Survey-Members-on-Drug-Shortage-Impact.aspx.
- 22.Catalano PJ, Ayanian JZ, Weeks JC, et al. Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the Surveillance, Epidemiology, and End Results Program. Med Care. 2013;51:e9–e15. doi: 10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 24.American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys (ed 5) Lenexa, KS: American Association for Public Opinion Research; 2008. [Google Scholar]
- 25.He Y, Zaslavsky AM, Landrum MB, et al. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19:653–670. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer (version 3.2014, 2014) www.ccn.org/professionals/physician_gls/pdf/colon.pdf. [DOI] [PubMed]
- 27.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer (version 3.2014, 2014) www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [DOI] [PubMed]
- 28.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (version 3.2014, 2014) www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 29.US Food and Drug Administration. Highlights of prescribing information: Alimta. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021462s021lbl.pdf.
- 30.Chuang VT, Suno M. Levoleucovorin as replacement for leucovorin in cancer treatment. Ann Pharmacother. 2012;46:1349–1357. doi: 10.1345/aph.1Q677. [DOI] [PubMed] [Google Scholar]
- 31.Kovoor PA, Karim SM, Marshall JL. Is levoleucovorin an alternative to racemic leucovorin? A literature review. Clin Colorectal Cancer. 2009;8:200–206. doi: 10.3816/CCC.2009.n.034. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg RM, Hatfield AK, Kahn M, et al. Prospectively randomized North Central Cancer Treatment Group trial of intensive-course fluorouracil combined with the l-isomer of intravenous leucovorin, oral leucovorin, or intravenous leucovorin for the treatment of advanced colorectal cancer. J Clin Oncol. 1997;15:3320–3329. doi: 10.1200/JCO.1997.15.11.3320. [DOI] [PubMed] [Google Scholar]
- 33.Scheithauer W, Kornek G, Marczell A, et al. Fluorouracil plus racemic leucovorin versus fluorouracil combined with the pure l-isomer of leucovorin for the treatment of advanced colorectal cancer: A randomized phase III study. J Clin Oncol. 1997;15:908–914. doi: 10.1200/JCO.1997.15.3.908. [DOI] [PubMed] [Google Scholar]
- 34.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. Fact sheet: Drug products in shortage in the United States, 2012. www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/ucm313121.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


