Abstract
A first-ever spinal cord imaging meeting was sponsored by the International Spinal Research Trust and the Wings for Life Foundation with the aim of identifying the current state-of-the-art of spinal cord imaging, the current greatest challenges, and greatest needs for future development. This meeting was attended by a small group of invited experts spanning all aspects of spinal cord imaging from basic research to clinical practice. The greatest current challenges for spinal cord imaging were identified as arising from the imaging environment itself; difficult imaging environment created by the bone surrounding the spinal canal, physiological motion of the cord and adjacent tissues, and small crosssectional dimensions of the spinal cord, exacerbated by metallic implants often present in injured patients. Challenges were also identified as a result of a lack of “critical mass” of researchers taking on the development of spinal cord imaging, affecting both the rate of progress in the field, and the demand for equipment and software to manufacturers to produce the necessary tools. Here we define the current state-of-the-art of spinal cord imaging, discuss the underlying theory and challenges, and present the evidence for the current and potential power of these methods. In two review papers (part I and part II), we propose that the challenges can be overcome with advances in methods, improving availability and effectiveness of methods, and linking existing researchers to create the necessary scientific and clinical network to advance the rate of progress and impact of the research.
Keywords: Magnetic resonance, Spinal cord, Functional MRI, Diffusion, Spinal cord injury, Pathology
Introduction
Our ability to research and understand human spinal cord function, its role in pain processing, the effects of traumatic injury or diseases such as multiple-sclerosis, and our understanding of pain processing, is all significantly hampered by the limited accessibility of the spinal cord. In the part I of our two part series we have described the current state-of-the-art of spinal cord imaging, the current greatest challenges, and the greatest needs for future development in order to support non-invasive human spinal cord research (Stroman et al., 2014). The general assumption is that providing increased sensitivity and specificity of spinal cord imaging in the context of well-defined clinical readouts will be instrumental to improve novel approaches in the diagnostic and treatment of spinal cord diseases. The objectives of this paper are to:
describe the current state-of-the-art and capabilities of human spinal cord imaging applications;
identify the greatest current needs, from a clinical point of view, that will drive forward future development.
In order to achieve these objectives we provide a general overview of two key techniques employed in clinical research, functional Magnetic Resonance Imaging (fMRI) and Diffusion Tensor Imaging (DTI), and then focus on specific applications of spinal cord imaging on four areas: investigations of cervical spondylotic myelopathy (CSM), spinal cord injury (SCI), pain and multiple-sclerosis (MS). Wherever applicable, reference to quantitative imaging methods other than fMRI and DTI, such as magnetic resonance spectroscopy (MRS) and magnetization transfer (MT) imaging, will also be mentioned. Issues related to spatial resolution, registration of subsequently acquired volumes, partial volume effects with the cerebrospinal fluid (CSF), as well as the lack of a standard common template and the effects of physiological noise are still limiting the adoption of many techniques into the clinical setting, confounding quantitative MRI of the spinal cord to research studies. The overall goal of this work is therefore to foster the development of novel and sensitive means of characterizing neural function and cellular structure in clinical populations that can supplement or surpass current methods for patient assessment, serve as clinical trial endpoints, and be used for monitoring of disease progression and efficacy of therapies.
General overview of fMRI and DTI
fMRI in the human spinal cord: Applications
A growing number of studies (summarized in Table 1) have been carried out to investigate spinal cord function in response to various sensory stimuli and motor tasks and to characterize the effects of traumatic injury and pathology.
Table 1.
Examples of studies employing spinal cord fMRI:.
Determining the sensitivity and reliability of spinal cord fMRI is a challenging task in that there is no “gold-standard” method that can be used to verify the results obtained in humans (Stroman et al., 2014). Even studies with animal models (Lawrence et al., 2004, 2007; Majcher et al., 2006, 2007; Malisza and Stroman, 2002; Malisza et al., 2003) can provide only limited validation because surgical interventions, anesthetics, and differences in emotional and cognitive factors can all significantly alter neural activity in the spinal cord (Hochman, 2007; Lawrence et al., 2004, 2007; van Eijsden et al., 2009). The reliability of spinal fMRI results must therefore be inferred from consistency across repeated studies, sensitivity to different stimuli or tasks, and correspondence with recorded responses and psychophysical ratings by the participants.
The studies listed in Table 1 have shown active regions in the spinal cord that are consistent across groups of participants, and that correspond with the region of the body being stimulated, or motor task being performed. A high degree of laterality has been demonstrated in comparisons of right-side and left-side motor activity or stimulation of the hand and shoulder (Maieron et al., 2007; Stroman et al., 2012). Although this is very encouraging, the relatively low resolution and consequent partial volume effects need to be considered when studying patients with severe spinal cord atrophy. Spinal fMRI studies in rats have provided further support by showing a correspondence between areas of activity detected with spinal fMRI and cells labeled with c-fos staining stimuli, when a noxious electrical stimulus was applied (Lawrence et al., 2004). This body of results provides strong evidence that signal changes related to neural activity in the spinal cord can indeed be detected with spinal fMRI methods. Several of the studies have further shown that spinal responses vary with the intensity, and modality of the stimulus. Most notably, applying thermal stimuli of between 32 °C and 10 °C to the leg revealed areas of activity in the lumbar spinal cord where signal intensity responses varied with the temperature, and notably included an abrupt transition to higher signal intensity changes when the stimulus went below 15 °C, a level considered painful (Stroman et al., 2002c). Patient studies provide further evidence of response sensitivity to pathological changes suggestive of a neuronal basis for spinal cord activity. Studies of people with SCI and MS have demonstrated altered activity in the spinal cord depending on the injury severity or disease state (Agosta et al., 2008a, 2008b; Kornelsen and Stroman, 2007; Stroman et al., 2004a; Valsasina et al., 2010).
From a clinical perspective, it is important to recognize that spinal fMRI has been demonstrated for group analyses but that there are still sources of variability or uncertainty that count against its use for individual studies. Once these sources of variability and errors are characterized and understood, it is expected that methods can be adapted to optimize the sensitivity to study and assess individuals.
DTI in the human spinal cord: Applications
Given the sensitivity and specificity of DTI to white matter integrity, this technique is sensitive to structural changes due to pathology. Despite the technical challenges associated with spinal cord DTI, this method has been applied in healthy and diseased spinal cord in humans in vivo, as summarized in Table 2.
Table 2.
Examples of studies employing cervical spinal cord DTI:.
| Healthy (uninjured) volunteers: | Smith et al. (2010), Vedantam et al. (2013), Wheeler-Kingshott et al. (2002) |
| Spinal cord compressions | Facon et al. (2005), Plank et al. (2007) |
| Inflammatory diseases | Renoux et al. (2006) |
| Arteriovenous malformation | Ozanne et al. (2007) |
| Ischemia | Thurnher and Bammer (2006) |
| SCI | Cohen-Adad et al. (2011, 2012), Ellingson et al. (2008), Freund et al. (2012b), Lammertse et al. (2007), Shen et al. (2007) |
| CSM | Sato et al. (2012), Uda et al. (2013) |
| Multiple sclerosis | Agosta et al. (2005, 2007a), Benedetti et al. (2010), Ciccarelli et al. (2007), Hesseltine et al. (2006), Oh et al. (2013a, 2013b), Ohgiya et al. (2007), Valsasina et al. (2005), Van Hecke et al. (2009) |
| Amyotrophic lateral sclerosis | Agosta et al. (2009a), Cohen-Adad et al. (2013), Nair et al. (2010), Pradat et al. (2011), Valsasina et al. (2007) |
DTI has also proven to be sensitive to Wallerian degeneration through animal models of pericontusional traumatic axonal injury (Mac Donald et al., 2007), demyelinating lesion (DeBoy et al., 2007) and dorsal root axotomy (Zhang et al., 2009). The specificity of diffusion measurements to white matter pathology has been studied in animal models of de/dysmyelination and suggests that axial diffusivity is more specific to axonal degeneration whereas radial diffusivity is more specific to demyelination (Budde et al., 2007, 2008, 2009; DeBoy et al., 2007; Hoffing et al., 2009; Kim et al., 2007; Kozlowski et al., 2008; Mac Donald et al., 2007; Song et al., 2002; Xie et al., 2010; Zhang et al., 2009). Other studies however reported possible interactions between axial and radial diffusivities, thereby limiting the specificity of these measures (Herrera et al., 2008; Sun et al., 2006; Wheeler-Kingshott and Cercignani, 2009; Wheeler-Kingshott et al., 2012). One argument refers to the pathophysiology of axon degeneration, as this process is known to be associated with demyelination in several pathologies such as in MS (Schmierer et al., 2007) or in SCI (Cohen-Adad et al., 2011; Zhang et al., 2009). Another argument is related to the biophysical properties of DTI, in which several physical parameters can influence diffusion metrics including myelination, axonal density, axonal diameter, or orientation of fiber bundles, as well as changes in the tissue matrix surrounding the axons (Beaulieu, 2002; Sen and Basser, 2005; Wheeler-Kingshott and Cercignani, 2009). Fig. 1 shows an example of corticospinal tract reconstruction from DTI data and their crossing in the medulla oblongata in a healthy subject. Ultimately, it is always important to remember that the diffusion tensor is an imperfect model used to explain data and one must always look at the data and make sure that interpretations in terms of axonal loss and demyelination are well supported by the behavior of the underlying model (Wheeler-Kingshott and Cercignani, 2009).
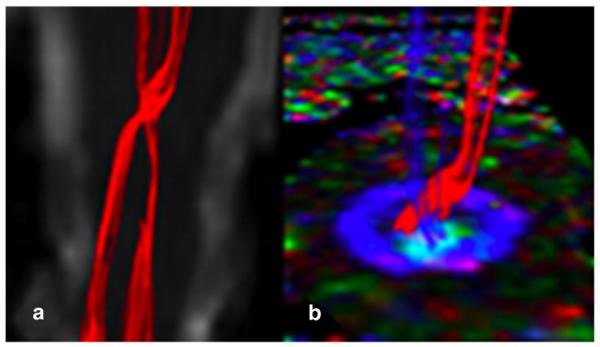
Fig. 1.
Selective reconstruction of the corticospinal tracts with crossing in the medulla oblongata in a healthy subject. a) Coronal view of the non-diffusion weighted scan with the CSTs in red. b) axial view of the spinal cord FA, color coded with the direction of the principal eigenvector of the diffusion tensor, with the right and left CSTs in red and blue, coming out of the axial section and showing their crossing point.
As extension of DTI, high angular resolution diffusion imaging (HARDI) and Q-Ball imaging (QBI) can represent more than one diffusion direction, and so alleviate some of the limitations of the diffusion tensor model in the presence of crossing fibers (Tuch, 2004). HARDI has proven to be efficient in the detection of subtle axonal connections in the spinal cord (Cohen-Adad et al., 2008; Lundell et al., 2009). The “model-free” q-space imaging approach appears to be feasible, if time-consuming, in the spinal cord and can be sensitive to pathological changes (Farrell et al., 2008). Advanced models instead are working towards characterizing axon diameter, density and dispersion in the spinal cord, in order to achieve higher specificity of the imaging biomarkers. These studies are innovative and far from being available clinically but have a huge potential in terms of characterizing spinal cord pathology and predicting patient's outcome and therefore need support from a network of scientists across centers in order to promote adoption into the clinical arena.
Spondylotic myelopathy
Spinal cord compression can be caused by various pathological processes such as neoplasms, degenerative changes, inflammatory processes and trauma. Degenerative spine disease is the most common and may have enormous effects on the patients' quality of life (Kara et al., 2011). Progressive compression of the spinal cord due to narrowing of the spinal canal is the main pathophysiology underlying the nontraumatic myelopathy. Early diagnosis and appropriately chosen treatment may prevent further damage to the spinal cord (Kara et al., 2011). Therefore, it is very important to visualize the on-going pathological process as early as possible. Careful quantitative analysis reveals that the combination of increased T2-weighted and reduced T1-weighted signal intensities, a long segment of increased T2-weighted signal intensity or patchy areas of increased T2-weighted signal intensity are predictive of reduced neurological recovery in patients undergoing surgery for CSM. These data can be used by clinicians to manage patient expectations and to assist in clinical decision-making (Arvin et al., 2013). Although at more advanced stages of disease, conventional T1- and T2-weighted MR images are quite sensitive for confirming the presence of myelopathy, in the early stages however, there are discrepancies between the actual clinical status and the imaging findings. Oligodendrocyte apoptosis and consequent demyelination are found to play a central role in neurologic deterioration as seen in cervical spondylomyelopathy at later stages (Kara et al., 2011). Spinal application of newly designed diffusion tensor imaging (DTI) sequences allows a more sensitive evaluation of the normal appearing white matter. Fractional Anisotropy (FA), Apparent Diffusion Coefficient (ADC) and Mean Diffusivity (MD) metrics derived from DTI are able to document and quantify orientation changes of water molecules in normal appearing white matter of the spinal cord (Budzik et al., 2011; Cui et al., 2011; Xiangshui et al., 2010). The findings of two subjects with cervical myelopathy are shown in Fig. 2 and Fig. 3. FA metrics have been found to be more sensitive to pathological changes in comparison with ADC and MD values (Budzik et al., 2011). In a recently published preliminary study using DTI on a 3 T MR system, Kara et al. (2011) found decreased FA values and increased ADC values in stenotic segments in comparison with nonstenotic ones (mean FA and ADC in stenotic segment: 0.65 and 1.01 with SD 0.04 and 0.19, respectively), suggestive of myelopathic changes even without T2 signal alterations. By application of orientation based entropy analysis in multi-level compression, it is possible to evaluate the contribution of each compression level to the overall picture (Cui et al., 2011). Another recent study at 3 T confirmed an increased MD and a decreased FA in patients compared to healthy controls. Here the authors assessed the diagnostic utility of DTI indices and found that in their study the receiver operating characteristic (ROC) analysis had higher sensitivity and specificity for prediction compared to FA and MD changes (Uda et al., 2013). ADC values have also been assessed to determine their ability to predict clinical recovery after decompression surgery in another study and showed promising results, suggesting that further work is warranted to determine the best way to include diffusion indices into the evaluation of cervical myelopathy (Sato et al., 2012).
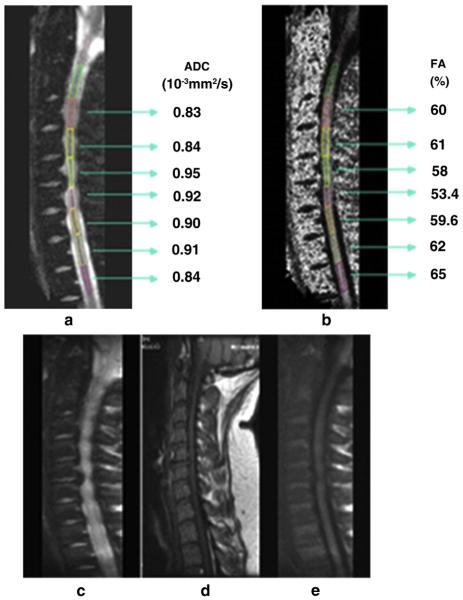
Fig. 2.
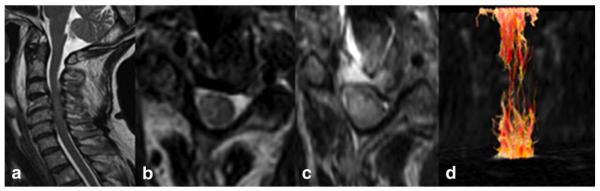
(a–d) Sagittal T2, axial T2, DWI and 3D reconstruction of the spinal cord fibres in cervical spondylomyelopathy. Although one observes faint signal changes on sagittal T2 (a), 3D fiber tractography reconstruction (d) shows diffuse nerve fiber destruction, eventually explaining the clinical status of a patient.
Fig. 3.
(a–e) C6/C7 root compression due to herniated disk, and its effect on neural tissue integrity. DTI metrics allows us to observe increment in ADC and decrement in FA not only in the affected area but also in near proximity in patients with disk herniation in comparison with healthy subjects (mean ADC of healthy volunteer: 0.78 ± 0.0; mean FA of healthy volunteer: 61 ± 5).
Spinal cord injury
Improving our ability to assess tissue viability and detect residual neuronal function in the spinal cord, in order to identify and distinguish morphological and functional changes, is key to advancing our capacity for clinical prognosis and management of spinal cord injury patients. A recently published prospective longitudinal study in chronic SCI investigated the motor system, including cervical spinal cord, cranial corticospinal tract (CST) and motor cortex, in particular correlating parameters indicative of axonal loss and myelin integrity to clinical outcome, providing a comprehensive assessment of progression using multi-modal MRI (Freund et al., 2013). A cross-sectional study of traumatic SCI patients using structural and functional MRI revealed associations between cross-sectional spinal cord area, gray and white matter volume as measured using voxel based morphometry of the brain, cortical activations following right-sided hand grip as well as median and tibial nerve stimulation and cortical thickness, and disability (Freund et al., 2011) confirming observed changes in the motor cortex and motor pathways following SCI (Wrigley et al., 2009). In light of the evidence of functional and structural changes of the motor system in SCI and the sensitivity of spinal cord quantitative measurements to damage (Cohen-Adad et al., 2011; Freund et al., 2011; Lundell et al., 2011; Petersen et al., 2012), one aim of spinal cord fMRI is to complement structure-sensitive techniques (e.g. atrophy, DTI, MT), by characterizing normal spinal circuits in healthy individuals and to delineate how these circuits become damaged after injury. The extension of this characterization is to predict progression and monitor the benefficial or deleterious effects of different treatment options.
Injury to the human spinal cord, regardless of the mechanism, results in a complex cascade of events at the cellular level that may or may not have an effect on the neurovascular coupling mechanisms that underlie the signal changes detected with spinal fMRI. It is important therefore, to bridge the research gap between our detailed understanding of the pathophysiology of SCI and the biophysical mechanisms of the signal changes that are exploited for spinal cord fMRI. Primary injury refers to a destructive force that directly leads to neural structure damage, as for example the sheer force, which tears an axon, or a direct compressive force occluding a blood vessel that results in ischemia. The primary destructive events initiate a series of cellular mechanisms that result in ongoing damage to the neural structures, termed secondary injury (Tator and Fehlings, 1991). The pathological processes that occur at the cellular level include ischemia, vasospasm, ion-mediated cellular damage, excitotoxicity, oxidative cellular damage, neuroinflammation, and cell death. Changes in the correspondence between fMRI signal changes and neural activity may therefore also depend on the time since the injury occurred, thus complicating interpretation.
There are a number of clinical outcomes that follow traumatic spinal cord injury as a consequence of either adaptive or maladaptive plasticity. Adaptive plasticity refers to the concept of natural recovery whereby an individual who sustained motor deficits, sensory impairment or both following SCI recovers to some degree in the months after injury (Fawcett et al., 2007). Maladaptive plasticity refers to neurological symptoms that arise as a result of spinal circuits reorganizing themselves in a way that provides no useful function such as neuropathic pain, spasticity, disruption of autonomic function and the lack of motor and sensory recovery (Gwak and Hulsebosch, 2011). One potential advantage of spinal fMRI will be to investigate the longitudinal effects of various stimulation paradigms as they relate to clinical symptoms such as the recovery of motor function, sensation or the development of neuropathic pain. A better understanding of the spinal circuit changes that are associated with maladaptive plasticity may lead to more accurate diagnoses and the ability to objectively monitor treatment.
Studies of the effects of spinal cord injury that have been carried out to date using spinal cord fMRI have all focused on the chronic stages at 1 year or more after the injury occurred. Potential confounding effects include on-going changes in cellular function and ionic metabolism, each of which may alter mechanisms of neurovascular coupling and the subsequent hemodynamic response. Nonetheless, the studies carried out to date have demonstrated that fMRI of the spinal cord can detect important changes as a result of traumatic injury in both the cervical and lumbar regions of the spinal cord (Goldfarb et al., 2011; Kornelsen and Stroman, 2007; Stroman et al., 2004a).
The first spinal cord fMRI study carried out on participants with spinal cord injuries investigated activity in the lumbar spinal cord, below the level of injury, in response to a noxious cold stimulus applied to the leg (L4 dermatome) (Stroman et al., 2004a). This study demonstrated significant activity in all participants, including SCI patients, in the lumbar segments of the spinal cord, below the level of injury. Moreover participants with SCI who could feel or had some altered sensation of the cold stimulus, had spatial patterns of activity that were similar to that observed in healthy participants.
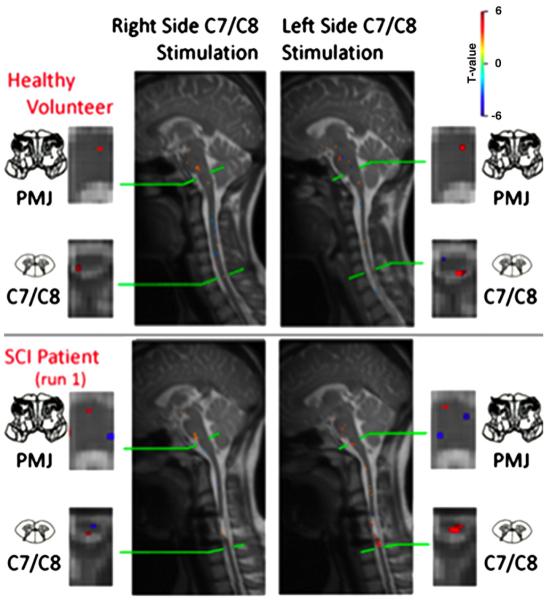
A more recent study of thermal responses in the cervical spinal cord and brainstem investigated responses to warm thermal stimuli in regions both above and below the level of injury (Saranathan et al., 2012). Results corresponded with the level and extent of injury, with responses above the level of injury being generally similar to those seen in healthy participants, and responses below the level of injury being altered depending on the injury severity. Right- and left-side differences were also demonstrated, as well as corresponding activity in the brainstem (Fig. 4).
Fig. 4.
Example of results obtained in a healthy female participant (top) and an age-matched participant with an incomplete (ASIA B) spinal cord injury (SCI) in the cervical level. Four dermatomes were stimulated, corresponding to the right and left C5 and C8 spinal cord segments, and results are shown in selected axial and sagittal slices showing the responses to right- and left-side stimulation of the little finger side of the palm (C8 dermatome). Results obtained in the healthy participant show activity in the ipsilateral dorsal horn of the spinal cord in the C8 segment, with a high degree of laterality corresponding to the side of the body being stimulated, as well as activity in the medulla of the brainstem. Results obtained in the participant with SCI show nearly normal responses on the left side of the spinal cord, and slightly altered responses on the right side, as well as corresponding activity in the medulla. In spite of an apparently extensive span of tissue damage in the cervical spinal cord, the neural activity detected is only slightly altered.
Collectively, these results demonstrate the sensitivity of the fMRI method to changes as a result of trauma and the feasibility of using fMRI as a research tool for studying the effects of injury. Future clinical applications will require demonstration that the results obtained in each participant are sufficiently sensitive and reliable to be suitable for diagnostic purposes or monitoring of treatment outcomes. As spinal fMRI emerges as a tool to investigate spinal cord injury, we must tread cautiously with the interpretation of results. One limitation, for example, is our lack of understanding of how the neurovascular coupling response may change after injury. Currently, the hemodynamic response function utilized in model-based fMRI data analysis is derived from healthy neural tissue. It will be important to investigate if damaged neural tissue also exhibits the same hemodynamic response function (both in temporal as well as spatial aspects) to a given stimulus, because the accuracy of the predicted response can impact on the sensitivity of the fMRI method for detecting neural activity. In addition, the technical challenges of spinal cord fMRI that have been detailed in part I of this review (Stroman et al., 2014) may need to be reduced or eliminated before its sensitivity and reliability are acceptable for clinical applications.
In complement to the evidence for responses to stimuli that spinal fMRI can provide, there is an important place for DT-MRI in providing information about the structural integrity of spinal cord tissue after injury. It has been shown for example, that diffusion indices, based on advanced acquisition and analysis protocols, are sensitive to severity of the damage, although no specific correlations were found with sensorimotor scores when looking at sensory-motor tracts. Correlation of DTI indices with electrophysiological measures have also been demonstrated (Petersen et al., 2012) as well as correlation of spinal cord DTI indices with retrograde CST changes and disability (Freund et al., 2012a). Wallerian degeneration of white matter pathways have been shown to reach the cortico-spinal-tract in the brain and to correlate with functional reorganization (Freund et al., 2012b). These few early studies indicate that structural changes following injury have an effect on the diffusion properties of the tissue, which should be investigated in larger patient studies to prove their true potential as imaging biomarkers of tissue damage or integrity. Furthermore, larger studies are needed to fully establish this technique as a clinical tool. It must be acknowledged however, that the challenges of spinal cord DT-MRI are worsened by the presence of metal implants in SCI patients and therefore large population studies may be very difficult to perform.
Clinical applications of spinal cord MRS to patients with SCI have been scarce and limited to a few studies that were carried out in patients with cervical spondylotic myelopathy (Holly et al., 2009), chronic whiplash (Elliott et al., 2012) and brachial plexus injury (Kachramanoglou et al., 2013). Although most of these studies are limited by small cohort sizes and technical limitations, they have shown the potential of MRS, which may provide information on the underlying mechanisms of the disease by reflecting pathological changes that are not detectable with conventional MRI techniques. Advanced diffusion imaging methods, such as diffusion kurtosis and q-space imaging have also shown great potential in correlating with disability in patients with spondylotic myelopathy (Hori et al., 2012), but are beyond the scope of the present review.
Pain
The spinal cord (and brainstem) is the first point in the central nervous system that processes nociceptive signals arriving from the body, and which ultimately may produce a sensation of pain. Functional imaging of the spinal cord aims to record this activity and can help to better understand how these signals are processed and whether altered spinal cord function underlies chronic or neuropathic pain states in humans. Nociceptors, free nerve endings located in skin, muscles and viscera, respond to potentially tissue-damaging stimuli such as temperature, pressure, extreme pH and other noxious stimuli by producing afferent volleys that signal the location, nature and intensity of the threat. Indeed, functional imaging studies have demonstrated widespread activity across the brain (Jones et al., 1991; Apkarian et al., 2005) in response to painful stimuli. The development of non-invasive imaging techniques to record spinal activity provides critical information needed to interpret nociceptive processing in health and disease, and may help explain the patterns of brain activity observed in response to noxious stimulation in healthy controls and in patients suffering from pain. Furthermore, it has already provided important information about the modulation of spinal nociceptive processing induced by analgesic drugs or by other non-pharmacological treatments.
Evidence for a spinal metabolic response to nociceptive stimulation
For a long time the focus on understanding how nociceptive signals are processed within the central nervous system has been targeted to the spinal cord (McMahon et al., 2006; Wall, 1964). Anatomists and electrophysiologists have described in great detail the laminar organization of the spinal cord (Watson, 2009), with peripheral nociceptors forming synaptic connections within different dorsal horn layers. More recently, the response of the spinal cord in awake or anesthetized animals has been studied using immunological and metabolic imaging techniques. Bullitt (1991) used c-fos immunolabelling (Hunt et al., 1987) to record the response to acute prolonged nociceptive stimulation (e.g. hindpaw pinch), which increased gene expression in response to neuronal activity in the ipsilateral dorsal horn, extending over several segments; these findings have been extended in other experimental models of nociception (see reviews in (Coggeshall, 2005; Porro and Cavazzuti, 1993)). Increased metabolic demand due to neuronal activity can be visualized using radiolabelled tracers such as 2-deoxyglucose (2-DG), and quantified using autoradiographic techniques (Coghill et al., 1991; Porro et al., 1991). An interesting observation is that, in the awake rat, responses were observed bilaterally. The contralateral activation observed in unanesthetized animals could be due both to motor behavior, and to “mirror” nociceptive processing (Aloisi et al., 1993). Note that the studies mentioned above utilized prolonged stimulation, and therefore the results may reflect changes in the tonic excitability of the cord (potentially reflecting pro-nociceptive input from the brainstem).
Human pain studies using fMRI
The first study in humans using stimulation likely to activate nociceptors (electrical stimulation of the median nerve), was performed by Backes et al. (2001). In response to electrical stimulation at the elbow, cardiac gated BOLD-sensitive echo-planar images acquired in the sagittal plane revealed signal increases (uncorrected p b 0.01) in lower cervical segments. Activity was not reliably detected in slices acquired in the transverse plane. Also in some instances bilateral activation was found. One issue relating to the use of supra-threshold (i.e. painful) electrical stimulation is that it excites a wide range of nerve fibers that convey information relating to sense of touch/vibration, limb position, and nociception, making interpretation of patterns of spinal activity difficult. Nonetheless, in other BOLD fMRI studies of the spinal cord (Brooks et al., 2012; Kong et al., 2012), patterns of cervical spinal activity were recorded in response to painful thermal and punctate stimulation and compared across a group of 18 subjects; a predominantly ipsilateral response was demonstrated using a mixed effects model and corrected (voxel-level) statistics (Brooks et al., 2012). However, not all reports of spinal activity in response to nociceptive stimuli have demonstrated lateralized activity in the cord. In response to painful laser stimulation of the dorsum of the left hand, activating predominantly Aδ fibers, activity was observed to be bilateral in the cervical spinal cord (Summers et al., 2010). Importantly, this was the first study to directly assess the false-positive detection rates in spinal cord activity, and the number of activated voxels in response to laser stimulation was found to exceed the false-positive level. Concerning the absence of lateralized signals in response to nociceptive stimulation, it should be noted that this assessment was made on the basis of sub-dividing the cord into right and left halves, so any bilateral motor-reflex activity in response to stimulation (involving activity in both anterior horns) might have concealed increased ipsilateral dorsal horn activity.
By using a combination of BOLD and SEEP contrast, Ghazni and co-workers reported changes in spinal cord and brainstem activity in response to painful and non-painful punctate stimuli (Ghazni et al., 2010). Activity in response to stimulation of the right thumb with a light (2 g) and heavier (15 g) von Frey filament was observed throughout the spinal cord, brainstem and thalamus. Surprisingly, activity in the ipsilateral cervical dorsal horn and brainstem was highest for the light-weight probe, which was interpreted as reflecting descending modulation from the brainstem. Of direct relevance to the study of descending pain modulation, two recent studies have recorded the brainstem and spinal cord response during endogenous analgesia due to placebo effects (Eippert et al., 2009a, 2009b). In response to thermal stimulation of the left forearm, in a region treated with an inert cream as a placebo, increased activity in periaqueductal gray matter (PAG) and rostral ventromedial medulla (RVM) was observed (Eippert et al., 2009a). Conversely, BOLD evoked signal increases were reduced in a restricted region of the spinal cord during placebo analgesia; this was interpreted as reflecting decreased nociceptive processing in the spinal cord due to descending pain modulation (Eippert et al., 2009b). The role of attention on patterns of brainstem and spinal cord activity in response to cold stimulation (18 or 15 °C) that produced “mild” to “strong discomfort”, was investigated by Stroman et al. (2011). Similar to previous data examining brainstem responses following painful thermal stimulation (Tracey et al., 2002), increased activity was found in the PAG during distraction conditions, perhaps relating to altered levels of discomfort. However, activity at the level of the spinal cord was opposite to what might be expected on the basis of descending inhibition of nociceptive input, with decreased activity during the no-distraction “rating” period when compared to the distraction condition. On the other hand, a recent study by Sprenger et al. (2012) has demonstrated that during a mentally demanding distraction task (n-back task), spinal cord activity in response to concurrent painful thermal stimulation is reduced when cognitive demand is high, and that the reduction in pain ratings (when compared to the low cognitive demand condition) is significantly correlated with the change in BOLD signal. It is worth noting that developments in MR data acquisition e.g. the use of slice dependent z-shimming (Finsterbusch et al., 2012) and region-selective radiofrequency (RF) excitation pulses (Finsterbusch, 2013; Finsterbusch et al., 2013) may improve our ability to record BOLD signal changes in the human spinal cord. In particular, z-shimming has been already used to study spinal responses to noxious stimulation (Eippert et al., 2009a, 2009b; Sprenger et al., 2012).
The studies of pain that have been reported to date demonstrate that by using spinal fMRI, researchers are already asking challenging questions relating to the interaction of brain, brainstem and spinal cord and of our ability to endogenously control pain. Future research assessing the impact of analgesic compounds on pain-related activity in the human spinal cord is already beginning.
Multiple sclerosis
Multiple sclerosis (MS) is the disease that has benefitted mostly from advanced quantitative spinal cord imaging techniques, spanning from cord atrophy measurements, fMRI, DTI and also magnetization transfer ratio (MTR), myelin imaging and proton MRS, as described in this section. Fig. 5 is showing an example of some of these techniques in a patient with MS at cervical level.
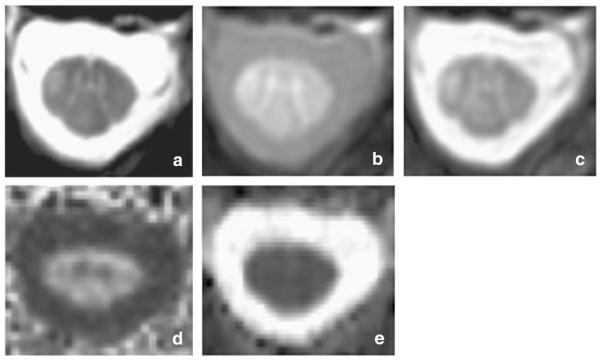
Fig. 5.
Spinal cord images acquired on a patient with multiple sclerosis. a) 3D Fast Field Echo (FFE) image acquired in the axial plane with the following parameters: 10 contiguous slices, FOV 240 × 180 mm2, TR 23 ms, TE 5 ms, flip angle α = 7°, number of averages = 8, voxel size = 0.5 × 0.5 × 5 mm3, total acquisition time = 13:34 min; b–c) Magnetization transfer imaging (b — MT pulse ‘ON’;c — MT pulse ‘OFF’) acquired with the following parameters: 3D slab-selective FFE sequence with two echoes (TR = 36 ms, TE1/TE2 = 3.5/5.9 ms, flip angle α = 9°), 22 axial slices, FOV = 240 × 180 mm2, voxel size 0.75 × 0.75 × 5 mm3 reconstructed to 0.5 × 0.5 × 5 mm3 to match the FFE scan resolution as in a), SENSE acceleration factor = 2, total acquisition time = 9 min. The MT pulse characteristics were: Sinc-Gaussian shaped MT saturating pulse of nominal α = 360º, offset frequency 1 kHz, duration 16 ms. d–e) Diffusion tensor imaging (DTI) derived maps (d — FA; e — MD). DW images were acquired axially with the following parameters: TE = 52 ms, TR = 12 RRs (cardiac gated), reduced FOV of 64 × 48 mm2, SENSE factor = 1.5, acquisition matrix 64 × 48, voxel size = 1 × 1 × 5 mm3. The DW imaging protocol consisted of 30 b = 1000 s mm−2 DWI volumes with gradient directions evenly distributed over the sphere and 3 non-DWI (b = 0) volumes.
Cord lesions in MS are more frequently observed in the cervical than in other regions, are usually peripheral, limited to two vertebral segments in length or less, occupy less than half the cross-sectional area of the cord, and typically are not T1-hypointense (Lycklama et al., 2003). Asymptomatic spinal cord lesions have been described in 30–40% of patients at presentation with clinically isolated syndromes (CIS) and in up to about 90% of patients with definite MS (Lycklama et al., 2003). Although significant reduction of cervical cord size can also be observed in the early phase of MS (Brex et al., 2001), cord atrophy is more severe in the progressive forms of MS (Lycklama et al., 2003). Some studies in MS patients found a correlation between atrophy of the cervical cord and disability, measured using the Expanded Disability Status Scale (EDSS) (Kidd et al., 1993; Lin et al., 2004; Losseff et al., 1996). Changes at a given time point and over time in cord cross-sectional area (CSA) correlate better with clinical disability than changes of T2 lesion burden (Losseff et al., 1996). As example of a novel development that could rapidly be adopted, recently, a new semi-automatic method based on an active surface (AS) model of the cord surface allowing segmentation of long portions of the cord (Horsfield et al., 2010) has shown to provide reproducible measures of cord CSA from C2 to C5. Its ability to give atrophy measures of potential clinical relevance has been demonstrated in a group of 40 patients with relapsing–remitting (RR) and secondary progressive (SP) MS (Horsfield et al., 2010). The use of this method in a multicenter study to investigate the correlation between cord atrophy and clinical disability in a large sample of MS patients has demonstrated that CSA differs significantly among the main MS clinical phenotypes and it is correlated with EDSS, with a differential effect among disease clinical phenotypes: no association in either CIS patients or in benign MS; association in RRMS, SPMS and primary progressive (PP) MS (Rocca et al., 2011). Combining the AS method with voxel based analysis of structural scans of the spinal cord has the potential to provide an insight into localized changes in patients with MS (Rocca et al., 2013; Valsasina et al., 2013). These results suggest that cervical cord atrophy provides a relevant and useful marker for the characterization of clinical heterogeneity of MS patients. The stability of this measure among different centers supports its use as a surrogate marker to monitor disease progression in multicenter trials.
Abnormal magnetization transfer and diffusion tensor MRI quantities from the cervical cord have been shown in patients with established MS, but not in those with clinically isolated syndromes (Agosta and Filippi, 2007). Conventional and diffusion tensor MRI of the cervical cord was obtained from relapse-onset MS patients at baseline and after a mean follow-up of 2.4 years (Agosta et al., 2007a): baseline cord cross-sectional area and FA correlated with increased disability at follow-up (Agosta et al., 2007a). Using magnetization transferweighted MRI a study showed that signal abnormalities in the dorsal and lateral columns of the spinal cord are correlated with vibration sensation and strength, respectively (Zackowski et al., 2009). Another study found that decreased magnetization transfer ratio (MTR) of the central gray matter of the cervical cord is related to EDSS score in patients with relapsing-remitting (RR) MS (Agosta et al., 2007b). In RRMS, baseline cervical cord MTR was correlated with relapse rate and EDSS changes over 18 months (Charil et al., 2006). Recent studies at 3 T have shown the sensitivity of DTI and MTR metrics to clinically relevant differences in patients with MS, beyond conventional imaging modalities (Oh et al., 2013a, 2013b).
A number of other quantitative MRI techniques have also been applied in MS, although not as extensively as CSA, MTR and DTI indices. For example, a two year longitudinal study of cervical cord myelin water in primary progressive multiple sclerosis found that at C2–C3 the myelin water fraction decreased by 5% per year (Laule et al., 2010). Studies to assess metabolite concentrations in MS in the cervical cord using MRS have been used both in cross-sectional and longitudinal studies. For example compared to controls, MS patients with a cervical cord relapse have reduced NAA (N-acetyl-aspartate) concentration and lower structural connectivity in the lateral CST and posterior tracts, and such abnormalities were correlated with disability (Ciccarelli et al., 2007). Interestingly, spinal cord NAA levels partially increased over time after an acute relapse, especially in patients who improved clinically (Ciccarelli et al., 2010a) suggesting that NAA may reffect changes in axonal integrity and/or metabolism which are clinically relevant. A recent paper has demonstrated that lower myo-inositol/creatine values, possibly suggesting astrocytic damage, were consistently found within the NMO (neuromyelitis optica) lesions in the spinal cord when compared with healthy controls and patients with MS, who showed at least one demyelinating lesion at the same cord level, suggesting that the in vivo quantification of myo-inositol may distinguish NMO from MS (Ciccarelli et al., in press).
Functional MRI has also been investigated in MS where an increased activation of the cervical cord has been demonstrated in all the major MS clinical phenotypes and has been related to the severity of clinical disability and the extent of tissue damage (Agosta et al., 2009b, 2009c; Valsasina et al., 2010). Future studies will investigate further the potential derived from statistical modeling imaging measures after the MRI post-processing, which may offer some information on the axonal, mitochondrial metabolism (Ciccarelli et al., 2010b).
Concluding statements
Significant advances in spinal cord imaging methods have been realized in the past decade. The great potential of such methods to support research into basic neuroscience and novel treatment strategies, as well as to improve clinical diagnoses, and the monitoring of treatment and rehabilitation outcomes have been well-demonstrated. The realization of methods to provide the desired research and clinical tools still requires technological development. In part I we reported the greatest technical challenges that are common across almost all of the spinal cord imaging methods that are currently being developed. In part II we have focused on some clinical application of cutting edge techniques that need adoption into the clinical scenario for testing their real impact on clinical practice (diagnosis and prognosis of several conditions). We propose that the fastest way to realize the potential of these methods is to make the most advanced techniques more widely accessible by engaging with equipment manufacturers and software developers, as well as accelerating the development of new methods by facilitating tools and data sharing across research groups.
Acknowledgments
This work is the result of the efforts of the International Spinal Research Trust and the Wings for Life Spinal Cord Research Foundation to bring together researchers with a common goal of developing non-invasive imaging tools for basic and clinical spinal cord research and to support advances in treatment and rehabilitation. The goal is to speed advances and make these imaging tools more widely available by promoting collaboration between researchers and by identifying the most important challenges and needs for spinal cord imaging.
Footnotes
Conflict of interest
The authors declare that they have no financial conflicts of interest that could have an actual or perceived influence over the work presented in this paper.
References
- Agosta F, Filippi M. MRI of spinal cord in multiple sclerosis. J. Neuroimaging. 2007;17(Suppl. 1):46S–49S. doi: 10.1111/j.1552-6569.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- Agosta F, Benedetti B, Rocca MA, Valsasina P, Rovaris M, Comi G, Filippi M. Quantification of cervical cord pathology in primary progressive MS using diffusion tensor MRI. Neurology. 2005;64:631–635. doi: 10.1212/01.WNL.0000151852.15294.CB. [DOI] [PubMed] [Google Scholar]
- Agosta F, Absinta M, Sormani MP, Ghezzi A, Bertolotto A, Montanari E, Comi G, Filippi M. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007a;130:2211–2219. doi: 10.1093/brain/awm110. [DOI] [PubMed] [Google Scholar]
- Agosta F, Pagani E, Caputo D, Filippi M. Associations between cervical cord gray matter damage and disability in patients with multiple sclerosis. Arch. Neurol. 2007b;64:1302–1305. doi: 10.1001/archneur.64.9.1302. [DOI] [PubMed] [Google Scholar]
- Agosta F, Valsasina P, Caputo D, Stroman PW, Filippi M. Tactile-associated recruitment of the cervical cord is altered in patients with multiple sclerosis. NeuroImage. 2008a;39:1542–1548. doi: 10.1016/j.neuroimage.2007.10.048. [DOI] [PubMed] [Google Scholar]
- Agosta F, Valsasina P, Rocca MA, Caputo D, Sala S, Judica E, Stroman PW, Filippi M. Evidence for enhanced functional activity of cervical cord in relapsing multiple sclerosis. Magn. Reson. Med. 2008b;59:1035–1042. doi: 10.1002/mrm.21595. [DOI] [PubMed] [Google Scholar]
- Agosta F, Rocca MA, Valsasina P, Sala S, Caputo D, Perini M, Salvi F, Prelle A, Filippi M. A longitudinal diffusion tensor MRI study of the cervical cord and brain in amyotrophic lateral sclerosis patients. J. Neurol. Neurosurg. Psychiatry. 2009a;80:53–55. doi: 10.1136/jnnp.2008.154252. [DOI] [PubMed] [Google Scholar]
- Agosta F, Valsasina P, Absinta M, Sala S, Caputo D, Filippi M. Primary progressive multiple sclerosis: tactile-associated functional MR activity in the cervical spinal cord. Radiology. 2009b;253:209–215. doi: 10.1148/radiol.2532090187. [DOI] [PubMed] [Google Scholar]
- Agosta F, Valsasina P, Caputo D, Rocca MA, Filippi M. Tactile-associated fMRI recruitment of the cervical cord in healthy subjects. Hum. Brain Mapp. 2009c;30:340–345. doi: 10.1002/hbm.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G. ‘Mirror pain’ in the formalin test: behavioral and 2-deoxyglucose studies. Pain. 1993;55:267–273. doi: 10.1016/0304-3959(93)90156-J. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Arvin B, Kalsi-Ryan S, Mercier D, Furlan JC, Massicotte EM, Fehlings MG. Preoperative magnetic resonance imaging is associated with baseline neurological status and can predict postoperative recovery in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38:1170–1176. doi: 10.1097/BRS.0b013e31828e23a8. [DOI] [PubMed] [Google Scholar]
- Backes WH, Mess WH, Wilmink JT. Functional MR imaging of the cervical spinal cord by use of median nerve stimulation and fist clenching. AJNR Am. J. Neuroradiol. 2001;22:1854–1859. [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Rocca MA, Rovaris M, Caputo D, Zaffaroni M, Capra R, Bertolotto A, Martinelli V, Comi G, Filippi M. A diffusion tensor MRI study of cervical cord damage in benign and secondary progressive multiple sclerosis patients. J. Neurol. Neurosurg. Psychiatry. 2010;81:26–30. doi: 10.1136/jnnp.2009.173120. [DOI] [PubMed] [Google Scholar]
- Brex PA, Leary SM, O'Riordan JI, Miszkiel KA, Plant GT, Thompson AJ, Miller DH. Measurement of spinal cord area in clinically isolated syndromes suggestive of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2001;70:544–547. doi: 10.1136/jnnp.70.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JC, Beckmann CF, Miller KL, Wise RG, Porro CA, Tracey I, Jenkinson M. Physiological noise modelling for spinal functional magnetic resonance imaging studies. NeuroImage. 2008;39:680–692. doi: 10.1016/j.neuroimage.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Brooks JCW, Kong Y, Lee MC, Warnaby CE, Wanigasekera V, Jenkinson M, Tracey I. Stimulus site and modality dependence of functional activity within the human spinal cord. J. Neurosci. 2012;32:6231–6239. doi: 10.1523/JNEUROSCI.2543-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn. Reson. Med. 2007;57:688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang H-F, Russell JH, Cross AH, Song S-K. Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed. 2008;21:589–597. doi: 10.1002/nbm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song S-K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J. Neurosci. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur. Radiol. 2011;21:426–433. doi: 10.1007/s00330-010-1927-z. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Somatotopy of spinal nociceptive processing. J. Comp. Neurol. 1991;312:279–290. doi: 10.1002/cne.903120210. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Stroman PW. Mapping of neural activity produced by thermal pain in the healthy human spinal cord and brain stem: a functional magnetic resonance imaging study. Magn. Reson. Imaging. 2011;29:342–352. doi: 10.1016/j.mri.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Charil A, Caputo D, Cavarretta R, Sormani MP, Ferrante P, Filippi M. Cervical cord magnetization transfer ratio and clinical changes over 18 months in patients with relapsing-remitting multiple sclerosis: a preliminary study. Mult. Scler. 2006;12:662–665. doi: 10.1177/1352458506070714. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Wheeler-Kingshott CA, McLean MA, Cercignani M, Wimpey K, Miller DH, Thompson AJ. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007;130:2220–2231. doi: 10.1093/brain/awm152. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Altmann DR, McLean MA, Wheeler-Kingshott CA, Wimpey K, Miller DH, Thompson AJ. Spinal cord repair in MS: does mitochondrial metabolism play a role? Neurology. 2010a;74:721–727. doi: 10.1212/WNL.0b013e3181d26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Toosy AT, De Stefano N, Wheeler-Kingshott CA, Miller DH, Thompson AJ. Assessing neuronal metabolism in vivo by modeling imaging measures. J. Neurosci. 2010b;30:15030–15033. doi: 10.1523/JNEUROSCI.3330-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Thomas D, De Vita E, Wheeler-Kingshott C, Kachramanoglou C, Kapoor R, Leary S, Matthews L, Palace J, Chard D, Miller D, Toosy A, Thompson A. Low myo-inositol indicating astrocytic damage in a case series of NMO. Ann. Neurol. 2013 doi: 10.1002/ana.23909. (in press, Electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Prog. Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Price DD, Hayes RL, Mayer DJ. Spatial distribution of nociceptive processing in the rat spinal cord. J. Neurophysiol. 1991;65:133–140. doi: 10.1152/jn.1991.65.1.133. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, Descoteaux M, Rossignol S, Hoge RD, Deriche R, Benali H. Detection of multiple pathways in the spinal cord using q-ball imaging. NeuroImage. 2008;42:739–749. doi: 10.1016/j.neuroimage.2008.04.243. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, El Mendili M-M, Lehéricy S, Pradat P-F, Blancho S, Rossignol S, Benali H. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. NeuroImage. 2011;55:1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J, Buchbinder B, Oaklander AL. Cervical spinal cord injection of epidural corticosteroids; comprehensive longitudinal study including multiparametric MRI. Pain. 2012;153:2292–2299. doi: 10.1016/j.pain.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Adad J, El Mendili MM, Morizot-Koutlidis R, Lehericy S, Meininger V, Blancho S, Rossignol S, Benali H, Pradat P-F. Involvement of spinal sensory pathway in ALS and specificity of cord atrophy to lower motor neuron degeneration. Amyotroph. Lateral Scler. Frontotemporal Degener. 2013;14(1):30–38. doi: 10.3109/17482968.2012.701308. [DOI] [PubMed] [Google Scholar]
- Cui JL, Wen CY, Hu Y, Mak KC, Mak KH, Luk KD. Orientation entropy analysis of diffusion tensor in healthy and myelopathic spinal cord. NeuroImage. 2011;58:1028–1033. doi: 10.1016/j.neuroimage.2011.06.072. [DOI] [PubMed] [Google Scholar]
- DeBoy CA, Zhang J, Dike S, Shats I, Jones M, Reich DS, Mori S, Nguyen T, Rothstein B, Miller RH, Griffin JT, Kerr DA, Calabresi PA. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130:2199–2210. doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009a;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009b;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am. J. Neuroradiol. 2008;29:1976–1982. doi: 10.3174/ajnr.A1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JM, Pedler AR, Cowin G, Sterling M, McMahon K. Spinal cord metabolism and muscle water diffusion in whiplash. Spinal Cord. 2012;50:474–476. doi: 10.1038/sc.2011.17. [DOI] [PubMed] [Google Scholar]
- Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am. J. Neuroradiol. 2005;26(6):1587–1594. [PMC free article] [PubMed] [Google Scholar]
- Farrell JA, Smith SA, Gordon-Lipkin EM, Reich DS, Calabresi PA, van Zijl PC. High b-value q-space diffusion-weighted MRI of the human cervical spinal cord in vivo: feasibility and application to multiple sclerosis. Magn. Reson. Med. 2008;59:1079–1089. doi: 10.1002/mrm.21563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- Figley CR, Stroman PW. Measurement and characterization of the human spinal cord SEEP response using event-related spinal fMRI. Magn. Reson. Imaging. 2012;30(4):471–484. doi: 10.1016/j.mri.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Finsterbusch J. Functional neuroimaging of inner fields-of-view with 2D-selective RF excitations. Magn. Reson. Imaging. 2013 doi: 10.1016/j.mri.2013.03.005. (in press, Electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- Finsterbusch J, Eippert F, Buchel C. Single, slice-specific z-shim gradient pulses improve T2*-weighted imaging of the spinal cord. NeuroImage. 2012;59:2307–2315. doi: 10.1016/j.neuroimage.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Finsterbusch J, Sprenger C, Buchel C. Combined T2*-weighted measurements of the human brain and cervical spinal cord with a dynamic shim update. NeuroImage. 2013;79:153–161. doi: 10.1016/j.neuroimage.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134:1610–1622. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Schneider T, Nagy Z, Hutton C, Weiskopf N, Friston K, Wheeler-Kingshott CA, Thompson AJ. Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. PLoS One. 2012a;7:e51729. doi: 10.1371/journal.pone.0051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Wheeler-Kingshott CA, Nagy Z, Gorgoraptis N, Weiskopf N, Friston K, Thompson AJ, Hutton C. Axonal integrity predicts cortical reorganisation following cervical injury. J. Neurol. Neurosurg. Psychiatry. 2012b;83:629–637. doi: 10.1136/jnnp-2011-301875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, Friston K, Thompson A, Curt A. Investigation of the sensorimotor cortex and corticospinal tract after acute spinal cord injury: a prospective longitudinal MRI study. Lancet Neurol. 2013 doi: 10.1016/S1474-4422(13)70146-7. (in press, Electronic publication ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazni NF, Cahill CM, Stroman PW. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional MR imaging. AJNR Am. J. Neuroradiol. 2010;31:661–667. doi: 10.3174/ajnr.A1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb JW, McLaughlin J, Gray CA, Han J. Cyclic CINE-balanced steady-state free precession image intensity variations: implications for the detection of myocardial edema. J. Magn. Reson. Imaging. 2011;33:573–581. doi: 10.1002/jmri.22368. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera JJ, Chacko T, Narayana PA. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J. Neurosci. Res. 2008;86:443–447. doi: 10.1002/jnr.21481. [DOI] [PubMed] [Google Scholar]
- Hesseltine SM, Law M, Babb J, Rad M, Lopez S, Ge Y, Johnson G, Grossman RI. Diffusion tensor imaging in multiple sclerosis: assessment of regional differences in the axial plane within normal-appearing cervical spinal cord. AJNR Am. J. Neuroradiol. 2006;27:1189–1193. [PMC free article] [PubMed] [Google Scholar]
- Hochman S. Spinal cord. Curr. Biol. 2007;17:R950–R955. doi: 10.1016/j.cub.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Hofling AA, Kim JH, Fantz CR, Sands MS, Song S-K. Diffusion tensor imaging detects axonal injury and demyelination in the spinal cord and cranial nerves of a murine model of globoid cell leukodystrophy. NMR Biomed. 2009;22:1100–1106. doi: 10.1002/nbm.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J. Neurosurg. Spine. 2009;10:194–200. doi: 10.3171/2008.12.SPINE08367. [DOI] [PubMed] [Google Scholar]
- Hori M, Fukunaga I, Masutani Y, Nakanishi A, Shimoji K, Kamagata K, Asahi K, Hamasaki N, Suzuki Y, Aoki S. New diffusion metrics for spondylotic myelopathy at an early clinical stage. Eur. Radiol. 2012;22:1797–1802. doi: 10.1007/s00330-012-2410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield MA, Sala S, Neema M, Absinta M, Bakshi A, Sormani MP, Rocca MA, Bakshi R, Filippi M. Rapid semi-automatic segmentation of the spinal cord from magnetic resonance images: application in multiple sclerosis. NeuroImage. 2010;50:446–455. doi: 10.1016/j.neuroimage.2009.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc. Biol. Sci. 1991;244:39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Kachramanoglou C, De Vita E, Thomas DL, Wheeler-Kingshott CA, Balteau E, Carlstedt T, Choi D, Thompson AJ, Ciccarelli O. Metabolic changes in the spinal cord after brachial plexus root re-implantation. Neurorehabil. Neural Repair. 2013;27:118–124. doi: 10.1177/1545968312457825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, Mutlu A, Ornek I, Sirvanci M, Hamzaoglu A. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011;53:609–616. doi: 10.1007/s00234-011-0844-4. [DOI] [PubMed] [Google Scholar]
- Kidd D, Thorpe JW, Thompson AJ, Kendall BE, Moseley IF, MacManus DG, McDonald WI, Miller DH. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology. 1993;43:2632–2637. doi: 10.1212/wnl.43.12.2632. [DOI] [PubMed] [Google Scholar]
- Kim JH, Loy DN, Liang HF, Trinkaus K, Schmidt RE, Song SK. Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn. Reson. Med. 2007;58:253–260. doi: 10.1002/mrm.21316. [DOI] [PubMed] [Google Scholar]
- Kong Y, Jenkinson M, Andersson J, Tracey I, Brooks JC. Assessment of physiological noise modelling methods for functional imaging of the spinal cord. NeuroImage. 2012;60:1538–1549. doi: 10.1016/j.neuroimage.2011.11.077. [DOI] [PubMed] [Google Scholar]
- Kornelsen J, Stroman PW. fMRI of the lumbar spinal cord during a lower limb motor task. Magn. Reson. Med. 2004;52:411–414. doi: 10.1002/mrm.20157. [DOI] [PubMed] [Google Scholar]
- Kornelsen J, Stroman PW. Detection of the neuronal activity occurring caudal to the site of spinal cord injury that is elicited during lower limb movement tasks. Spinal Cord. 2007;45:485–490. doi: 10.1038/sj.sc.3102019. [DOI] [PubMed] [Google Scholar]
- Kozlowski P, Raj D, Liu J, Lam C, Yung AC, Tetzlaff W. Characterizing white matter damage in rat spinal cord with quantitative MRI and histology. J. Neurotrauma. 2008;25:653–676. doi: 10.1089/neu.2007.0462. [DOI] [PubMed] [Google Scholar]
- Lammertse D, Dungan D, Dreisbach J, Falci S, Flanders A, Marino R, Schwartz E. Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2007;30:205–214. doi: 10.1080/10790268.2007.11753928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C, Vavasour IM, Zhao Y, Traboulsee AL, Oger J, Vavasour JD, MacKay AL, Li DKB. Two year study of cervical cord volume and myelin water in primary progressive multiple sclerosis. Mult. Scler. 2010;16:670–677. doi: 10.1177/1352458510365586. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Stroman PW, Bascaramurty S, Jordan LM, Malisza KL. Correlation of functional activation in the rat spinal cord with neuronal activation detected by immunohistochemistry. NeuroImage. 2004;22:1802–1807. doi: 10.1016/j.neuroimage.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Stroman PW, Malisza KL. Comparison of functional activity in the rat cervical spinal cord during alpha-chloralose and halothane anesthesia. NeuroImage. 2007;34:1665–1672. doi: 10.1016/j.neuroimage.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Stroman PW, Kollias SS. Functional magnetic resonance imaging of the human spinal cord during vibration stimulation of different dermatomes. Neuroradiology. 2008;50:273–280. doi: 10.1007/s00234-007-0338-6. [DOI] [PubMed] [Google Scholar]
- Leitch J, Cahill CM, Ghazni N, Figley CR, Stroman PW. Spinal cord and brainstem activation in carpal tunnel syndrome patients in response to noxious stimuli: a spinal fMRI study; International Society for Magnetic Resonance in Medicine 17th Annual meeting; Honolulu, Hawaii, U.S.A.. May, 2009. pp. 18–25. [Google Scholar]
- Leitch JK, Figley CR, Stroman PW. Applying functional MRI to the spinal cord and brainstem. Magn. Reson. Imaging. 2010;28:1225–1233. doi: 10.1016/j.mri.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Lin X, Tench CR, Evangelou N, Jaspan T, Constantinescu CS. Measurement of spinal cord atrophy in multiple sclerosis. J. Neuroimaging. 2004;14:20S–26S. doi: 10.1177/1051228404266265. [DOI] [PubMed] [Google Scholar]
- Losseff NA, Kingsley DP, McDonald WI, Miller DH, Thompson AJ. Clinical and magnetic resonance imaging predictors of disability in primary and secondary progressive multiple sclerosis. Mult. Scler. 1996;1:218–222. [PubMed] [Google Scholar]
- Lundell H, Dyrby T, Ptito M, Nielsen JB. Crossing fibers in lateral white matter of the cervical spinal cord detected with diffusion MRI in monkey postmortem; Proceedings of the 17th Annual Meeting of ISMRM; Honolulu, USA. 2009. p. 1497. [Google Scholar]
- Lundell H, Barthelemy D, Skimminge A, Dyrby TB, Biering-Sorensen F, Nielsen JB. Independent spinal cord atrophy measures correlate to motor and sensory deficits in individuals with spinal cord injury. Spinal Cord. 2011;49:70–75. doi: 10.1038/sc.2010.87. [DOI] [PubMed] [Google Scholar]
- Lycklama G, Thompson A, Filippi M, Miller D, Polman C, Fazekas F, Barkhof F. Spinal-cord MRI in multiple sclerosis. Lancet Neurol. 2003;2:555–562. doi: 10.1016/s1474-4422(03)00504-0. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maieron M, Iannetti GD, Bodurka J, Tracey I, Bandettini PA, Porro CA. Functional responses in the human spinal cord during willed motor actions: evidence for side- and rate-dependent activity. J. Neurosci. 2007;27:4182–4190. doi: 10.1523/JNEUROSCI.3910-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majcher K, Tomanek B, Jasinski A, Foniok T, Stroman PW, Tuor UI, Kirk D, Hess G. Simultaneous functional magnetic resonance imaging in the rat spinal cord and brain. Exp. Neurol. 2006;197:458–464. doi: 10.1016/j.expneurol.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Majcher K, Tomanek B, Tuor UI, Jasinski A, Foniok T, Rushforth D, Hess G. Functional magnetic resonance imaging within the rat spinal cord following peripheral nerve injury. NeuroImage. 2007;38:669–676. doi: 10.1016/j.neuroimage.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Stroman PW. Functional imaging of the rat cervical spinal cord. J. Magn. Reson. Imaging. 2002;16:553–558. doi: 10.1002/jmri.10185. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Gregorash L, Turner A, Foniok T, Stroman PW, Allman AA, Summers R, Wright A. Functional MRI involving painful stimulation of the ankle and the effect of physiotherapy joint mobilization. Magn. Reson. Imaging. 2003;21:489–496. doi: 10.1016/s0730-725x(03)00074-2. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Koltzenburg M, Wall PD. Wall and Melzack's Textbook of Pain. 5th Elsevier Churchill Livingstone; Edinburgh: 2006. [Google Scholar]
- Nair G, Carew JD, Usher S, Lu D, Hu XP, Benatar M. Diffusion tensor imaging reveals regional differences in the cervical spinal cord in amyotrophic lateral sclerosis. NeuroImage. 2010;53:576–583. doi: 10.1016/j.neuroimage.2010.06.060. [DOI] [PubMed] [Google Scholar]
- Oh J, Saidha S, Chen M, Smith SA, Prince J, Jones C, Diener-West M, van Zijl PC, Reich DS, Calabresi PA. Spinal cord quantitative MRI discriminates between disability levels in multiple sclerosis. Neurology. 2013a;80(6):540–547. doi: 10.1212/WNL.0b013e31828154c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Zackowski K, Chen M, Newsome S, Saidha S, Smith SA, Diener-West M, Prince J, Jones CK, Van Zijl PC, Calabresi PA, Reich DS. Multiparametric MRI correlates of sensorimotor function in the spinal cord in multiple sclerosis. Mult. Scler. 2013b;19(4):427–435. doi: 10.1177/1352458512456614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgiya Y, Oka M, Hiwatashi A, Liu X, Kakimoto N, Westesson PL, Ekholm SE. Diffusion tensor MR imaging of the cervical spinal cord in patients with multiple sclerosis. Eur. Radiol. 2007;17:2499–2504. doi: 10.1007/s00330-007-0672-4. [DOI] [PubMed] [Google Scholar]
- Ozanne A, Krings T, Facon D, Fillard P, Dumas JL, Alvarez H, Ducreux D, Lasjaunias P. MR diffusion tensor imaging and fiber tracking in spinal cord arteriovenous malformations: a preliminary study. AJNR Am. J. Neuroradiol. 2007;28(7):1271–1279. doi: 10.3174/ajnr.A0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, Dietz V, Kollias S. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J. Neurotrauma. 2012;29:1556–1566. doi: 10.1089/neu.2011.2027. [DOI] [PubMed] [Google Scholar]
- Plank C, Koller A, Mueller-Mang C, Bammer R, Thurnher MM. Diffusion-weighted MR imaging (DWI) in the evaluation of epidural spinal lesions. Neuroradiology. 2007;49(12):977–985. doi: 10.1007/s00234-007-0275-4. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M. Spatial and temporal aspects of spinal cord and brainstem activation in the formalin pain model. Prog. Neurobiol. 1993;41:565–607. doi: 10.1016/0301-0082(93)90044-s. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M, Galetti A, Sassatelli L, Barbieri GC. Functional activity mapping of the rat spinal cord during formalin-induced noxious stimulation. Neuroscience. 1991;41:655–665. doi: 10.1016/0306-4522(91)90357-t. [DOI] [PubMed] [Google Scholar]
- Pradat PF, Cohen-Adad J, El-Mendili MM, Lehericy S, Blancho S, Meininger V, Rossignol S, Benali H. Diffusion and magnetization transfer imaging detects spinal cord lesions in amyotrophic lateral sclerosis; Proceedings of the 19th Annual Meeting of ISMRM; Montreal, Canada. 2011. p. 2465. [Google Scholar]
- Renoux J, Facon D, Fillard P, Huynh I, Lasjaunias P, Ducreux D. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. AJNR Am. J. Neuroradiol. 2006;27:1947–1951. [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, Horsfield MA, Sala S, Copetti M, Valsasina P, Mesaros S, Martinelli V, Caputo D, Stosic-Opincal T, Drulovic J, Comi G, Filippi M. A multicenter assessment of cervical cord atrophy among MS clinical phenotypes. Neurology. 2011;76:2096–2102. doi: 10.1212/WNL.0b013e31821f46b8. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Valsasina P, Damjanovic D, Horsfield MA, Mesaros S, Stosic-Opincal T, Drulovic J, Filippi M. Voxel-wise mapping of cervical cord damage in multiple sclerosis patients with different clinical phenotypes. J. Neurol. Neurosurg. Psychiatry. 2013;84:35–41. doi: 10.1136/jnnp-2012-303821. [DOI] [PubMed] [Google Scholar]
- Saranathan M, Bayram E, Worters PW, Glockner JF. A 3D balanced-SSFP Dixon technique with group-encoded k-space segmentation for breath-held noncontrast-enhanced MR angiography. Magn. Reson. Imaging. 2012;30:158–164. doi: 10.1016/j.mri.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Horikoshi T, Watanabe A, Uchida M, Ishigame K, Araki T, Kinouchi H. Evaluation of cervical myelopathy using apparent diffusion coefficient measured by diffusion-weighted imaging. AJNR Am. J. Neuroradiol. 2012;33:388–392. doi: 10.3174/ajnr.A2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Tozer DJ, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, Miller DH. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J. Magn. Reson. Imaging. 2007;26:41–51. doi: 10.1002/jmri.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys. J. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Tang Y, Huang L, Yang R, Wu Y, Wang P, Shi Y, He X, Liu H, Ye J. Applications of diffusion-weighted MRI in thoracic spinal cord injury without radiographic abnormality. Int. Orthop. 2007;31(3):375–383. doi: 10.1007/s00264-006-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Jones CK, Gifford A, Belegu V, Chodkowski B, Farrell JA, Landman BA, Reich DS, Calabresi PA, McDonald JW, van Zijl PC. Reproducibility of tract-specific magnetization transfer and diffusion tensor imaging in the cervical spinal cord at 3 tesla. NMR Biomed. 2010;23(2):207–217. doi: 10.1002/nbm.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Curr. Biol. 2012;22:1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Stroman PW. Spinal fMRI investigation of human spinal cord function over a range of innocuous thermal sensory stimuli and study-related emotional influences. Magn. Reson. Imaging. 2009;27:1333–1346. doi: 10.1016/j.mri.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Cahill CM. Functional magnetic resonance imaging of the human spinal cord and brainstem during heat stimulation; Proceedings of the International Society for Magnetic Resonance in Medicine 14th Annual Meeting; Seattle, USA, May. 2006. 2006. pp. 6–12. [Google Scholar]
- Stroman PW, Ryner LN. Functional MRI of motor and sensory activation in the human spinal cord. Magn. Reson. Imaging. 2001;19:27–32. doi: 10.1016/s0730-725x(01)00226-0. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Nance PW, Ryner LN. BOLD MRI of the human cervical spinal cord at 3 tesla. Magn. Reson. Med. 1999;42:571–576. doi: 10.1002/(sici)1522-2594(199909)42:3<571::aid-mrm20>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Krause V, Malisza KL, Frankenstein UN, Tomanek B. Characterization of contrast changes in functional MRI of the human spinal cord at 1.5 T. Magn. Reson. Imaging. 2001;19:833–838. doi: 10.1016/s0730-725x(01)00409-x. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Krause V, Malisza KL, Frankenstein UN, Tomanek B. Extravascular proton-density changes as a non-BOLD component of contrast in fMRI of the human spinal cord. Magn. Reson. Med. 2002a;48:122–127. doi: 10.1002/mrm.10178. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Krause V, Malisza KL, Frankenstein UN, Tomanek B. Functional magnetic resonance imaging of the human cervical spinal cord with stimulation of different sensory dermatomes. Magn. Reson. Imaging. 2002b;20:1–6. doi: 10.1016/s0730-725x(02)00468-x. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Tomanek B, Krause V, Frankenstein UN, Malisza KL. Mapping of neuronal function in the healthy and injured human spinal cord with spinal fMRI. NeuroImage. 2002c;17:1854–1860. doi: 10.1006/nimg.2002.1305. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Krause V, Malisza KL, Kornelsen J, Bergman A, Lawrence J, Tomanek B. Spinal fMRI of Spinal Cord Injury in Human Subjects; Proceedings of the International Society for Magnetic Resonance in Medicine 11th Annual Meeting; Toronto, Canada. Jul 10-16, 2003. 2005. p. 13. [Google Scholar]
- Stroman PW, Kornelsen J, Bergman A, Krause V, Ethans K, Malisza KL, Tomanek B. Noninvasive assessment of the injured human spinal cord by means of functional magnetic resonance imaging. Spinal Cord. 2004a;42:59–66. doi: 10.1038/sj.sc.3101559. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Kornelsen J, Lawrence J, Malisza KL. A novel technique for spinal fMRI with large volume three-dimensional coverage of the spinal cord; Proceedings of the International Society for Magnetic Resonance in Medicine 12th Annual Meeting; Kyoto, Japan. May 15-21, 2004b. 2004. p. 2541. [Google Scholar]
- Stroman PW, Coe BC, Munoz DP. Influence of attention focus on neural activity in the human spinal cord during thermal sensory stimulation. Magn. Reson. Imaging. 2011;29:9–18. doi: 10.1016/j.mri.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Bosma RL, Tsyben A. Somatotopic arrangement of thermal sensory regions in the healthy human spinal cord determined by means of spinal cord functional MRI. Magn. Reson. Med. 2012;68(3):923–931. doi: 10.1002/mrm.23292. [DOI] [PubMed] [Google Scholar]
- Stroman PW, Wheeler-Kingshott C, Bacon M, Schwab JM, Bosma R, Brooks J, Cadotte D, Carlstedt T, Ciccarelli O, Cohen-Adad J, Curt A, Evangelou N, Fehlings MG, Filippi M, Kelly B, Kollias S, Mackay A, Porro CA, Smith S, Strittmatter SM, Summers P, Tracey I. The current state-of-the-art of spinal cord imaging: methods. NeuroImage. 2014;84:1070–1081. doi: 10.1016/j.neuroimage.2013.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers PE, Ferraro D, Duzzi D, Lui F, Iannetti GD, Porro CA. A quantitative comparison of BOLD fMRI responses to noxious and innocuous stimuli in the human spinal cord. NeuroImage. 2010;50:1408–1415. doi: 10.1016/j.neuroimage.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Sun S-W, Liang H-F, Trinkaus K, Cross AH, Armstrong RC, Song S-K. Non-invasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- Thurnher MM, Bammer R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology. 2006;48(11):795–801. doi: 10.1007/s00234-006-0130-z. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS. Q-ball imaging. Magn. Reson. Med. 2004;52:1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- Uda T, Takami T, Tsuyuguchi N, Sakamoto S, Yamagata T, Ikeda H, Nagata T, Ohata K. Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla. Spine (Phila Pa 1976) 2013;38:407–414. doi: 10.1097/BRS.0b013e31826f25a3. [DOI] [PubMed] [Google Scholar]
- Valsasina P, Rocca MA, Agosta F, Benedetti B, Horsfield MA, Gallo A, Rovaris M, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histogram analysis of the cervical cord in MS patients. NeuroImage. 2005;26:822–828. doi: 10.1016/j.neuroimage.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Valsasina P, Agosta F, Benedetti B, Caputo D, Perini M, Salvi F, Prelle A, Filippi M. Diffusion anisotropy of the cervical cord is strictly associated with disability in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2007;78:480–484. doi: 10.1136/jnnp.2006.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsasina P, Agosta F, Absinta M, Sala S, Caputo D, Filippi M. Cervical cord functional MRI changes in relapse-onset MS patients. J. Neurol. Neurosurg. Psychiatry. 2010;81:405–408. doi: 10.1136/jnnp.2009.187526. [DOI] [PubMed] [Google Scholar]
- Valsasina P, Rocca MA, Horsfield MA, Absinta M, Messina R, Caputo D, Comi G, Filippi M. Regional cervical cord and disability in multiple sclerosis: a voxel-based analysis. Radiology. 2013;266:853–861. doi: 10.1148/radiol.12120813. [DOI] [PubMed] [Google Scholar]
- van Eijsden P, Hyder F, Rothman DL, Shulman RG. Neurophysiology of functional imaging. NeuroImage. 2009;45:1047–1054. doi: 10.1016/j.neuroimage.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke W, Nagels G, Emonds G, Leemans A, Sijbers J, Van Goethem J, Parizel P. A diffusion tensor imaging group study of the spinal cord in multiple sclerosis patients with and without T(2) spinal cord lesions. J. Magn. Reson. Imaging. 2009;30:25–34. doi: 10.1002/jmri.21817. [DOI] [PubMed] [Google Scholar]
- Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN. Characterization and limitations of diffusion tensor imaging metrics in the cervical spinal cord in neurologically intact subjects. J. Magn. Reson. Imaging. 2013 doi: 10.1002/jmri.24039. (in press, Electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- Wall PD. Presynaptic control of impulses at the first central synapse in the cutaneous pathway. Prog. Brain Res. 1964;12:92–118. doi: 10.1016/s0079-6123(08)60619-6. [DOI] [PubMed] [Google Scholar]
- Watson C. The Spinal Cord. A Christopher and Dana Reeve Foundation Text and Atlas. Academic; London: 2009. [Google Scholar]
- Wheeler-Kingshott C, Cercignani M. About “axial” and “radial” diffusivities. Magn. Reson. Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Hickman SJ, Parker GJ, Ciccarelli O, Symms MR, Miller DH, Barker GJ. Investigating cervical spinal cord structure using axial diffusion tensor imaging. NeuroImage. 2002;16:93–102. doi: 10.1006/nimg.2001.1022. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Ciccarelli O, Schneider T, Alexander DC, Cercignani M. A new approach to structural integrity assessment based on axial and radial diffusivities. Funct. Neurol. 2012;27:85–90. [PMC free article] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb. Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- Xiangshui M, Xiangjun C, Xiaoming Z, Qingshi Z, Yi C, Chuanqiang Q, Xiangxing M, Chuanfu L, Jinwen H. 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin. Radiol. 2010;65:465–473. doi: 10.1016/j.crad.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Xie M, Tobin JE, Budde MD, Chen C-I, Trinkaus K, Cross AH, Mcdaniel DP, Song S-K, Armstrong RC. Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J. Neuropathol. Exp. Neurol. 2010;69:704–716. doi: 10.1097/NEN.0b013e3181e3de90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Piche M, Khoshnejad M, Perlbarg V, Chen JI, Hoge RD, Benali H, Rossignol S, Rainville P, Cohen-Adad J. Reduction of physiological noise with independent component analysis improves the detection of nociceptive responses with fMRI of the human spinal cord. NeuroImage. 2012;63:245–252. doi: 10.1016/j.neuroimage.2012.06.057. [DOI] [PubMed] [Google Scholar]
- Zackowski KM, Smith SA, Reich DS, Gordon-Lipkin E, Chodkowski BA, Sambandan DR, Shteyman M, Bastian AJ, van Zijl PC, Calabresi PA. Sensorimotor dysfunction in multiple sclerosis and column-specific magnetization transfer-imaging abnormalities in the spinal cord. Brain. 2009;132:1200–1209. doi: 10.1093/brain/awp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jones M, DeBoy CA, Reich DS, Farrell JA, Hoffman PN, Griffin JW, Sheikh KA, Miller MI, Mori S, Calabresi PA. Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J. Neurosci. 2009;29:3160–3171. doi: 10.1523/JNEUROSCI.3941-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]