Abstract
Purpose
To assess safety and efficacy of presurgical bevacizumab in patients with metastatic renal cell carcinoma (mRCC), and to explore the hypothesis that pretreatment of patients with antiangiogenic therapy will select patients who benefit most from cytoreductive nephrectomy.
Patients and Methods
Patients with newly diagnosed, clear cell mRCC whose primary tumors were considered resectable were enrolled. In this single-arm, phase II trial, patients received bevacizumab plus erlotinib (first patients, n = 23) or bevacizumab alone (n = 27 patients) for 8 weeks followed by restaging. If patients demonstrated progressive disease and had declining performance statuses after 8 weeks, nephrectomy procedures were deferred. Postoperatively, patients continued on the study drug or drugs if disease stabilization or regression had occurred.
Results
Between March 2005 and March 2008, 52 patients were enrolled on study, and 50 were included in the analysis. By Memorial Sloan-Kettering Cancer Center criteria, 82% of patients had intermediate-risk, and 18% had poor-risk, features. Forty-two patients underwent nephrectomy. Median progression-free survival was 11.0 months (95% CI, 5.5 to 15.6 months). Median overall survival was 25.4 months (95% CI, 11.4 months to not estimable). Two perioperative deaths occurred; neither was attributable to study drug. Wound dehiscence resulted in treatment discontinuation for three patients and treatment delay for two others.
Conclusion
Presurgical treatment with bevacizumab therapy yields clinical outcomes comparable to post-surgical treatment with antiangiogenic therapy in patients with mRCC, but it may result in wound-healing delays. Prospective, randomized trials to test the use of presurgical therapy as a method to select appropriate patients for cytoreductive nephrectomy are warranted.
INTRODUCTION
Renal cell carcinoma (RCC) affects greater than 40,000 patients per year in the United States and is responsible for close to 13,000 deaths.1 Once metastatic RCC (mRCC) develops, treatment is difficult, and median survival is between 1 to 2 years.2 Several systemic treatment modalities, including immunotherapy,3,4 chemotherapy,5,6 and biologically based targeted therapy, have been used to treat mRCC. With the advent of antiangiogenic and targeted agents, including sorafenib,7 sunitinib,8 bevacizumab,9 and temsirolimus,10 outcomes have modestly improved for patients with mRCC. Nevertheless, few patients achieve cure, and most patients will die as a result of the disease. Thus, therapy for patients with mRCC must be improved.
The role of cytoreductive nephrectomy in patients with mRCC who receive treatment with antiangiogenic therapy is not well established. Although cytoreductive nephrectomy improved survival in two randomized, clinical trials, patients had received immunotherapy in both studies.11,12 Furthermore, the timing of nephrectomy relative to systemic therapy has received little attention. Use of a lead-in course of systemic immunotherapy to select patients for cytoreductive nephrectomy was evaluated in a small, pilot study.13 Eleven of 16 patients demonstrated either stable disease or response in their metastatic lesions and underwent cytoreductive nephrectomy with no evidence of increased morbidity. To definitively establish the role of cytoreductive nephrectomy in patients who receive targeted agents, randomized trials are needed. Nevertheless, it is clear that preliminary efficacy data on patients who undergo delayed nephrectomy after receiving antiangiogenic therapy will be helpful to decide whether or not to proceed with larger randomized trials, and these data will influence the trial designs.
Emerging data exist on the safety of surgery after antiangiogenic therapy. Gruenberger et al14 described surgical outcomes of patients who underwent surgical resection of hepatic metastases after treatment with bevacizumab, capecitabine, and oxaliplatin in a prospective trial; minimal perioperative morbidity was reported in this trial. Margulis et al5 reported a retrospective analysis of perioperative complications in patients with mRCC who underwent cytoreductive nephrectomy after receiving various antiangiogenic agents; this analysis did not report excessive morbidity.5 Prospective data in the context of RCC will assist in better defining the toxicity profile seen with antiangiogenic agents.
This prospective study sets out to answer the following clinical questions: Is it safe to perform a cytoreductive nephrectomy after pretreatment with antiangiogenic therapy in patients with mRCC? Are the outcomes similar or dissimilar to those seen with nephrectomy followed by antiangiogenic therapy? Can pretreatment with systemic therapy also select for individuals who should not undergo cytoreductive nephrectomy? This report summarizes the safety profiles and clinical outcomes of patients with newly diagnosed, untreated mRCC who received bevacizumab or bevacizumab and erlotinib before undergoing cytoreductive nephrectomy.
PATIENTS AND METHODS
Patient Selection
Before enrolling on this study, patients were required to sign informed consent that was approved by The University of Texas M. D. Anderson Cancer Center (MDACC) institutional review board. Patients were accrued at a single center (MDACC). Eligibility criteria included the following: histologically or cytologically confirmed metastatic, clear cell RCC with the primary tumor in place; primary tumor deemed resectable by the treating urologist; measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST), not including primary tumor; Eastern Cooperative Oncology Group performance status of zero or one; adequate organ and marrow function within 14 days, including absolute neutrophil count ≥ 1,500/μL, platelets ≥ 75,000/μL, hemoglobin greater than 9.0 g/d (transfusion allowed), total bilirubin ≤ 2.0 mg/dL, albumin greater than 3.0 g/dL, serum creatinine ≤ 2.0 mg/dL, AST and/or ALT ≤ 2.5 times the institutional upper limit of normal for participants without evidence of liver metastases, and AST and/or ALT ≤ five times institutional upper limit of normal for participants with documented liver metastases; absence of brain metastases; and no prior systemic therapy. Biopsy could be performed either via fine-needle aspiration or core biopsy, and both primary and metastatic lesions could be biopsied for diagnostic purposes.
Study Design
Patients received bevacizumab 10 mg/kg intravenously every 14 days, which was considered one cycle, for four cycles. The first 23 patients also received erlotinib 150 mg orally daily. The protocol then was amended in December 2006 to remove erlotinib from the treatment regimen of the next cohort of 27 patients after the disclosure of randomized, phase II data by Bukowski et al2 that showed no progression-free survival (PFS) or overall survival (OS) differences with the addition of erlotinib to bevacizumab therapy.
Evaluation of response was performed as per RECIST. Primary lesions were not included in response assessment. Patients underwent restaging at week 8 and, if indicated, cytoreductive nephrectomy at week 10, which was 4 weeks after their last doses of bevacizumab and 2 weeks after their last doses of erlotinib in the first cohort. The decision to proceed with nephrectomy was made by a team with input from urology surgeons and genitourinary medical oncologists. Overall performance status and disease progression in metastatic and primary site were key determining factors. At 4 weeks postnephrectomy, patients were restaged and were restarted on protocol therapy. Patients were restaged every 8 weeks. Patients came off study for toxicity or for disease progression.
The primary end points were time to disease progression and safety of the therapy. Secondary clinical end points included response rate, duration of response, and OS. All patients who completed the first cycle of bevacizumab were considered assessable for clinical response and toxicity. Patients who died as a result of unrelated cause during therapy or who were lost to follow-up were censored. The hypothesis for the trial was that bevacizumab therapy achieves improvement in PFS, which is demonstrated as a 50% increase from a baseline of 4 months to 6 months. These estimates were based on the study by Yang et al,15 in which patients with mRCC who received bevacizumab 10 mg/kg had a PFS of 4.8 months and those who received placebo had a PFS of 2.5 months; additional improvement in PFS is expected in this study because of the frontline status of the patient population receiving bevacizumab. A total sample of 50 patients was required to test these hypotheses at 80% power with a two-sided significance level of 5%. For discrete or categoric data, descriptive statistics included tabulations of frequencies. For continuous data, summary statistics, including n, mean, standard deviation, median, minimum, and maximum, were computed. Kaplan-Meier methodology and Cox proportional hazards regression modeling served to analyze time to progression, duration of response, and OS. Time to progression was recorded from time of first treatment. A 95% exact binomial CI on response rate was computed for objective (ie, partial and complete) response. All statistical tests were performed at a significance level of 5% by using SAS (SAS Institute, Cary, NC) or S-Plus (Statistical Sciences, Seattle, WA), as appropriate.
RESULTS
Patient Characteristics
Between March 2005 and March 2008, a total of 52 patients were enrolled on the trial. Two patients received no therapy; therefore, the assessable sample size was 50 patients, as defined in the statistical requirements. Patient characteristics are summarized in Table 1. The median age of patients on study was 61 years. All patients possessed either intermediate- or poor-risk features by the Memorial Sloan-Kettering Cancer Center (MSKCC) risk stratification. Eighty-four percent of patients had two or more sites of metastatic disease. The most common site of metastatic disease was lung, followed by lymph node and bone. Three patients required transfusion to meet eligibility criteria.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | Patients (N = 50) |
|
|---|---|---|
| No. | % | |
| Age | ||
| Median | 61 | |
| Range | 35-83 | |
| Sex | ||
| Male | 37 | 74 |
| Female | 13 | 26 |
| MSKCC prognostic risk | ||
| Intermediate | 41 | 82 |
| Poor | 9 | 18 |
| No. of metastatic sites | ||
| None | 2* | 4 |
| 1 | 6 | 12 |
| 2 | 23 | 46 |
| ≥ 3 | 19 | 38 |
| Sites of metastatic disease | ||
| Lung | 41 | 82 |
| Lymph nodes | 29 | 58 |
| Bone | 14 | 28 |
| Liver | 7 | 14 |
| Other | 23 | 46 |
Abbreviation: MSKCC, Memorial Sloan-Kettering Cancer Center.
False positive for metastatic disease; confirmed at nephrectomy.
Clinical Outcomes
Of the 50 assessable patients on study, forty-two (84%) underwent nephrectomy. Reasons for not undergoing nephrectomy included death unrelated to study (n = 1; by motor vehicle accident), coming off study for toxicity before first restaging (n = 1; poorly controlled, bevacizumab-related hypertension); in addition, six patients (12%) who showed either clinical or radiographic progression were not deemed suitable candidates to undergo cytoreductive nephrectomy.
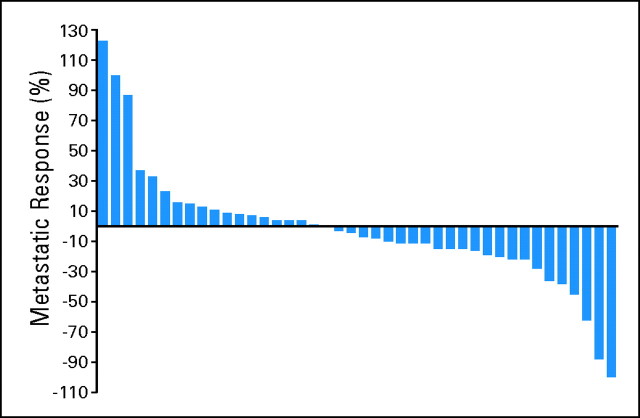
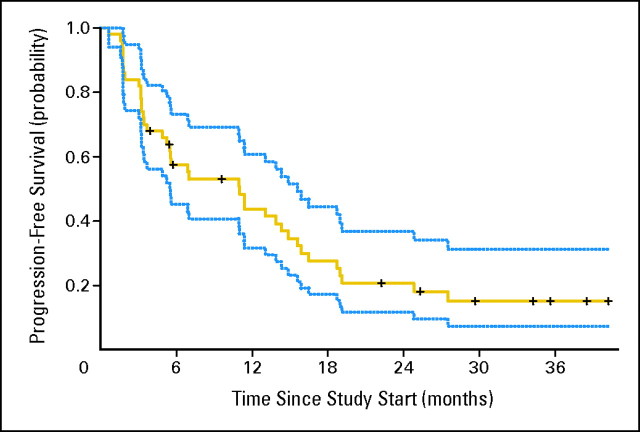
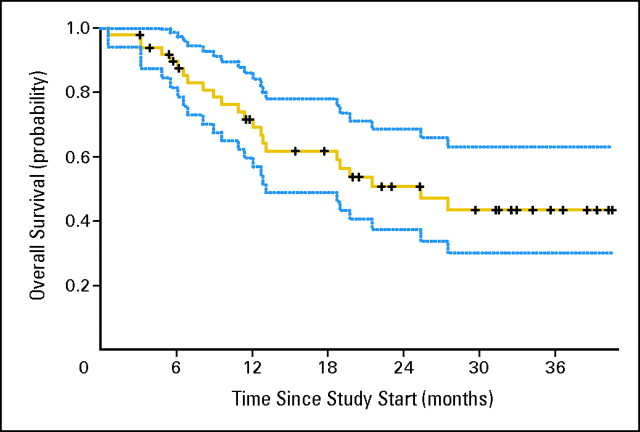
As outlined in Table 2, the median PFS for the 50 assessable patients was 11.0 months (95% CI, 5.5 to 15.6 months). The overall response rate was 12% (95% CI, 5% to 24%). Median OS was 25.4 months (95% CI, 11.4 months to unreached). When divided by MSKCC risk group, there were no statistically significant differences among different risk groups for PFS, overall response rate, or OS. Figure 1 shows a waterfall plot of best response in metastatic lesions.
Table 2.
Clinicopathologic and Perisurgical Outcomes
| Outcome Variable | Patients (N = 50) |
|
|---|---|---|
| No. | % | |
| Best response | ||
| CR | 1 | 2 |
| PR | 5 | 10 |
| SD | 29 | 58 |
| PD | 10 | 20 |
| Inevaluable | 5 | 10 |
| Survival | ||
| PFS, months | ||
| Median | 11.0 | |
| 95% CI | 5.5 to 15.6 | |
| OS, months | ||
| Median | 25.4 | |
| 95% CI | 11.4 to NR | |
| Response duration, months | ||
| Median | 8.3 | |
| 95% CI | 3.7 to 15.9 | |
| Pathologic stage* | ||
| pT1 | 10 | |
| pT3aN0 | 12 | |
| pT3aN2 | 1 | |
| pT3bN0 | 9 | |
| pT3bN2 | 7 | |
| pT4N0 | 2 | |
| pT4N2 | 1 | |
| Surgical outcome* | ||
| Estimated blood loss, mL | ||
| Median | 400 | |
| Range | 0-7,000 | |
| Length of stay, days | ||
| Median | 5 | |
| Range | 1-70 | |
| Surgical time, hours | ||
| Median | 2.8 | |
| Range | 2.0 to 9.0 | |
| Transfusion volume, mL | ||
| Median | 0 | |
| Range | 0-14,186 | |
| Primary tumor regression† | ||
| Growth, % | ||
| > 20 | 1 | 2 |
| 10-20 | 2 | 4 |
| 0-10 | 19 | 42 |
| Reduction, % | ||
| 1-10 | 13 | 29 |
| 11-20 | 7 | 16 |
| 20-30 | 3 | 7 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression-free survival; OS, overall survival; NR, not reached.
Total No. of patients = 42.
Total No. of patients = 45.
Fig 1.
Waterfall plot of best response in metastatic sites.
Univariable and multivariable analyses were performed by using the following covariates: lactate dehydrogenase (LDH), serum calcium, MSKCC risk factors, treatment with erlotinib, and nephrectomy. In a multivariable Cox proportional hazards model for PFS, LDH greater than 1.5 times the normal upper limit was associated with a higher risk of progression or death (hazard ratio, 3.91; P = .001) In the multivariable Cox model for OS, only LDH greater than 1.5 times the normal upper limit and nephrectomy remained in the model, with P = .06 and .0009, respectively.
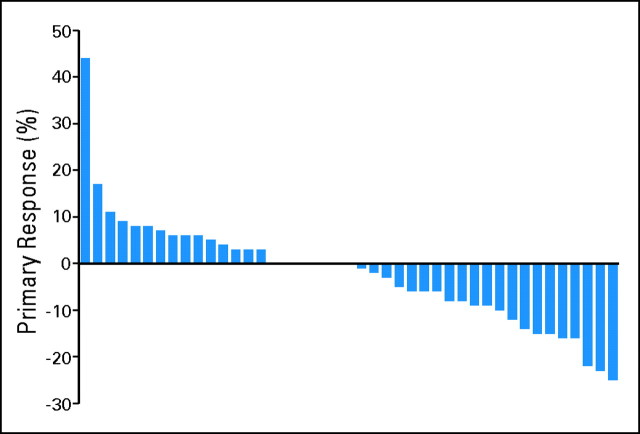
Primary tumor size reduction is listed in Table 2. Of the 45 patients with first restaging scans, 23 patients (52%) had some degree of tumor size reduction. None of the patients showed greater than 30% reduction in primary tumor size, but 23% of patients showed at least 10% reduction by using RECIST methodology after 8 weeks of therapy. Figure 2 shows a waterfall plot of primary tumor reduction after 8 weeks of therapy.
Fig 2.
Waterfall plot of best response in primary tumor site.
Pathology Findings
Two patients were found not to have metastases at nephrectomy. One had a presumed liver metastasis that was biopsied at nephrectomy and was proven benign. A second patient was found to have disseminated Hodgkin's disease but no mRCC. Both patients had T1 primary kidney tumors. When the patient with Hodgkin's disease was included as a biopsy failure, concordance between biopsy and nephrectomy specimens existed in 39 (93%) of 42 patients who underwent nephrectomy. Discordant findings in the remaining included two patients who had non–clear cell histology (n = 1 with papillary RCC; n = 1 with chromophobe RCC). In addition, eight patients (19%) had sarcomatoid elements in their nephrectomy specimens that were not detected on pretreatment biopsy. Pathologic tumor T stage is summarized in Table 2. Pretreatment histologic diagnoses were obtained via fine-needle aspiration in 43 patients, and core biopsies were obtained in seven patients. Biopsies were of primary kidney (n = 23), lung (n = 8), and other sites (n = 19).
Perioperative Outcomes and Complications
Perioperative outcomes are listed in Table 2. Median length of hospital stay was 5 days (range, 1 to 70 days), and median estimated operative blood loss was 400 mL (range, 0 to 7,000 mL). No intraoperative or perioperative complications attributable to study drug were reported. Two patients died within 2 months of nephrectomy. The first patient had a pT4N2 primary tumor that resulted in a prolonged and technically challenging operation; greater than 7,000 mL of blood required a 14-liter blood transfusion intraoperatively. Postoperatively, the patient developed an intra-abdominal infection and ultimately died 70 days postoperatively from a massive pulmonary embolus. The second patient had progressive disease at week 8 but proceeded with nephrectomy because of continued good performance status. She had a T4 tumor that resulted in a prolonged and technically challenging operation; then she developed multiorgan failure in the first postoperative week and died 4 weeks postoperatively.
Toxicity
Therapy-related toxicities are listed in Table 3. The most common adverse effect was hypertension, followed by diarrhea and anemia. A total of 10 patients (20%) came off study because of toxicity. Patients who did not receive erlotinib had lower rates of skin toxicities and diarrhea. Toxicities that resulted in treatment discontinuation are highlighted in Table 4.
Table 3.
Adverse Events
| Event | No. of Patients per Event Grade by Treatment Group |
|||||
|---|---|---|---|---|---|---|
| Bevacizumab and Erlotinib (n = 23) |
Bevacizumab Only (n = 27) |
|||||
| 1 to 2 | 3 to 4 | 5 | 1 to 2 | 3 to 4 | 5 | |
| Hypertension | 17 | 3 | 0 | 21 | 5 | 0 |
| Diarrhea | 24 | 6 | 0 | 9 | 0 | 0 |
| Anemia | 20 | 0 | 0 | 40 | 5 | 0 |
| Fatigue | 33 | 2 | 0 | 42 | 4 | 0 |
| Hyperglycemia | 23 | 1 | 0 | 33 | 2 | 0 |
| Elevated creatinine | 18 | 3 | 0 | 12 | 1 | 0 |
| Abdominal pain | 7 | 3 | 0 | 9 | 0 | 0 |
| Hypoalbuminemia | 7 | 0 | 0 | 14 | 3 | 0 |
| Nausea | 20 | 2 | 0 | 14 | 1 | 0 |
| Bone pain | 14 | 2 | 0 | 14 | 0 | 0 |
| Vomiting | 12 | 2 | 0 | 10 | 1 | 0 |
| Allergic reaction | 0 | 0 | 0 | 0 | 2 | 0 |
| Anorexia | 4 | 0 | 0 | 7 | 2 | 0 |
| Rash | 35 | 3 | 0 | 13 | 0 | 0 |
| Thrombus/embolus | 0 | 1 | 0 | 0 | 2 | 0 |
| Wound healing delay/dehiscence | 4 | 0 | 0 | 6 | 2 | 0 |
| Hyperbilirubinemia | 8 | 0 | 0 | 4 | 1 | 0 |
| Dehydration | 0 | 1 | 0 | 2 | 1 | 0 |
| Dyspnea | 7 | 0 | 0 | 5 | 2 | 0 |
| Hemorrhage | 16 | 0 | 0 | 5 | 2 | 0 |
| Hypotension | 0 | 1 | 0 | 0 | 0 | 0 |
| Appendicitis | 0 | 1 | 0 | 0 | 0 | 0 |
| Neuropathy | 2 | 0 | 0 | 1 | 1 | 0 |
| Diverticulitis | 0 | 1 | 0 | 0 | 0 | 0 |
| Pancreatitis | 0 | 1 | 0 | 0 | 0 | 0 |
| Hypophosphatemia | 1 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 0 | 1 | 0 | 0 | 0 | 0 |
| Hypokalemia | 0 | 0 | 0 | 1 | 1 | 0 |
| Proteinuria | 15 | 0 | 0 | 18 | 1 | 0 |
| Supraventricular arrhythmia | 0 | 0 | 0 | 1 | 1 | 0 |
| Hyperuricemia | 13 | 1 | 0 | 6 | 2 | 0 |
| Dysarthria | 4 | 0 | 0 | 2 | 1 | 0 |
| Weight gain | 2 | 1 | 0 | 1 | 0 | 0 |
| Death not associated with study drug | 0 | 0 | 2 | 0 | 0 | 1 |
Table 4.
Toxicities That Resulted in Treatment Discontinuation
| Toxicity | No. of Patients per Treatment Group |
|
|---|---|---|
| Bevacizumab/Erlotinib | Bevacizumab | |
| Appendicitis | 1 | 0 |
| Pancreatitis | 1 | 0 |
| Pneumonitis | 1 | 0 |
| Proteinuria | 0 | 1 |
| Diverticulitis | 1 | 0 |
| Hypertension | 1 | 0 |
| Pulmonary embolus | 0 | 1 |
| Delayed wound healing/dehiscence | 0 | 3 |
Nine patients (20.9%) demonstrated delayed wound healing 4 weeks postoperatively, and one patient experienced fascial dehiscence that required reoperation. In five patients, open areas were less than 3 cm in length and healed spontaneously with no treatment delay. Two patients experienced a treatment delay of 20 and 21 days, respectively, and eventually experienced complete wound healing on study. Two patients had grade 3, delayed wound healing that prevented them from restarting trial therapy. These patients started sunitinib 15 weeks and 20 weeks postoperatively, respectively. One patient developed fascial dehiscence 3 months after restarting bevacizumab therapy, and it required surgical intervention. Comparison to an historic cohort of 101 patients matched according to metastatic disease burden and primary tumor size was performed. Comparison of multiple perioperative and postoperative variables showed that delayed superficial wound healing was higher in the bevacizumab-treated group (20.9% v 2%; P < .001). Fascial dehiscence and perioperative death rates were not significantly different between the two groups.
DISCUSSION
This prospective, phase II study was performed to explore the hypothesis that presurgical treatment with antiangiogenic therapy can select for patients who will benefit from cytoreductive nephrectomy. Because of the relatively small sample size and the lack of randomization, definitive statements cannot be made about the hypothesis or the clinical outcomes observed in this study. However, in an intermediate- and poor-risk population of patients with large primary tumors, the 95% CIs of the observed PFS outcomes fall within the prospectively anticipated range of this patient group. Indeed, these OS data are comparable to contemporary studies in which patients are treated in the frontline setting.8,10 This was despite the poorer risk features of the patient group at baseline, the lack of preselection of patients by nephrectomy before enrollment, and the inclusion of the time to undergo and recover from nephrectomy in the PFS and OS data. The statistical design of this trial was challenging because of the presence of only one other trial that described outcomes in RCC after antiangiogenic therapy15 and because of the lack of information at the time this study was conceived on how presurgical therapy could influence outcomes.
As per study design, a total of six patients (12%) did not undergo nephrectomy because of clinical or radiographic progression and overall functional decline at the first restaging scans. Five of the six patients had at least three organ sites involved, and four patients had liver metastases. One might speculate that these individuals would have had better outcomes if they had undergone upfront nephrectomy. However, it is equally likely that these patients would not have fared well in the postoperative period because of rapid tumor growth. All six patients were switched to alternate therapies, but no patient achieved disease response or stabilization with salvage systemic therapy, and none ultimately underwent nephrectomy. The disease feature most plausibly associated with poorer outcome was the presence of liver metastases. It is important to note that, of the seven patients on the study with liver metastases, only three underwent nephrectomy.
Concern has been raised about the safety of administering antiangiogenic therapy to patients with scheduled surgical intervention. In this study, the incidence of wound dehiscence or delayed wound healing was 20.9%. This rate is higher than those published in other studies5 and is higher than historic data from MDACC. These findings clearly need to be considered when presurgical bevacizumab therapy for patients with mRCC is evaluated. In addition, two patients died in the postoperative period. Both of these patients had T4 primary tumors, and both patients had protracted and difficult surgeries. The perioperative mortality rate was not significantly different from the control group and speaks to the surgical challenges in patients with larger primary tumors. For patients with radiographic evidence of T4 disease, continued treatment with systemic therapy until a stage reduction is accomplished may reduce perioperative risk.
Few data exist on the impact of antiangiogenic therapy on the primary RCC tumor. A report by van der Veldt et al16 describes primary tumor response in a retrospective analysis of 17 patients who received sunitinib therapy. Four patients experienced a 30% or greater response, 12 experienced stable disease, and one experienced tumor growth.16 Additionally, a case report of vena cava thrombus regression after therapy with sunitinib was reported in the literature.17 In this study, all but one patient demonstrated stable disease by RECIST, and greater than 50% of RCC primaries from the patients in this study showed some degree of tumor size reduction. Whether a small amount of size reduction facilitates surgical management is doubtful, but it does point toward a possible paradigm shift if a higher degree of cytoreduction could be achieved with systemic therapy. We hypothesize that, when robust stage reduction is consistently achieved, surgical difficulty likely will be commensurately reduced. The optimal timing of nephrectomy in patients also is not known. A trial to perform nephrectomy at time of maximal cytoreduction of the primary tumor could be considered.
Because all patients underwent an upfront diagnostic biopsy, some observations can be made on the accuracy of the pathologic diagnosis made by using fine-needle biopsies, which were performed in the large majority of the patients on study entry. Three patients were given a diagnosis other than clear cell RCC at the time of pathologic review of the nephrectomy specimen. Of those three, one patient had a correctly diagnosed clear cell RCC primary, but the presumed metastatic disease was determined to be Hodgkin's disease. The remaining two patients had papillary and chromophobe RCC, respectively. Additionally, a total of eight patients demonstrated previously undocumented sarcomatoid features. These findings underscore the general accuracy of needle biopsies used for diagnostic purposes in RCC but also point out the limitations from a standpoint of subcategorization of tumor histology and treatment assignment.
This trial underwent the unanticipated modification of removing erlotinib from the treatment regimen after the first 23 patients were treated. As described above, the presence or absence of erlotinib did not have a significant effect on PFS or OS in univariable or multivariable Cox models. Future tissue-based studies will answer the question of whether there are pharmacodynamic changes present in the nephrectomy specimens that reflect the addition of epidermal growth factor blockade to the regimen.
In conclusion, we show that presurgical treatment of patients who have mRCC with antiangiogenic therapy meets predetermined outcome measures and is associated with an increased rate of delayed wound healing compared with historical controls. The questions of whether this treatment algorithm is superior to upfront nephrectomy and whether nephrectomy is required in patients with mRCC who are treated with antiangiogenic therapy will require prospective, randomized trials. Phase III trials are being planned or are underway to address some of these questions. A clinical trial for patients with newly diagnosed mRCC that randomly assigns individuals between upfront nephrectomy and delayed nephrectomy will test the following hypotheses: that pretreatment with systemic therapy selects for patients most likely to benefit from surgery, and that pretreatment with systemic therapy increases the number of patients who benefit from surgery by decreasing the impact of disease burden on postsurgical recovery.
Appendix
Fig A1.
Progression-free survival and 95% CI (N = 50).
Fig A2.
Overall survival and 95% CI (N = 50).
Footnotes
Supported by Genentech.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Eric Jonasch, Genentech (C); Christopher G. Wood, Pfizer (C), Ethicon (C) Stock Ownership: None Honoraria: Christopher G. Wood, Pfizer, Ethicon Research Funding: Eric Jonasch, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eric Jonasch, Christopher G. Moore, Surena F. Matin, Nizar Tannir
Administrative support: Eric Jonasch, Nizar Tannir
Provision of study materials or patients: Eric Jonasch, Christopher G. Wood, Surena F. Matin, Shi-Ming Tu, Lance C. Pagliaro, Ana Aparicio, John C. Araujo, Wadih Arap, Nizar Tannir
Collection and assembly of data: Eric Jonasch, Christopher G. Wood, Xuemei Wang, Nizar Tannir
Data analysis and interpretation: Randall E. Millikan, Nizar Tannir
Manuscript writing: Eric Jonasch, Christopher G. Wood, Surena F. Matin, Paul G. Corn, Randall E. Millikan, Wadih Arap, Nizar Tannir
Final approval of manuscript: Eric Jonasch, Christopher G. Wood, Surena F. Matin, Shi-Ming Tu, Lance C. Pagliaro, Paul G. Corn, Ana Aparicio, Pheroze Tamboli, Randall E. Millikan, Xuemei Wang, John C. Araujo, Wadih Arap, Nizar Tannir
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25:4536–4541. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 3.Pyrhönen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol. 1999;17:2859–2867. doi: 10.1200/JCO.1999.17.9.2859. [DOI] [PubMed] [Google Scholar]
- 4.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 5.Margulis V, Matin SF, Tannir N, et al. Surgical morbidity associated with administration of targeted molecular therapies before cytoreductive nephrectomy or resection of locally recurrent renal cell carcinoma. J Urol. 2008;180:94–98. doi: 10.1016/j.juro.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Vogelzang NJ, Dumas MC, et al. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18:2419–2426. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 10.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 11.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 12.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 13.Bex A, Horenblas S, Meinhardt W, et al. The role of initial immunotherapy as selection for nephrectomy in patients with metastatic renal cell carcinoma and the primary tumor in situ. Eur Urol. 2002;42:570–574. doi: 10.1016/s0302-2838(02)00404-9. discussion 575-576. [DOI] [PubMed] [Google Scholar]
- 14.Gruenberger B, Scheithauer W, Punzengruber R, et al. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. doi: 10.1186/1471-2407-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. Sunitinib for treatment of advanced renal cell cancer: Primary tumor response. Clin Cancer Res. 2008;14:2431–2436. doi: 10.1158/1078-0432.CCR-07-4089. [DOI] [PubMed] [Google Scholar]
- 17.Shuch B, Riggs SB, LaRochelle JC, et al. Neoadjuvant targeted therapy and advanced kidney cancer: Observations and implications for a new treatment paradigm. BJU Int. 2008;102:692–696. doi: 10.1111/j.1464-410X.2008.07660.x. [DOI] [PubMed] [Google Scholar]