Abstract
After the first observation of the immunosuppressive effects of ultraviolet (UV) irradiation was reported in 1974, therapeutic modification of immune responses by UV irradiation began to be investigated in the context immunization. UV-induced immunosuppression is via the action of regulatory T cells (Tregs). Antigen-specific Tregs were induced by high-dose UV-B irradiation before antigen immunization in many studies, as it was considered that functional alteration and/or modulation of antigen-presenting cells by UV irradiation was required for the induction of antigen-specific immunosuppression. However, it is also reported that UV irradiation after immunization induces antigen-specific Tregs. UV-induced Tregs are also dominantly transferable, with interleukin-10 being important for UV-induced immunosuppression. Currently, various possible mechanisms involving Treg phenotype and cytokine profile have been suggested. UV irradiation accompanied by alloantigen immunization induces alloantigen-specific transferable Tregs, which have potential therapeutic applications in the transplantation field. Here we review the current status of UV-induced antigen-specific immunosuppression on the 40th anniversary of its discovery.
Keywords: Alloantigen, Ultraviolet irradiation, Donor-specific immunosuppression, Interleukin-10, Regulatory T cells
Core tip: The perception of immunological changes induced by ultraviolet (UV) exposure has changed over the past several years. Although carcinogenesis and immunosuppression due to UV irradiation are regarded as detrimental, UV irradiation is also currently considered a useful tool to induce alloantigen-specific regulatory T cells (Tregs). There is great enthusiasm for the potential to develop strategies that can use Tregs for therapeutic interventions. Alloantigen-specific immunosuppression is an ideal therapy for allotransplant recipients. Although the full mechanism has yet to be determined, UV irradiation accompanied by alloantigen immunization produces a beneficial effect in transplant immunity via the induction of alloantigen-specific transferable Tregs.
INTRODUCTION
Intermittent exposure to ultraviolet (UV) light, especially the mid-wave range (UV-B, 280-320 nm), is an important environmental factor affecting human health[1]. Although primary carcinogenesis is the most common problem[2], UV irradiation also impairs immune responses to oncogenic and infectious antigens[3,4]. Paradoxically, the immunosuppressive effects induced by UV irradiation may have therapeutic potential[5-8].
Immunosuppressants have revolutionized clinical transplantation, but have many side effects including pan-immunosuppression[9]. Infectious complications are mostly fatal for transplant recipients[10]. After organ transplantation, patients on immunosuppressants face a dilemma between infectious morbidity and graft rejection. Therefore, alloantigen-specific immunosuppression is an ideal therapy for transplant recipients[11,12]. Research has focused on the immune modulating effects of UV-B irradiation in conjunction with alloantigen immunization to induce donor alloantigen-specific immunosuppression.
Here, we review the application of UV irradiation accompanied by alloantigen immunization to induce alloantigen-specific immunosuppression and discuss the therapeutic potential of UV-induced regulatory T cells (Tregs) in the transplant immunology field.
HISTORY AND BACKGROUND
The initial observations on the immunosuppressive effects of UV irradiation were documented in 1974[13]. Thereafter, many researchers have pushed the frontiers of photopheresis and photoimmunosuppression. Two models of contact hypersensitivity and delayed-type hypersensitivity have been developed to clarify the immunological mechanisms involving UV irradiation[14-18]. The therapeutic capacity of UV irradiation to modify immune responses began to be investigated in the late 1970s[13,19,20]. By the late 1980s, many researchers had reported that antigen-specific Tregs were induced by high-dose UV-B irradiation before antigen immunization[15,21,22]. At this time it was thought that functional alteration and/or modulation of antigen-presenting cells (APCs) by UV irradiation was required for the induction of antigen-specific immunosuppression[23].
METHODOLOGY FOR SUCCESSFUL UV-INDUCED IMMUNE EFFECTS
Many researchers have used mice in their studies on the immunosuppressive effect of UV irradiation. Animal care during and after UV irradiation is critically important for successful UV irradiation experiments[24,25]. Murine skin must be carefully shaved without any injuries. To prevent unevenness of UV irradiation, mice are anesthetized during UV exposure with their feet fixed to a metal plate. Thus, the shaved abdominal wall is sufficiently extended with even exposure to the UV lamps. Therefore careful shaving of the irradiation area and adequate anesthesia and restraint are very important for stable UV irradiation with even exposure. If challenge with antigen or graft beds for transplantation is required after UV irradiation, these sites should be protected from UV irradiation. High-dose UV-B exposure is very damaging, therefore post-irradiation care is also crucial. Adequate analgesic medication is thus a serious requirement after UV irradiation. UV-irradiated mice should be placed in separate cages to avoid scratching of irradiated skin by cage mates. They are also fed with a supply of Ringer’s lactate solution. As irradiated skin undergoes contraction to become scar tissue, post-irradiated stiffening severely restricts movement and activity in mice. Therefore, some ingenuity to prevent unexpected death and post-irradiation dehydration (such as a raised floor for easy access to food and water and availability of gels containing sugar, water and dietary supplements to ensure a steady supply of nutrients and water) is required.
ALTERATION AND/OR MODULATION OF APC FUNCTION BY UV IRRADIATION
UV irradiation alters APC function[23]. UV-induced DNA damage has been recognized as the major molecular trigger for photoimmunosuppression[26-28]. Interleukin (IL)-12 reduces DNA damage and prevents the generation of UV-induced Tregs[28,29]. Langerhans cells (LCs) were initially regarded as the most important APC in the epidermis[18,30,31], and it was believed that LCs were killed by UV irradiation. However, it is now accepted that the primary APC in the skin is not LC but dermal dendritic cell (DC)[32-34], and UV irradiation destroys the DC network of LC in the skin[31]. LCs appear to be involved in down-regulating immune responses[35], and inducing and activating Tregs[36,37]. Recently, the functional role of LCs was redefined, and it was shown that UV-damaged LCs in the regional lymph nodes were required for Treg induction[28]. Damaged but viable LCs will present antigen in a nonprofessional manner, which will induce Tregs rather than effector T cells[27].
ANTIGEN-SPECIFIC IMMUNOSUPPRESSION
Many researchers have reported that antigen-specific Tregs were induced by high-dose UV-B irradiation prior to antigen immunization[15,21,22]. At the time it was thought that UV-induced functional alteration and/or modulation of APC function was required for the induction of antigen-specific immunosuppression[23]. This may explain why previous researchers documented that antigen immunization must follow UV-B irradiation and not vice versa[15,21,22]. However, the successful use of UV irradiation after antigen immunization has also been reported[24,25,38-41]. In both models, with UV irradiation before or after antigen immunization, antigen presentation in a nonprofessional manner is the key to inducing antigen-specific Tregs[23,27].
UV-induced Tregs and their phenotypes
UV-induced antigen-specific immunosuppression is attributable to T cells with suppressive activity (formerly called, “suppressor T cells”)[42,43], and currently these T cells are referred to as Tregs[17,44,45]. A number of studies have investigated the phenotype and mechanism of UV-induced Tregs. UV-induced Tregs express CD4, CD25 and CTLA4[17,46,47] and the lymph node-homing receptor CD62L and therefore migrate into the lymph nodes[46,48].
The early inflammatory phase in the skin has been well studied[49]. When we investigate UV-induced Treg subsets, the role of natural killer T (NKT) cells and mast cells should also be considered. NKT cells are a unique class of T cells. They express T-cell receptor molecules and co-express surface antigens normally found on natural killer (NK) cells. NKT cells have a critical role in UV-induced tumor immune responses[37,50], and they appear to be dependent on IL-4[37]. Researchers have also focused on the role of mast cells in UV-induced immunosuppression[51,52]. Although mast cells were formerly ignored in the field of UV-induced immunosuppression, it has been suggested that they may have immunosuppressive potential[53]. The concept that LCs, mast cells and NKT cells can act in an unconventional manner is now well accepted in the communities of photobiology and immunology[26,27]. The LCs transmit an immunosuppressive signal from the skin to lymph nodes, where they activate NKT cells to secrete regulatory cytokines[26].
Dominant transferability of UV-induced Tregs
As described above, UV irradiation accompanied by antigen immunization induces antigen-specific Tregs. Moreover, these Tregs are dominantly transferable[19]. This transferability confirms that UV-induced immunosuppression is mediated by Tregs. Moreover, this transferability is an advantage for alloantigen-specific immunosuppression in the transplantation field as UV-induced Tregs dominantly have the same immune effect in recipients[24,25].
Role of cytokine milieu
CD4+ Th2 lymphocytes secrete pro-inflammatory cytokines (IL-4, IL-5 and IL-13)[54,55]. IL-4 is thought to promote the induction of transplantation tolerance and alloantigen-specific Tregs[56]. IL-4 also promotes both regulatory and effector T cells in the initial immune response. Moreover, IL-4 activation of effector cells can mediate rejection and will not support alloantigen-specific Tregs that could transfer specific tolerance[56]. Transforming growth factor (TGF)-β is a growth and differentiation factor that displays multiple functions[57]. It is known that the combined use of IL-10 and TGF-β effectively generates CD4+ Tregs[57,58].
Immunosuppression induced by UV irradiation and immunization is dependent on CD4+ Tregs[59-61] and cytokines play an important role[17,62]. The immunosuppressive effects induced by UV irradiation before immunization were explained by a shift in the activation of T cells from a Th1 to a Th2 immune response[63-67]. However, alloantigen-specific immunosuppression induced by UV irradiation after immunization depends not on IL-4, IL-5, IL-13 or TGF-β but on IL-10[24,25,38-41]. Thus, the mechanism of immunosuppression by UV irradiation after immunization cannot be simply explained only by a Th2 shift[24,25,38-41].
Role of IL-10
IL-10 is a well-known immunosuppressive cytokine[68,69], and is important for UV-induced immunosuppression[70-73]. The inhibitory capacity of UV-induced Tregs depends on IL-10 expression[46]. Antigen-specific activation of Tregs by APCs induces the release of IL-10[46,47], which mediates the inhibitory activity of UV-induced Tregs[47,74]. The source of IL-10 in UV-induced immunosuppression is therefore UV-induced Tregs themselves[17,71], although mast cell[52,75] and CD11b+ macrophages[76] have also been suggested. IL-10 is crucial for both the induction[25,72,77] and effector phases[46,78] of UV-induced Tregs, though some researchers reported that IL-10 is not required for Treg induction by UV irradiation[73].
CD4+ T cells with cytokine profiles displaying a large amount of IL-10, but no IL-4, are labelled regulatory T cell type 1 cells (Tr1)[79]. The presence of IL-10 gives rise to CD4+ T-cell clones with a low proliferative capacity that in turn produce high levels of IL-10, low levels of IL-2 and no IL-4[69,79]. These antigen-specific T-cell clones suppress the proliferation of effector CD4+ T cells in response to antigen[69,79]. Thus, IL-10 drives the generation of a CD4+ T-cell subset, designated Tr1, which suppresses antigen-specific immune responses and actively down-regulates pathological immune responses in vivo[69,79]. As described above, UV irradiation before immunization induces CD4+ Tregs, and resulted in a shift to Th2 immune response[63-67], and IL-10 plays an important role in this. However, in immunosuppression induced by UV irradiation after immunization, CD4+ Tr1-like cells with high expression of IL-10 are important for alloantigen-specific immunosuppression[24,25,38-41].
Panoptic finding in alloantigen-specific immunosuppressiton induced by UV-B irradiation
As described above, UV-B irradiation accompanied with alloantigen immunization is a useful tool to induce alloantigen-specific immunosuppression. Here, we reviewed previous documents which have described the possible mechanisms in achieving alloantigen-specific immunosuppression induced by UV-B irradiation[17,26,27,38,39,44,50,72], and summarized the postulated reactions in Figure 1.
Figure 1.
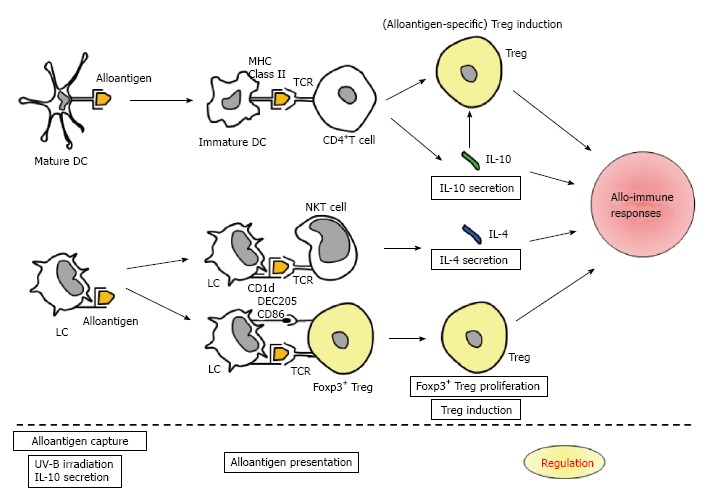
Schema illustrating the postulated reactions in achieving alloantigen-specific immunosuppression by ultraviolet-B irradiation accompanied with alloantigen immunization. APCs, such as mature DC and LC, capture alloantigen. UV-B irradiation and subsequent IL-10 secretion will cause antigen presentation in a nonprofessional manner to induce antigen-specific immunosuppression. Immature DC presents alloantigen to CD4+ T cell, and then, Treg induction and IL-10 secretion arise. LC presents alloantigen to NKT cell, and IL-4 secretion subsequently occurs. Also, LC presents alloantigen to Foxp3+ Treg, and thereafter, Foxp3+ Treg proliferation and Treg induction are triggered. Hence, alloantigen-specific Treg, Foxp3+ Treg, IL-10 and IL-4 will regulate allo-immune responses. MHC: Major histocompatibility complex; TCR: T cell receptor; CD: Cluster of differentiation; DEC: Dendritic and epithelial cells; APCs: Antigen-presenting cells; UV: Ultraviolet; DC: Dendritic cell; LC: Langerhans cell; IL: Interleukin; NKT: Natural killer T.
In brief, APCs, such as mature DC and LC, capture alloantigen. UV-B irradiation and subsequent IL-10 secretion will cause antigen presentation in a nonprofessional manner to induce antigen-specific immunosuppression[23,27]. Immature DC presents alloantigen to CD4+T cell, and then, Treg induction and IL-10 secretion arise. LC presents alloantigen to NKT cell, and IL-4 secretion subsequently occurs. Also, LC presents alloantigen to Foxp3+Treg, and thereafter, Foxp3+Treg proliferation and Treg induction are triggered. Hence, alloantigen-specific Treg, Foxp3+Treg, IL-10 and IL-4 will regulate allo-immune responses.
So-called "bystander immunosuppression" or "linked suppression"
UV-induced Tregs will demonstrate unique behavior referred to as “bystander suppression”. Antigen specificity appears to be restricted to the activation of UV-induced Tregs and not to the suppressive activity itself, as once activated by their cognate antigen, they release IL-10 and thereby suppress other immune reactions nonspecifically[46,80]. Previous researchers also demonstrated a rigor rule for activation of UV-induced Tregs[46,71,80]. Migratory behavior of UV-induced Tregs can be reprogrammed by APCs[81], and UV-induced Tregs switch APCs from a stimulatory to a regulatory phenotype[81]. This alteration of APC function may help to explain bystander suppression. In summary, once IL-10 is released upon antigen-specific activation by UV-induced Tregs, IL-10 suppresses other immune responses in a nonspecific fashion through bystander suppression[74]. The therapeutic potential for Tregs generated in response to antigens that are not necessarily the same antigen driving the pathogenic process has been reported in the literature[74,80].
Possibilities for clinical use, and some future perspectives in human
The view of photoimmunology has changed over the past several years[26,27]. The mechanisms involved are much more complex than those many researchers initially thought. The skin is an organ close to immunity, and many autoimmune diseases affect the skin. One of the best routes to immunize is via the skin. The majority of these reactions are T cell-driven[82]. Therefore, many researchers focused on the tentative theory that UV-induced T cells may not always be beneficial, but more often harmful[26,27]. Nowadays, many researchers assume that a fine-tuned balance is optimal[26,27]. Hence, suppression may be as relevant as induction, and replacing the negatively perceived term “suppression” with “regulation” is preferable[17].
Clinical physicians recognized that UV-induced immunosuppression has a therapeutic potential in human, and therefore, UV-irradiation itself have been already applied for actual clinical use[5-8]. Experimental studies demonstrated that UV-induced immunosuppression supports the exacerbation of skin infections and the suppression of T-cell reactions against microbial antigens[83]. However, the clinical experience differs. The risk for infections, in particular bacterial infections, after UV-B exposure is low[27]. Atopic dermatitis is frequently superinfected with Staphylococcus aureus, but can be improved by UV-B irradiation even without antiseptic or antibiotic measures.
A strong association of UV-susceptible and UV-resistant phenotypes in humans with single-nucleotide polymorphisms in the tumor necrosis factor region was found, suggesting this region to contain genes that determine the outcome of an UV response[84]. Human volunteers developed tolerance when the hapten was initially painted onto UV-treated skin[85]. UV-B irradiation not only depleted LCs but also induced CD11b+ macrophages, which released IL-10[76].
Experimental studies demonstrated that high-dose UV-B irradiation accompanied with antigen immunization is required for antigen-specific Tregs. From the viewpoint of transplant immunity, a simple question arises. How do we establish an actual regimen without severe rejection and intractable infection? Moreover, the development of tolerance versus suppressed contact hypersensitivity appears to correlate with the timing of antigen application after UV-B exposure[27]. How do we consolidate the timing of alloantigen immunization, in an emergent case of available allograft from a deceased-donor. Hence, in current status, translational researched and clinical trials are seriously required, and we should carefully attempt those studies for the further developments.
CONCLUSION
Paradoxically, although high-dose UV exposure is toxic, it is suggested that photopheresis and photoimmunosuppression may have therapeutic potential. The perception of UV-induced immunological changes has thus changed over the past several years[26,27]. Carcinogenesis and immunosuppression due to UV irradiation were regarded as detrimental; however, a finely-tuned therapeutic dose may be possible[26,27]. To induce alloantigen-specific transferable CD4+ Tregs, UV irradiation is a very useful tool[15,19,21,22,24,25,39-41]. Clinically, there is great enthusiasm for the potential to develop strategies that can use Tregs for therapeutic interventions[71]. Alloantigen-specific immunosuppression is an ideal therapy for transplant recipients[86-88]. Although the full mechanism has not yet been determined, UV irradiation accompanied by alloantigen immunization to induce alloantigen-specific Tregs may have great benefits in the transplant immunology field.
Footnotes
P- Reviewer: Fujino Y, Sugawara Y S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Conflict-of-interest: The authors have no financial conflicts of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 18, 2014
First decision: October 28, 2014
Article in press: January 19, 2015
References
- 1.Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 2.Osmola-Mańkowska A, Silny W, Dańczak-Pazdrowska A, Olek-Hrab K, Mańkowski B, Osmola K, Hojan-Jezierska D, Kubisz L. The sun--our friend or foe? Ann Agric Environ Med. 2012;19:805–809. [PubMed] [Google Scholar]
- 3.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz T. Mechanisms of UV-induced immunosuppression. Keio J Med. 2005;54:165–171. doi: 10.2302/kjm.54.165. [DOI] [PubMed] [Google Scholar]
- 5.Norval M. The challenges of UV-induced immunomodulation for children’s health. Prog Biophys Mol Biol. 2011;107:323–332. doi: 10.1016/j.pbiomolbio.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Norval M, Halliday GM. The consequences of UV-induced immunosuppression for human health. Photochem Photobiol. 2011;87:965–977. doi: 10.1111/j.1751-1097.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 7.Norval M, Woods GM. UV-induced immunosuppression and the efficacy of vaccination. Photochem Photobiol Sci. 2011;10:1267–1274. doi: 10.1039/c1pp05105a. [DOI] [PubMed] [Google Scholar]
- 8.Ullrich SE. Ultraviolet carcinogenesis and Immune suppression. Weber R, Moore B, editors. Cutaneous Malignancy of the Head and Neck: A Multidisciplinary Approach. San Diego: Plural Publishing; 2011. pp. 57–78. [Google Scholar]
- 9.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191:5785–5791. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 10.Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. 2011;17 Suppl 3:S34–S37. doi: 10.1002/lt.22378. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham EC, Sharland AF, Bishop GA. Liver transplant tolerance and its application to the clinic: can we exploit the high dose effect? Clin Dev Immunol. 2013;2013:419692. doi: 10.1155/2013/419692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers NM, Isenberg JS, Thomson AW. Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance? Am J Transplant. 2013;13:1125–1133. doi: 10.1111/ajt.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 14.Aubin F, Mousson C. Ultraviolet light-induced regulatory (suppressor) T cells: an approach for promoting induction of operational allograft tolerance? Transplantation. 2004;77:S29–S31. doi: 10.1097/01.TP.0000112969.24120.64. [DOI] [PubMed] [Google Scholar]
- 15.Magee MJ, Kripke ML, Ullrich SE. Suppression of the elicitation of the immune response to alloantigen by ultraviolet radiation. Transplantation. 1989;47:1008–1013. doi: 10.1097/00007890-198906000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Nghiem DX, Walterscheid JP, Kazimi N, Ullrich SE. Ultraviolet radiation-induced immunosuppression of delayed-type hypersensitivity in mice. Methods. 2002;28:25–33. doi: 10.1016/s1046-2023(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 18.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 19.Fisher MS, Kripke ML. Further studies on the tumor-specific suppressor cells induced by ultraviolet radiation. J Immunol. 1978;121:1139–1144. [PubMed] [Google Scholar]
- 20.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullrich SE. Suppression of the immune response to allogeneic histocompatibility antigens by a single exposure to ultraviolet radiation. Transplantation. 1986;42:287–291. doi: 10.1097/00007890-198609000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Ullrich SE, Magee M. Specific suppression of allograft rejection after treatment of recipient mice with ultraviolet radiation and allogeneic spleen cells. Transplantation. 1988;46:115–119. doi: 10.1097/00007890-198807000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Greene MI, Sy MS, Kripke M, Benacerraf B. Impairment of antigen-presenting cell function by ultraviolet radiation. Proc Natl Acad Sci USA. 1979;76:6591–6595. doi: 10.1073/pnas.76.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori T, Kuribayashi K, Uemoto S, Saito K, Wang L, Torii M, Shibutani S, Taniguchi K, Yagi S, Iida T, et al. Alloantigen-specific prolongation of allograft survival in recipient mice treated by alloantigen immunization following ultraviolet-B irradiation. Transpl Immunol. 2008;19:45–54. doi: 10.1016/j.trim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Hori T, Kuribayashi K, Saito K, Wang L, Torii M, Uemoto S, Kato T. Induction of alloantigen-specific CD4+ T regulatory Type 1 cells by alloantigen immunization and ultraviolet-B irradiation: a pilot study in murine transplantation models with skin and cardiac allografts. Ann Transplant. 2014;19:519–536. doi: 10.12659/AOT.890890. [DOI] [PubMed] [Google Scholar]
- 26.Ullrich SE, Byrne SN. The immunologic revolution: photoimmunology. J Invest Dermatol. 2012;132:896–905. doi: 10.1038/jid.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz T, Beissert S. Milestones in photoimmunology. J Invest Dermatol. 2013;133:E7–E10. doi: 10.1038/skinbio.2013.177. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarz A, Ständer S, Berneburg M, Böhm M, Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J, Schwarz T. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 30.Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol. 2012;132:872–881. doi: 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- 31.Streilein JW, Toews GT, Gilliam JN, Bergstresser PR. Tolerance or hypersensitivity to 2,4-dinitro-1-fluorobenzene: the role of Langerhans cell density within epidermis. J Invest Dermatol. 1980;74:319–322. doi: 10.1111/1523-1747.ep12543557. [DOI] [PubMed] [Google Scholar]
- 32.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 33.Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J Immunol. 2008;180:3057–3064. doi: 10.4049/jimmunol.180.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein P, Rechtsteiner G, Warger T, Bopp T, Fuhr T, Prüfer S, Probst HC, Stassen M, Langguth P, Schild H, et al. UV exposure boosts transcutaneous immunization and improves tumor immunity: cytotoxic T-cell priming through the skin. J Invest Dermatol. 2011;131:211–219. doi: 10.1038/jid.2010.254. [DOI] [PubMed] [Google Scholar]
- 35.Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz A, Noordegraaf M, Maeda A, Torii K, Clausen BE, Schwarz T. Langerhans cells are required for UVR-induced immunosuppression. J Invest Dermatol. 2010;130:1419–1427. doi: 10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 37.Fukunaga A, Khaskhely NM, Ma Y, Sreevidya CS, Taguchi K, Nishigori C, Ullrich SE. Langerhans cells serve as immunoregulatory cells by activating NKT cells. J Immunol. 2010;185:4633–4640. doi: 10.4049/jimmunol.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato T, Wang L. UV-induced immune suppression that promotes skin cancer development and progression. In: La Porta C., editor. Skin cancers-Risk factors, Prevention and Therapy. Rijeka: InTech; 2011. pp. 27–52. [Google Scholar]
- 39.Toda M, Wang L, Ogura S, Torii M, Kurachi M, Kakimi K, Nishikawa H, Matsushima K, Shiku H, Kuribayashi K, et al. UV irradiation of immunized mice induces type 1 regulatory T cells that suppress tumor antigen specific cytotoxic T lymphocyte responses. Int J Cancer. 2011;129:1126–1136. doi: 10.1002/ijc.25775. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Saito K, Toda M, Hori T, Torii M, Ma N, Katayama N, Shiku H, Kuribayashi K, Kato T. UV irradiation after immunization induces type 1 regulatory T cells that suppress Th2-type immune responses via secretion of IL-10. Immunobiology. 2010;215:124–132. doi: 10.1016/j.imbio.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Toda M, Saito K, Hori T, Horii T, Shiku H, Kuribayashi K, Kato T. Post-immune UV irradiation induces Tr1-like regulatory T cells that suppress humoral immune responses. Int Immunol. 2008;20:57–70. doi: 10.1093/intimm/dxm124. [DOI] [PubMed] [Google Scholar]
- 42.Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J Exp Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 44.Loser K, Beissert S. Regulatory T cells: banned cells for decades. J Invest Dermatol. 2012;132:864–871. doi: 10.1038/jid.2011.375. [DOI] [PubMed] [Google Scholar]
- 45.Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J Invest Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 46.Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, Beissert S, Vestweber D, Schwarz T. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz A, Beissert S, Grosse-Heitmeyer K, Gunzer M, Bluestone JA, Grabbe S, Schwarz T. Evidence for functional relevance of CTLA-4 in ultraviolet-radiation-induced tolerance. J Immunol. 2000;165:1824–1831. doi: 10.4049/jimmunol.165.4.1824. [DOI] [PubMed] [Google Scholar]
- 48.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190:970–976. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamatas GN, Morello AP, Mays DA. Early inflammatory processes in the skin. Curr Mol Med. 2013;13:1250–1269. doi: 10.2174/15665240113139990047. [DOI] [PubMed] [Google Scholar]
- 50.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 51.Byrne SN, Limón-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–4655. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alard P, Kurimoto I, Niizeki H, Doherty JM, Streilein JW. Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin requires mast cell degranulation. Eur J Immunol. 2001;31:1736–1746. doi: 10.1002/1521-4141(200106)31:6<1736::aid-immu1736>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 53.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Köhler A, Peschke K, Vöhringer D, Waskow C, Krieg T, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 54.Blom L, Poulsen LK. In vitro Th1 and Th2 cell polarization is severely influenced by the initial ratio of naïve and memory CD4+ T cells. J Immunol Methods. 2013;397:55–60. doi: 10.1016/j.jim.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55 Suppl 61:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 56.Plain KM, Verma ND, Tran GT, Nomura M, Boyd R, Robinson CM, Hodgkinson SJ, Hall BM. Cytokines affecting CD4(+) T regulatory cells in transplant tolerance. Interleukin-4 does not maintain alloantigen specific CD4(+)CD25(+) Treg. Transpl Immunol. 2013;29:51–59. doi: 10.1016/j.trim.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 58.Cottrez F, Groux H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J Immunol. 2001;167:773–778. doi: 10.4049/jimmunol.167.2.773. [DOI] [PubMed] [Google Scholar]
- 59.Mottram PL, Mirisklavos A, Clunie GJ, Noonan FP. A single dose of UV radiation suppresses delayed type hypersensitivity responses to alloantigens and prolongs heart allograft survival in mice. Immunol Cell Biol. 1988;66(Pt 5-6):377–385. doi: 10.1038/icb.1988.49. [DOI] [PubMed] [Google Scholar]
- 60.Krasteva M, Aubin F, Laventurier S, Kehren J, Assossou O, Kanitakis J, Kaiserlian D, Nicolas JF. MHC class II-KO mice are resistant to the immunosuppressive effects of UV light. Eur J Dermatol. 2002;12:10–19. [PubMed] [Google Scholar]
- 61.Nghiem DX, Kazimi N, Mitchell DL, Vink AA, Ananthaswamy HN, Kripke ML, Ullrich SE. Mechanisms underlying the suppression of established immune responses by ultraviolet radiation. J Invest Dermatol. 2002;119:600–608. doi: 10.1046/j.1523-1747.2002.01845.x. [DOI] [PubMed] [Google Scholar]
- 62.Walterscheid JP, Nghiem DX, Ullrich SE. Determining the role of cytokines in UV-induced immunomodulation. Methods. 2002;28:71–78. doi: 10.1016/s1046-2023(02)00212-8. [DOI] [PubMed] [Google Scholar]
- 63.Ullrich SE. Does exposure to UV radiation induce a shift to a Th-2-like immune reaction? Photochem Photobiol. 1996;64:254–258. doi: 10.1111/j.1751-1097.1996.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 64.Leitenberger J, Jacobe HT, Cruz PD. Photoimmunology--illuminating the immune system through photobiology. Semin Immunopathol. 2007;29:65–70. doi: 10.1007/s00281-007-0063-6. [DOI] [PubMed] [Google Scholar]
- 65.Garssen J, Vandebriel RJ, De Gruijl FR, Wolvers DA, Van Dijk M, Fluitman A, Van Loveren H. UVB exposure-induced systemic modulation of Th1- and Th2-mediated immune responses. Immunology. 1999;97:506–514. doi: 10.1046/j.1365-2567.1999.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dupont E, Craciun L. UV-induced immunosuppressive and anti-inflammatory actions: mechanisms and clinical applications. Immunotherapy. 2009;1:205–210. doi: 10.2217/1750743X.1.2.205. [DOI] [PubMed] [Google Scholar]
- 67.Shreedhar VK, Pride MW, Sun Y, Kripke ML, Strickland FM. Origin and characteristics of ultraviolet-B radiation-induced suppressor T lymphocytes. J Immunol. 1998;161:1327–1335. [PubMed] [Google Scholar]
- 68.Miyamoto T, Kaneko T, Yamashita M, Tenda Y, Inami M, Suzuki A, Ishii S, Kimura M, Hashimoto K, Shimada H, et al. Prolonged skin allograft survival by IL-10 gene-introduced CD4 T cell administration. Int Immunol. 2005;17:759–768. doi: 10.1093/intimm/dxh256. [DOI] [PubMed] [Google Scholar]
- 69.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beissert S, Hosoi J, Kühn R, Rajewsky K, Müller W, Granstein RD. Impaired immunosuppressive response to ultraviolet radiation in interleukin-10-deficient mice. J Invest Dermatol. 1996;107:553–557. doi: 10.1111/1523-1747.ep12582809. [DOI] [PubMed] [Google Scholar]
- 71.Maeda A, Beissert S, Schwarz T, Schwarz A. Phenotypic and functional characterization of ultraviolet radiation-induced regulatory T cells. J Immunol. 2008;180:3065–3071. doi: 10.4049/jimmunol.180.5.3065. [DOI] [PubMed] [Google Scholar]
- 72.Loser K, Apelt J, Voskort M, Mohaupt M, Balkow S, Schwarz T, Grabbe S, Beissert S. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 73.Ghoreishi M, Dutz JP. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+CD25+ T regulatory cells and is dependent on host-derived IL-10. J Immunol. 2006;176:2635–2644. doi: 10.4049/jimmunol.176.4.2635. [DOI] [PubMed] [Google Scholar]
- 74.Maeda A, Schwarz A, Bullinger A, Morita A, Peritt D, Schwarz T. Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells. J Immunol. 2008;181:5956–5962. doi: 10.4049/jimmunol.181.9.5956. [DOI] [PubMed] [Google Scholar]
- 75.Chacón-Salinas R, Limón-Flores AY, Chávez-Blanco AD, Gonzalez-Estrada A, Ullrich SE. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J Immunol. 2011;186:25–31. doi: 10.4049/jimmunol.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang K, Hammerberg C, Meunier L, Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J Immunol. 1994;153:5256–5264. [PubMed] [Google Scholar]
- 77.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 78.Tomimori Y, Ikawa Y, Oyaizu N. Ultraviolet-irradiated apoptotic lymphocytes produce interleukin-10 by themselves. Immunol Lett. 2000;71:49–54. doi: 10.1016/s0165-2478(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 79.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 80.Groux H, Powrie F. Regulatory T cells and inflammatory bowel disease. Immunol Today. 1999;20:442–445. doi: 10.1016/s0167-5699(99)01510-8. [DOI] [PubMed] [Google Scholar]
- 81.Schwarz A, Schwarz T. UVR-induced regulatory T cells switch antigen-presenting cells from a stimulatory to a regulatory phenotype. J Invest Dermatol. 2010;130:1914–1921. doi: 10.1038/jid.2010.59. [DOI] [PubMed] [Google Scholar]
- 82.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 83.Chapman RS, Cooper KD, De Fabo EC, Frederick JE, Gelatt KN, Hammond SP, Hersey P, Koren HS, Ley RD, Noonan F. Solar ultraviolet radiation and the risk of infectious disease: summary of a workshop. Photochem Photobiol. 1995;61:223–247. doi: 10.1111/j.1751-1097.1995.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 84.Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 85.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, Terhune M, LeVee G, Anderson T, Koren H. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci USA. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rezaee F, Peppelenbosch M, Dashty M. Donor chimera model for tolerance induction in transplantation. Hum Immunol. 2013;74:550–556. doi: 10.1016/j.humimm.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Csencsits K, Wood SC, Lu G, Magee JC, Eichwald EJ, Chang CH, Bishop DK. Graft rejection mediated by CD4+ T cells via indirect recognition of alloantigen is associated with a dominant Th2 response. Eur J Immunol. 2005;35:843–851. doi: 10.1002/eji.200425685. [DOI] [PubMed] [Google Scholar]
- 88.Brinkman C, Burrell B, Scalea J, Bromberg JS. Transplantation tolerance. Methods Mol Biol. 2013;1034:85–101. doi: 10.1007/978-1-62703-493-7_4. [DOI] [PubMed] [Google Scholar]


