Abstract
Background
Glioma patients are not only confronted with the diagnosis and treatment of a brain tumor, but also with changes in cognitive and neurological functioning that can profoundly affect their daily lives. At present, little is known about the relationship between cognitive functioning and health-related quality of life (HRQOL) during the disease trajectory. We studied this association in low-grade glioma (LGG) patients with stable disease at an average of 6 years after diagnosis.
Methods
Patients and healthy controls underwent neuropsychological testing and completed self-report measures of generic (MOS SF36) and disease-specific (EORTC BN20) HRQOL. Associations were determined with Pearson correlations, and corrections for multiple testing were made.
Results
We analyzed data gathered from 190 LGG patients. Performance in all cognitive domains was positively associated with physical health (SF36 Physical Component Summary). Executive functioning, processing speed, working memory, and information processing were positively associated with mental health (SF36 Mental Component Summary). We found negative associations between a wide range of cognitive domains and disease-specific HRQOL scales.
Conclusions
In stable LGG patients, poorer cognitive functioning is related to lower generic and disease-specific HRQOL. This confirms that cognitive assessment of LGG patients should not be done in isolation from assessment of its impact on HRQOL, both in clinical and in research settings.
Keywords: brain tumor, cognitive functioning, health-related quality of life, low-grade glioma
Gliomas are the most common primary malignant brain tumors, with an incidence of 5 to 7 per 100 000 persons.1 A minority of gliomas can be histologically defined as low-grade (WHO grade I or II).
Patients diagnosed with low-grade glioma (LGG) have a more favorable prognosis than those diagnosed with more rapidly progressing tumors;2,3 however, the diagnosis and treatment can have a great impact on their lives. In addition, LGG patients find themselves confronted with focal neurological limitations, including loss of motor functioning, visual-perceptual deficits, sensory loss,4 and epilepsy, which affects ∼85% of LGG patients.5 Moreover, cognitive impairment is often associated with LGGs6,7 with patients experiencing deterioration in a broad array of cognitive domains (eg, information processing, attention, psychomotor speed, and memory) when compared with control groups.6–8
While the prognostic value of cognitive functioning has been demonstrated for survival in glioma patients,9–12 relatively little is known about its relationship to patients' daily functioning. A small study among long-term survivors of malignant supratentorial brain tumors suggests that even subtle cognitive deficits might hamper a patient's autonomy and professional life.13 In addition, indices of neurological functioning, such as epilepsy burden, have been shown to be related to both lower objective cognitive functioning and self-reported health-related quality of life (HRQOL) in LGG patients.14 With the high incidence of cognitive and neurological deficits and poorer self-reported HRQOL in LGG patients,15,16 a relationship between cognitive functioning and generic and disease-specific HRQOL would be expected. However, to our knowledge, these associations have not yet been examined in depth. Previous studies that examined both cognitive functioning and HRQOL did not formulate these associations as their primary study objective and consequently yielded only brief reports with little detail.14,15 However, it is of particular importance to know the clinical and functional significance of cognitive impairment for clinicians and patients. The clinical relevance of cognitive deficits cannot be fully appreciated without assessing their impact on the patient's quality of life (QOL). Apart from these possible clinical implications, a separate investigation into the nature and strength of the correlation between these factors is also merited because of the increased value being attributed to both cognitive functioning and HRQOL as secondary endpoints in glioma clinical trials.17,18
Materials and Methods
Participants
Data for this study were collected as part of a nationwide study of cognitive functioning and HRQOL of glioma patients. The methodology of these studies has been described in detail elsewhere.7 In short, LGG patients were diagnosed an average of 6 years prior to data collection and were included in the study if they had (i) been diagnosed with a histologically confirmed low-grade astrocytoma, oligodendroglioma, or oligoastrocytoma at least 1 year prior to study entry; (ii) no clinical signs of tumor recurrence for at least 1 year after diagnosis and primary treatment; (iii) no radiological signs of recurrence within 3 months before the first assessments were performed, (iv) no current treatment with corticosteroids; and (v) basic proficiency in the Dutch language.
In addition, we included data from 2 samples of healthy controls. Specifically, for comparison on cognitive performance, we employed a reference sample from the Maastricht Aging Study,19 a large cross-sectional study on the biological and psychological determinants of cognitive aging. Reference data for the HRQOL assessments were selected from a national study aimed at constructing a Dutch version of the Short-Form Health Survey.20 All healthy controls were matched to the participant group for age, sex, and educational level.
Procedure
Participants were asked to provide information about their sociodemographic background via a structured interview. Clinical data were obtained from the medical records. Participants completed the self-report measures of generic (SF36) and disease-specific (BN20) HRQOL and the neuropsychological tests either at home or at their treating hospital. Neuropsychological assessments were performed by a trained test assistant, who was supervised by a board certified neuropsychologist (M.K.). The institutional review boards of the participating centers approved the research protocol, and all participants provided written, informed consent.
Outcome Measures
Cognitive performance was assessed using an extensive battery of standardized neuropsychological tests, described in detail in Table 1.21–26 Tests included measures of executive functioning (categoric word fluency task,21 concept shifting task25), processing speed (concept shifting task,25 letter digit substitution test24), verbal memory (visual verbal learning test23), working memory (memory scanning test26), information processing (letter digit substitution test24), and attention (Stroop color word test22).
Table 1.
Neuropsychological tests and corresponding cognitive domains
| Cognitive domain | Content |
|---|---|
| Executive functioning | Categoric Word Fluency Task21 |
| Measures executive functioning and semantic memory. Outcome variable: number of animals in 60 seconds | |
| Concept Shifting Test25 | |
| Measures attention, visual search, mental processing speed, and ability to mentally control simultaneous stimulus patterns. Outcome variables: CST A, CST B, CST C. | |
| Processing speed | Concept Shifting Test25 |
| Outcome variable: CST 0 | |
| Letter Digit Substitution Test24 | |
| Measures psychomotor speed that is relatively unaffected by a decline in intellectual ability. Outcome variable: LDST Delta (ie, number of substitutions read minus number of substitutions written). | |
| Verbal memory | Visual Verbal Learning Test23 |
| Examines verbal learning capacity and consolidation of verbal information into long-term memory. Outcome variables: Trial 1, delayed recall, delayed recognition, and difference between maximum score and trials 1, total score trial 1-5) | |
| Working memory | Memory Scanning Test26 |
| Measures the speed and efficiency of memory retrieval processes. Outcome variables/items to be stored in working memory: symbol ‘%’, 1, 2, 3, and 4 letters, successively. | |
| Information processing | Letter Digit Substitution Test24 |
| Outcome variables: number of substitutions read and written. | |
| Attention | Stroop Color Word Test22 |
| Examines information processing speed, selective attention, and mental control. | |
| Outcome variables: Stroop card I, Stroop card II, Stroop card III. |
Self-reported HRQOL was measured with the Dutch version of the 36-Item Short-Form Health Survey (SF36).20 The SF36 yields 2 component summary scores: one for physical health (PCS) and one for mental health (MCS). The PCS and MCS employ norm-based scoring, with a mean of 50 and a standard deviation of 10. The Dutch version of the SF-36 is a valid and reliable instrument, yielding a mean coefficient alpha of 0.84 across scales.20
Disease-specific HRQOL was measured with the Dutch version of the EORTC brain cancer module (EORTC QLQ-BN20).27 This module contains 4 multi-item scales (future uncertainty, visual disorders, motor dysfunctions, communication deficits) and 7 single items assessing headaches, seizures, drowsiness, hair loss, itching, weakness in the legs, and difficulties with bladder control. Scores range from 0 to 100, with higher scores indicating more symptoms. The BN20 scales have high internal consistency reliability (alpha > 0.70) and show overall adequate psychometric properties.27 Although the BN20 is often administered alongside the EORTC QLQ-C30, unfortunately, we have no data regarding this cancer-specific HRQOL questionnaire.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Science version 20.0 (SPSS). Standard scoring rules were used to convert the data from the questionnaires. The neuropsychological test scores were transformed into Z scores using the mean and standard deviations (SDs) of the healthy controls, and 6 cognitive domains were created for the purpose of data reduction (Table 1). To calculate each domain, z scores of the outcome variables were summed up and divided by the number of variables per domain. Higher scores indicate better performance in all domains.
Sociodemographic characteristics, HRQOL, and cognitive functioning of the LGG group and the control groups were compared using univariate analysis of variance (ANOVA) and the chi-square statistic. A 2-sided P value < .05 was considered significant. To examine the associations between cognitive functioning and both generic (SF36 component summaries MCS and PCS) and disease-specific HRQOL (BN20 scales future uncertainty, visual disorders, motor dysfunctions, communication deficits, headaches, seizures, and drowsiness), Pearson correlations were calculated. To adjust for multiple testing, corrections were applied for the 6 cognitive outcome measures. A 2-sided P value < .0083 was required as evidence of statistical significance for all Pearson correlations shown.
Results
Demographic Characteristics
In total, 239 eligible LGG patients were invited for participation, of whom 82% (n = 195) were included in the study. The main reasons reported for declining participation were the perceived burden of participating and not wanting to be confronted with their disease history. In 5 cases, data were incomplete, leaving 190 LGG participants for the present analyses. No statistically significant differences between the participants and the healthy controls were found for age, sex, and educational level, indicating an adequate matching procedure (Table 2). Most LGG participants were men (61.5%), and most received middle to high levels of education. The majority of participants were married or lived together with their partner (63.6%).
Table 2.
Demographic characteristics of low-grade glioma patients and healthy controls
| LGG Patients (n = 195) | Healthy Controls (cognition; n = 195) | Healthy Controls (HRQOL; n = 195) | P value | |
|---|---|---|---|---|
| Age in years M (SD) | 40.80 (11.62) | 40.55 (12.01) | 39.68 (2.32) | .494 |
| Sex | ||||
| Male | 120 (61.5%) | 121 (62.1%) | 122 (62.6%) | .978 |
| Female | 75 (38.5%) | 74 (37.9%) | 73 (37.7%) | |
| Educational level n (%) | .285 | |||
| Low | 58 (29.7%) | 55 (28.2%) | 61 (31.3%) | |
| Middle | 74 (37.9%) | 76 (39.0%) | 80 (41.0%) | |
| High | 60 (30.8%) | 64 (32.8%) | 54 (27.7%) | |
| Other | 3 (1.5%) | N/A | N/A | |
| Marital status n (%) | <.001 | |||
| Single | 56 (28.7%) | 27 (13.8%) | 29 (14.9%) | |
| Married/living with partner | 124 (63.6%) | 161 (82.6%) | 164 (84.1%) | |
| Divorced | 6 (3.1%) | 5 (2.6%) | 0 (0%) | |
| Widow(er) | 6 (3.1%) | 2 (1.0%) | 0 (0%) | |
| Tumor grade n (%) | N/A | N/A | N/A | |
| Grade I | 21 (10.8%) | |||
| Grade II | 174 (89.2%) | |||
| Tumor location n (%) | N/A | N/A | N/A | |
| Frontal | 47 (24.1%) | |||
| Temporal | 33 (16.9%) | |||
| Parietal | 19 (9.7%) | |||
| Occipital | 5 (2.6%) | |||
| Mixed | 89 (45.6%) | |||
| Other | 2 (1.0%) | |||
| Tumor lateralization n (%)* | N/A | N/A | N/A | |
| Left | 85 (43.6%) | |||
| Right | 87 (44.6%) | |||
| Bilateral | 9 (4.6%) | |||
| Time since diagnosis | Months | N/A | N/A | N/A |
| M (SD) | 66.99 (43.96) | |||
| (range) | 0–258 | |||
*Information on tumor lateralization was missing in 14 cases.
Abbreviations: LGG, low-grade glioma; M (SD), mean, standard deviation; N/A, not applicable.
Cognitive Functioning and Health-related Quality of Life
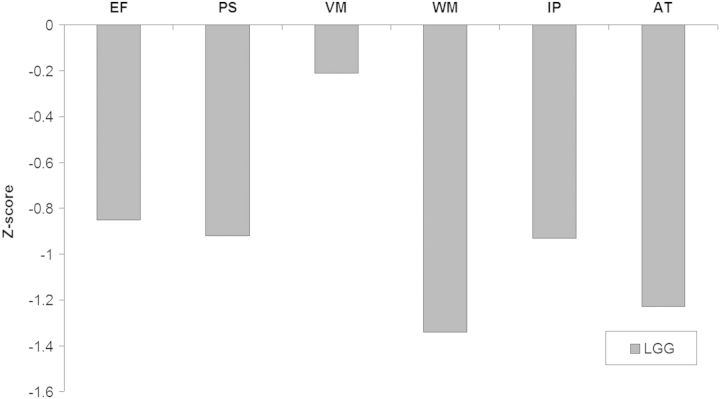
LGG participants had lower scores than healthy controls on all cognitive domains that were assessed (P < .001 for all domains except the verbal memory domain (P = .009); see Fig. 1. Furthermore, we found lower self-reported mental health in LGG patients (MCS, M = 46.09; SD = 9.81) than in healthy controls (M = 49.91; SD = 9.92; P < .001). No statistically significant differences were observed in physical health between LGG patients and healthy controls (PCS, M = 49.92; SD = 9.11 vs M = 51.28; SD = 7.86; P = .119).
Fig. 1.
Cognitive performance of low-grade glioma patients relative to their healthy controls at the 0-line. Abbreviations: EF, executive functioning; PS, processing speed; VM, verbal memory; WM, working memory; IP, information processing; AT, attention.
Associations Between Cognitive Functioning and Generic (SF36) and Disease-specific (BN20) Health-related Quality of Life
Cognitive Functioning and Generic (SF36) Health-related Quality of Life
Better performance on all of the cognitive domains that we assessed was associated with significantly better self-reported physical health (Table 3 PCS; all P < .001). Furthermore, better performance on executive functioning, processing speed, working memory capacity, and information processing speed was associated with better mental health (MCS, r = 0.270, r = 0.318, r = 0.250, and r = 0.267, respectively; all P ≤ .001).
Table 3.
Associations between cognitive functioning and generic health-related quality of life in low-grade glioma patients
| Low-grade gliomas (n = 190) |
||
|---|---|---|
| Physical Health (PCS) | Mental Health (MCS) | |
| Executive functioning | r = 0.427, P < .001* | r = 0.270, P < .001* |
| Processing speed | r = 0.455, P < .001* | r = 0.318, P < .001* |
| Verbal memory | r = 0.265, P < .001* | r = 0.184, P = .012 |
| Working memory | r = 0.393, P < .001* | r = 0.250, P = .001* |
| Information processing | r = 0.436, P < .001* | r = 0.267, P < .001* |
| Attention | r = 0.336, P < .001* | r = 0.157, P = .036 |
*P < .00833.
Cognitive Functioning and Disease-specific (BN20) Health-related Quality of Life
Regarding cognitive functioning and disease-specific HRQOL as assessed by the BN20, many negative correlations of weak to moderate strength were found (Table 4). All cognitive domains were negatively correlated with the BN20 scales for uncertainty concerning the future, motor dysfunctions, and seizures. This indicates that worse cognitive performance is associated with more symptoms, as assessed by these scales.
Table 4.
Associations between cognitive functioning and disease-specific health-related quality of life in low-grade glioma patients (n = 190)
| Future Uncertainty | Visual Disorder | Motor Dysfynction | Communication Deficit | Headaches | Seizures | Drowsiness | |
|---|---|---|---|---|---|---|---|
| EF | r = −0.325 P < .001* | r = −0.226 P = .002* | r = −0.386 P < .001* | r = −0.156 P = .034 | r = −0.106 P = .152 | r = −0.316 P < .001* | r = −0.181 P = .014 |
| PS | r = −0.383 P < .001* | r = −0.316 P < .001* | r = −0.388 P < .001* | r = −0.136 P = .065 | r = 0.174 P = .018 | r = −0.254 P = .001* | r = −0.276 P < .001* |
| VM | r = −0.252 P = .001* | r = −0.188 P = .011 | r = −0.271 P < .001* | r = −0.187 P = .011 | r = −0.149 P = .044 | r = −0.244 P = .001* | r = −0.161 P = .029 |
| WM | r = −0.287 P < .001* | r = −0.295 P < .001* | r = −0.426 P < .001* | r = −0.225 P = .002 | r = −0.154 P = .036 | r = −0.315 P < .001* | r = −0.186 P = .011 |
| IP | r = −0.345 P < .001* | r = −0.325 P < .001* | r = −0.405 P < .001* | r = −0.255 P < .001* | r = −0.175 P = .018 | r = −0.255 P = .001* | r = −0.209 P = .004 |
| AT | r = −0.270 P < .001* | r = −0.248 P = .001* | r = −0.445 P < .001* | r = −0.355 P < .001* | r = −0.059 P = .433 | r = −0.311 P < .001* | r = −0.113 P = .130 |
*P < .00833.
Abbreviations: EF, executive functioning; PS, processing speed; VM, verbal memory; WM, working memory; P, information processing; AT, attention.
Participants who had lower executive functioning, processing speed, working memory capacity, information processing speed, and attentional functioning were characterized by more symptoms of visual disorders. Furthermore, worse performance on information processing tasks and attention tasks was related to more difficulty with communication. Patients who had a lower information processing speed also reported more drowsiness.
Discussion
It is often assumed, but has never actually been demonstrated, that cognitive functioning in brain tumor patients is related to their HRQOL. We tested this assumption in a large cohort of low-grade glioma participants with stable disease, at an average of 6 years after diagnosis. We found that many aspects of physical functioning, as measured with the SF36 and BN20, were associated with many, if not all, cognitive domains. Furthermore, poorer mental health (MCS) and more uncertainty concerning the future were related to lower cognitive functioning. These results suggest that LGG patients in a stable phase of their disease may be bothered by cognitive deficits that negatively affect their everyday life functioning. The present study outcomes concur with those of Giovagnoli and Boiardi,13 who reported that asymptomatic, long-term glioma survivors may experience limitations in their autonomy, even with subtle cognitive deficits. In addition, severe cognitive dysfunction was related to worse levels of HRQOL in patients with a benign (WHO grade I) meningioma.28
This report, as well as our previous report on this LGG patient cohort,7 demonstrates that cognitive deficits are present in LGG patients in a period of stable disease and that their performance on cognitive tests is statistically significantly worse than that of healthy controls. However, the deficits found are, on a group level, relatively mild. In fact, the z scores on all domains tested did not exceed 1.5 SD below the mean of healthy controls (the threshold often used in the patient context to define clinically significant cognitive dysfunction). Memory deficits in particular seemed less prominently present in our cohort than in other publications on glioma patients.29,30 One explanation for this particular difference could be the use of a different neuropsychological test. We tested verbal memory using visually presented stimuli, while other reports frequently used verbal auditory-presented stimuli. While still measuring the same construct (ie, verbal memory), a bias in results based on this difference cannot be excluded.
In addition, the reduction found in mental health does not exceed a standard deviation below the mean and hence probably reflects only subtle compromise. Nevertheless, while cognitive deficits and compromise in HRQOL may be subtle in nature, the present report demonstrates the highly correlated relationship of cognitive functioning and both generic and disease-specific HRQOL. With most correlations being of moderate strength, it seems likely that LGG patients with stable disease, who resumed their daily activities, may be more aware of subtle or more pronounced negative changes in their cognitive abilities. We suspect that the priorities of LGG patients may shift along with their view of the immediate and more distant future. However, these hypotheses cannot be confirmed by the present study due to its cross-sectional nature. Thus, additional longitudinal studies are needed.
Alternatively, in part, the associations found may be explained by the nature of the neuropsychological tests and the neurological disabilities of the participants. Visual and motor deficits in particular may contribute to poorer performance on certain cognitive tasks that depend on these skills, such as tests assessing attentional functioning. Indeed, poor performance on timed tasks in these patients can be attributed, in large part, to visual and motor deficits.31 Where possible, interventions to improve functioning in these areas may potentially contribute to better cognitive functioning as well as better HRQOL.
We only investigated the association between HRQOL and cognitive functioning in this study; it is likely that this association was confounded by other patient-related factors such as fatigue, sleep quality, anxiety, depression, and instrumental activities of daily living (IADL), which have been reported to affect the daily lives of patients as well.8,32–34 Although it is not yet available as a validated instrument, a brain tumor-specific measure of IADL is currently being developed at our institution. Because this test includes adapted items based on IADL assessment in patients with dementia and focuses on their everyday functional impairment resulting from cognitive deficits, it could prove to be a highly relevant tool in the future in both clinical practice and the research setting.
In conclusion, our results indicate that when cognitive functioning is worse, LGG patients who are in a stable phase of their disease experience worse physical and mental HRQOL. Furthermore, LGG patients who experience more cognitive deficits also report more issues with disease-specific HRQOL, which is most pronounced in the scales of future uncertainty, motor dysfunction, visual disorders, and seizures. Future longitudinal studies should include measures of anxiety and depression, fatigue, IADL, demographic characteristics, and clinical variables in order to assess which other factors have an effect on these associations. While beyond the scope of the present study, examining associations between cognitive functioning and subscales, rather than summary scales of generic HRQOL, could provide additional information in future studies. Maintaining or even improving HRQOL by preventing long-term cognitive sequelae, or rehabilitation of cognitive deficits if prevention is not feasible, is an important goal in the treatment of glioma patients. It is important to understand the functional significance of cognitive impairments in the everyday lives of LGG patients. Cognitive assessment of patients with gliomas cannot—or rather, should not—be performed in isolation from assessment of its impact on psychosocial functioning and HRQOL.
Funding
This study was supported by grant no. VU96–1155 from the Dutch Cancer Society.
Acknowledgments
These data were previously presented at the International Neuropsychological Society Mid-Year 2013 Meeting in Amsterdam, Netherlands, and at the 4th Quadrennial Meeting of the World Federation of Neuro-Oncology, held in conjunction with the 18th Scientific Meeting of the Society for Neuro-Oncology in San Francisco, California.
Conflict of interest statement. None declared.
References
- 1.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15:ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54(7):1442–1448. doi: 10.1212/wnl.54.7.1442. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Mukand JA, Blackinton DD, Crincoli MG, et al. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehab. 2001;80(5):346. doi: 10.1097/00002060-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lote K, Stenwig AE, Skullerud K, et al. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34(1):98–102. doi: 10.1016/s0959-8049(97)00374-2. [DOI] [PubMed] [Google Scholar]
- 6.Bosma I, Douw L, Bartolomei F, et al. Synchronized brain activity and neurocognitive function in patients with low-grade glioma: a magnetoencephalography study. Neuro Oncol. 2008;10(5):734–744. doi: 10.1215/15228517-2008-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. doi: 10.1016/s0140-6736(02)11398-5. [DOI] [PubMed] [Google Scholar]
- 8.Taphoorn MJ, Heimans JJ, Snoek FJ, et al. Assessment of quality of life in patients treated for low-grade glioma: a preliminary report. J Neurol Neurosurg Psychiatry. 1992;55(5):372–376. doi: 10.1136/jnnp.55.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PD, Buckner JC, O'Fallon JR, et al. Importance of baseline mini-mental state examination as a prognostic factor for patients with low-grade glioma. Int J Radiat Oncol Biol Phys. 2004;59(1):117–125. doi: 10.1016/j.ijrobp.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DR, Sawyer AM, Meyers CA, et al. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2012;14(6):808–816. doi: 10.1093/neuonc/nos082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein M, Postma TJ, Taphoorn MJB, et al. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology. 2003;61(12):1796–1798. doi: 10.1212/01.wnl.0000098892.33018.4c. [DOI] [PubMed] [Google Scholar]
- 12.Meyers CA, Hess KR, Yung WA, et al. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18(3):646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 13.Giovagnoli AR, Boiardi A. Cognitive impairment and quality of life in long-term survivors of malignant brain tumors. Ital J Neurol Sci. 1994;15(9):481–488. doi: 10.1007/BF02334609. [DOI] [PubMed] [Google Scholar]
- 14.Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low–grade gliomas: The impact on cognitive function and quality of life. Ann Neurol. 2003;54(4):514–520. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson NK, Taphoorn MJ, Heimans JJ, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. doi: 10.1200/JCO.2011.35.5750. [DOI] [PubMed] [Google Scholar]
- 16.Taphoorn MJ, van den Bent MJ, Mauer ME, et al. Health-related quality of life in patients treated for anaplastic oligodendroglioma with adjuvant chemotherapy: results of a European Organisation for Research and Treatment of Cancer randomized clinical trial. J Clin Oncol. 2007;25(36):5723–5730. doi: 10.1200/JCO.2007.12.7514. [DOI] [PubMed] [Google Scholar]
- 17.Butowski N, Chang SM. Endpoints for clinical trials and revised assessment in neuro-oncology. Curr Opin Neurol. 2012;25(6):780–785. doi: 10.1097/WCO.0b013e328359b45e. [DOI] [PubMed] [Google Scholar]
- 18.Van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 19.van Boxtel MP, Buntinx F, Houx PJ, et al. The relation between morbidity and cognitive performance in a normal aging population. J Gerontol : Biol Sci Med Sci. 1998;53(2):M147–M154. doi: 10.1093/gerona/53a.2.m147. [DOI] [PubMed] [Google Scholar]
- 20.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–1068. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 21.Luteijn F, Van der Ploeg FAE. Handleiding Groninger intelligentietest (git)[manual Groningen intelligence test] Lisse, The Netherlands: Swetz and Zeitlinger. 1983 [Google Scholar]
- 22.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643. [Google Scholar]
- 23.Van Der Elst WIM, van Boxtel MP, van Breukelen GJ, et al. Rey's verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11(3):290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- 24.van der Elst W, van Boxtel MP, van Breukelen GJ, et al. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24–81 from the Maastricht Aging Study (MAAS): Influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28(6):998–1009. doi: 10.1080/13803390591004428. [DOI] [PubMed] [Google Scholar]
- 25.van der Elst W, van Boxtel MP, van Breukelen GJ, et al. The Concept Shifting Test: adult normative data. Psychol Assess. 2006;18(4):424. doi: 10.1037/1040-3590.18.4.424. [DOI] [PubMed] [Google Scholar]
- 26.van der Elst W, van Boxtel MP, van Breukelen GJ, et al. Assessment of information processing in working memory in applied settings: the paper & pencil memory scanning test. Psychol Med. 2007;37(9):1335. doi: 10.1017/S0033291707000360. [DOI] [PubMed] [Google Scholar]
- 27.Taphoorn MJ, Claassens L, Aaronson NK, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. doi: 10.1016/j.ejca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Waagemans ML, van ND, Dijkstra M, et al. Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery. 2011;69(1):72–78. doi: 10.1227/NEU.0b013e318212badb. [DOI] [PubMed] [Google Scholar]
- 29.Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol. 2007;81(2):175–184. doi: 10.1007/s11060-006-9212-3. [DOI] [PubMed] [Google Scholar]
- 30.Kayl AE, Meyers CA. Does brain tumor histology influence cognitive function? Neuro Oncol. 2003;5(4):255–260. doi: 10.1215/S1152851703000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein M, Taphoorn MJ, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19(20):4037–4047. doi: 10.1200/JCO.2001.19.20.4037. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson M, Edvardsson T, Ahlström G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support Care Cancer. 2006;14(12):1205–1212. doi: 10.1007/s00520-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 33.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76. doi: 10.1093/jnci/djq458. [DOI] [PubMed] [Google Scholar]
- 34.Struik K, Klein M, Heimans JJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2009;92(1):73–78. doi: 10.1007/s11060-008-9738-7. [DOI] [PubMed] [Google Scholar]