Abstract
Sleep deprivation (SD) following hippocampus-dependent learning in young mice impairs memory when tested the following day. Here, we examined the effects of SD on remote memory in both young and aged mice. In young mice, we found that memory is still impaired 1 mo after training. SD also impaired memory in aged mice 1 d after training, but, by a month after training, sleep-deprived and control aged animals performed similarly, primarily due to remote memory decay in the control aged animals. Gene expression analysis supported the finding that SD has similar effects on the hippocampus in young and aged mice.
Two major concerns in developed countries are the aging population and the prevalence of sleep deprivation (SD) (Luyster et al. 2012). Both aging and SD have clear negative impacts on cognitive function, including specific forms of memory. In particular, aging is associated with deficits in hippocampus-dependent episodic memories with associative, contextual, or spatial components (Moscovitch et al. 1986; Spencer and Raz 1995; Eichenbaum 1999; Old and Naveh-Benjamin 2008). Rodent studies show that hippocampus-dependent memory formation and synaptic plasticity are impaired by both SD (Graves et al. 2003; Guan et al. 2004; Ruskin et al. 2004; Vecsey et al. 2009) and aging (Barnes and McNaughton 1985; Tanila et al. 1997; Burke and Barnes 2006; Robitsek et al. 2008; Foster et al. 2012). Further, aging causes abnormalities in the amount, quality, and timing of sleep (Bonnet and Arand 1989; Stone 1989; Wimmer et al. 2013; Pace-Schott and Spencer 2014), particularly nonrapid eye movement (NREM) sleep (Cajochen et al. 2006). Thus, it is possible that aging induces a form of SD that could mediate some of the effects of aging on memory (Hornung et al. 2005). A goal of this study was therefore to assess how aging and SD interact to affect memory.
Studies of post-training SD have shown clear memory impairments in hippocampus-dependent tasks such as contextual fear conditioning (CFC), but memory is typically not abolished completely (Graves et al. 2003; Ruskin et al. 2004; Vecsey et al. 2009). Therefore, we were interested to determine what happens to the remaining memory after longer post-training intervals. Memory has several stages (Abel and Lattal 2001), including acquisition, consolidation, retrieval, reconsolidation, and systems consolidation, in which memory is transformed such that its storage and recall become dependent on different brain systems (Winocur et al. 2010). Systems consolidation may involve transfer of the memory from one brain area to another for storage (Frankland and Bontempi 2005), or at least reorganization of the memory traces such that recall involves a new set of brain regions (Winocur et al. 2010; Sutherland and Lehmann 2011). In the case of CFC, the hippocampus appears to be required for all of the stages of memory occurring during the first few weeks following learning. When memory is assessed at more “remote” time points, the hippocampus is less strongly required for retrieval, indicating that systems consolidation has taken place (Scoville and Milner 1957; Kim and Fanselow 1992; Anagnostaras et al. 1999; Bontempi et al. 1999). However, recent evidence indicates that the hippocampus is likely to continue to play a role in remote memory storage and recall (Moscovitch et al. 2005; Goshen et al. 2011). Using CFC, we studied the effects of SD and aging on recent and remote memory to determine how these two factors interacted. We used CFC for a number of reasons. First, because SD had been shown to cause deficits in the memory consolidation for this task, as described above. Second, because the process of systems consolidation was best characterized for this task. And third, because this learning task has the advantage of inducing very long-lasting memory with a single training trial, it allowed us to carry out SD following training and to assess memory at different intervals following learning. This meant that we could target the memory consolidation stage with SD while leaving acquisition and systems consolidation unaffected. Last, we compared the molecular effects of aging and SD by assessing gene expression in the hippocampus.
Young adult (2-mo old) and aged (22- to 23-mo old) male C57BL/6 mice from the National Institute of Aging (NIA) were used for behavioral experiments testing memory and for gene expression analysis. Mice were individually housed in a temperature-controlled environment, which was on a 12 h/12 h light/dark schedule, and had ad libitum access to food and water. All experiments were conducted according to National Institutes of Health Guidelines for Animal Care and Use and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
All behavioral experiments were carried out during the first half of the light period, as previously described (Vecsey et al. 2009). Mice were trained in one of four chambers (Med Associates), two of which had circular floors and two of which had square floors. The chambers had walls made of clear Plexiglas and shock grid floors. Blue and white striped paper was placed behind two of the four walls of the square chambers and around half of the circular chambers. There were also X's of varying size made with red tape on the same walls covered with paper (two per wall for square chambers, three for circular chamber). The chambers were located in a windowless, dimly lit room. Each mouse was handled for 2–3 min daily for 5–6 d prior to training, at approximately the same time that they were trained. On the training day, each mouse was carried to the testing room in its cage and was placed in one of the four chambers for 2 min and 28 sec. For the next 2 sec, a 1.5-mA foot shock was given. The mice remained in the chambers for another 30 sec following the shock and were then returned to their home cages. Four mice were trained simultaneously using the four chambers. Between each training or testing set, the chambers were cleaned with 70% ethanol.
Half of the mice were tested after 24 h (Recent test). They were placed in the same chambers they were trained in for 5 min. The fear response was measured at intervals of 5 sec by recording levels of freezing, which is the absence of all movement except for respiration (Blanchard and Blanchard 1969; Fanselow 1980). The same mice were retested in an altered context 24 h after testing. For the altered chamber, smooth, black panels covered the floor, laminated cardboard served as the top, and a metal sheet was placed in the chamber to divide it in half (diagonally for the square shape). Mice were placed in a chamber with the opposite shape than the one in which they were trained and initially tested. The chambers were cleaned with a 15% Lemon Joy (Procter & Gamble)/water mixture. The other half of the mice were tested in the trained context 30 d after training (Remote test), in the same manner as the mice tested after 24 h, followed by altered context testing the following day.
In behavioral experiments, SD began immediately following behavioral training. In gene expression analysis experiments, SD in handled but untrained animals began 2 h after lights-on. Sleep-deprived mice were kept awake for 5 h by gentle handling (Vecsey et al. 2013), which consisted of tapping of the cage, lifting of the cage lids, and uncovering of the cages for short periods of time. The young and aged nonsleep-deprived mice were left undisturbed in their home cages and allowed to rest. Although quantitative measurements were not made, no qualitative differences were observed in the degree of manipulation required to keep young and old mice awake.
Freezing levels were scored by an observer blind to condition, by tallying the number of times the mice were freezing, at 5-sec intervals, during the 5-min tests. The percent freezing was calculated by dividing the freezing levels by the number of 5-sec intervals during testing (Abel et al. 1997). Freezing levels and percent freezing were calculated for both sets of mice for the original and altered context. A “delta” score was also calculated by subtracting the percent freezing in the altered context from the percent freezing in the original context for each animal (Paylor et al. 1994). This score reflects freezing that is specific to the original context (Frankland et al. 1998). For behavioral experiments, young mice were trained and tested in separate experimental cohorts from aged mice. We performed two-way ANOVAs with treatment (FC alone versus FCSD) and testing time (Recent versus Remote) as factors, run separately for each age. We also performed two-way ANOVAs with age (Young versus Old) and treatment (FC alone versus FCSD) as factors, run separately for each of the two test times. All behavioral data ANOVAs showing a significant effect were followed by post hoc Tukey tests, and were carried out using JMP8 and JMP10 (SAS Institute).
For gene expression analysis, RNA preparation, cDNA synthesis, and qPCR analysis was performed as previously described (Vecsey et al. 2012). Quantitative real-time RT-PCR (qPCR) reactions were carried out in the ABI Prism 7000 with an initial activation at 50°C for 2 min followed by incubation at 95°C for 15 min and 40 subsequent cycles of 95°C for 15 sec, 56°C for 30 sec, and 72°C for 30 sec. Primer sequences, data analysis, and relative quantification of gene expression have been previously described (Vecsey et al. 2012). Hippocampal samples from young and aged mice were tested simultaneously. A two-way ANOVA was performed to compare fold change values for each gene, with age (young versus aged) and treatment (sleep-deprived versus nonsleep-deprived), followed by post hoc Tukey tests (Statistica 7, Statsoft, Inc.).
Sleep deprivation following contextual fear conditioning impairs memory when tested 1 or 30 d after training in young mice
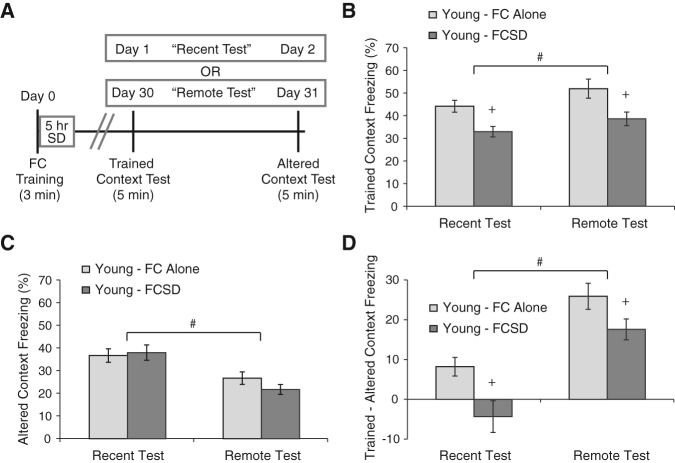
In young mice, SD for 5 h immediately following CFC impaired memory during both the Recent and Remote tests, as seen in Figure 1B for freezing in the trained context (F(1,79) = 15.8, P = 0.0002) and in 1D for memory specificity (delta score) (F(1,79) = 11.8, P = 0.0009). In contrast, freezing in the altered context (indicative of fear memory that is not specific for the trained context) was not affected by SD (F(1,79) = 0.45, P = 0.51), but instead showed a significant overall decrement with time after training (F(1,79) = 22.43, P = <0.0001), with no interaction between SD and test time (F(1,79) = 1,31 P = 0.26). There was no interaction between the effects of SD and test time on either freezing in the trained context (F(1,79) = 0.12, P = 0.74) or on the delta score (F(1,79) = 0.49, P = 0.49), indicating that SD caused comparable deficits at either time of testing. The deficits in 30-d remote memory support the idea that SD after learning impairs memory consolidation and does not simply disrupt recall during the test the following day.
Figure 1.
Sleep deprivation after training in young mice impairs memory tested 1 or 30 d after training. (A) A timeline of experimental procedures used for both young and old mice. C57BL/6 NIA mice (either 2-mo old (young) or 22- to 23-mo old (aged)) received single-trial contextual fear conditioning and were immediately either sleep-deprived for 5 h by gentle handling (FCSD) or left undisturbed in their home cages (FC Alone). Half of the mice from each group were then tested for retention, as assessed by percent time spent freezing, in the same environment (trained context) 1 d later, followed by testing in a different environment (altered context) the following day (Recent tests). The other half of the mice were tested in the trained context 30 d following training, again followed by testing in the altered context the next day (Remote tests). The specificity of memory was calculated by subtracting the amount of freezing in the altered context from the freezing in the trained context. (B) In young mice, SD significantly impaired memory for the trained context during the Recent and Remote tests. (C) SD did not have a significant effect on freezing in the altered context, which instead lessened over time in both the FC Alone and FCSD groups. (D) SD impaired the specificity of memory for the trained context during the Recent and Remote tests. Notably, young mice showed an improvement in the specificity of memory from the Recent to the Remote test. Data are graphed as the mean ± SEM. (#) A significant overall effect of testing time, and (+) a significant overall effect of SD (P ≤ 0.05 was considered significant). Number of animals: FC Alone Recent = 20, FCSD Recent = 20, FC Alone Remote = 21, FCSD Remote = 22.
This experiment also demonstrated that memory in the trained context tends to improve over time in young mice (F(1,79) = 4.72, P = 0.033). This, combined with the decrease in freezing in the altered context, led to a pronounced improvement in memory specificity, as evident in the delta score in Figure 1D (F(1,79) = 42.7, P < 0.0001). Although memory was highly unspecific in young fear-conditioned and sleep-deprived (FCSD) animals during the Recent memory tests, this did improve over time, suggesting that systems consolidation in the absence of continuing SD is able to improve the quality and accuracy of memory (compare darker gray bars in Fig. 1B). This effect has been observed previously, and has been termed memory “incubation” (Oler and Markus 1998; Houston et al. 1999; Pickens et al. 2009), Despite this improvement, FCSD animals still showed a memory deficit compared with the FC alone group, suggesting that the long-term quality of the memory was still dependent on the initial consolidation event, which had been impaired by the SD occurring immediately post-training.
Sleep deprivation in aged animals causes memory impairments at 1 d, but not at 30 d
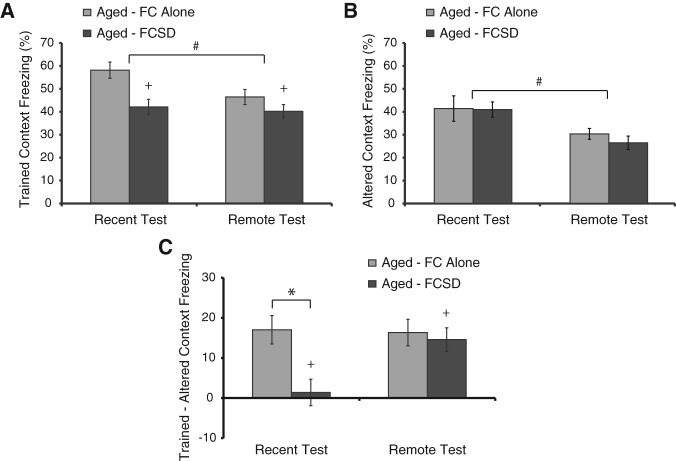
When the same experiment was carried out in aged mice, SD significantly impaired memory during the Recent test. This was true both for freezing in the trained context alone, and most strikingly in the delta score, where memory specificity was essentially null in sleep-deprived mice of both ages. However, in contrast to what was observed in young mice, SD did not impact freezing levels during the Remote test in aged mice. As in young mice, freezing in the altered context decreased significantly with testing time (F(1,83) = 11.64, P = 0.001), but showed no significant effect of SD (F(1,83) = 0.34, P = 0.56), and no interaction between SD and testing time (F(1,83) = 0.21, P = 0.65). For the delta score, there was a significant overall effect of SD (F(1,83) = 7.0, P = 0.01). There was also a trend toward an interaction between SD and testing time (F(1,83) = 3.8, P = 0.05), with a stronger effect of SD at the Recent test than at the Remote test. Post hoc tests found a significant deficit in memory specificity due to SD during the 1-d test, but no deficit at the Remote test.
Unlike young mice, in which overall freezing and specificity for the trained context increased over time following learning (F(1,79) = 4.7, P = 0.03), aged mice did not show an improvement over time. In fact, freezing in the trained context showed a trend toward a decline in aged mice from 1 to 30 d (F(1,83) = 3.9, P = 0.05), reflective of an age-dependent impairment in “systems” memory consolidation (compare light gray bars in Fig. 2A). This is reminiscent of previously published reports of age-related deficits in memory tests performed at remote time points, but not soon after training (Moscovitch et al. 1986; Winocur 1988; Oler and Markus 1998; Houston et al. 1999). Interestingly, in aged mice, the specificity of memory shows a significant improvement over time in the FCSD group, but not in the FC Alone group (Fig. 2B). It should be noted that the aged, FC Alone mice showed evidence for a strong memory that was highly specific for the trained context during the 1-d tests (Fig. 2A,C). The lack of a further enhancement in memory specificity in the aged mice may indicate a lower saturation point in aged animals than in young animals, such that no further improvement in the specificity of memory was possible in the FC Alone group. In contrast, in the FCSD group, because SD initially impaired memory specificity during the Recent tests (Fig. 2A,C), there was ample room for improvement by the time of the Remote tests.
Figure 2.
Sleep deprivation after training in aged mice impairs memory at 1 d, but not 30 d, after training. Training, SD, and testing procedures for aged (22- to 23-mo old) C57BL/6 NIA mice were the same as shown in Figure 1A. (A) In aged mice, SD significantly reduced freezing in the trained context during the 1-d test, but had no significant effect on freezing during the Remote test. (B) SD did not have a significant effect on freezing in the altered context, which lessened over time in both the FC Alone and FCSD groups, similar to what was observed in young mice. (C) SD impaired the specificity of memory for the trained context during the Recent memory test, but had no effect during the Remote test. Aged FCSD mice showed a significant improvement in the specificity of memory over time, whereas aged FC Alone mice did not. Data are graphed as the mean ± SEM. (#) A significant overall effect of testing time, (+) a significant overall effect of SD, and (*) a significant post hoc comparison following a significant interaction between SD and testing time (P ≤ 0.05 was considered significant). Number of animals: FC Alone Recent = 20, FCSD Recent = 19, FC Alone Remote = 23, FCSD Remote = 24.
Sleep deprivation has equivalent effects on hippocampal gene expression in young and aged mice
We had previously shown that SD in young mice causes widespread changes in hippocampal gene expression (Vecsey et al. 2012). Therefore, in this study we examined whether aging altered the effects of SD on the expression of a subset of those previously identified genes, chosen to represent many of the functional clusters of SD target genes (Vecsey et al. 2012). Across young and aged mice, SD significantly increased the expression levels of Prkab2 (energy metabolism and regulation of sleep Carling 2005; Chikahisa et al. 2009; Dworak et al. 2010), Arc (synaptic plasticity Tzingounis and Nicoll 2006), Tsc22d3, Hspa5, and Hspb1, (stress response Gething 1999; Gusev et al. 2002; Naidoo et al. 2005, 2007; Ayroldi et al. 2007) (Fig. 3A, two-way ANOVA for each, P < 0.05). SD also significantly decreased expression levels of Rbm3 and Hnrpdl (mRNA metabolism and trafficking (Kawamura et al. 2002; Smart et al. 2007)) and Usp2 (deubiquitination and control of circadian rhythms (Metzig et al. 2011; Scoma et al. 2011; Yang et al. 2012)) (Fig. 3B; two-way ANOVA for each, P < 0.05).
Figure 3.
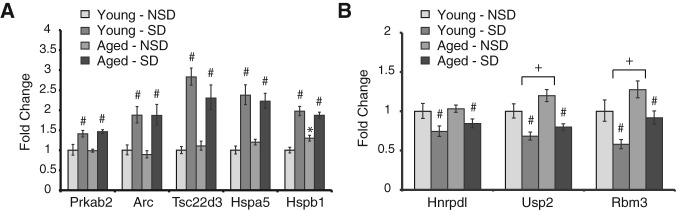
Sleep deprivation causes similar changes in hippocampal gene expression in young and aged mice. Young (2 mo) and aged (22–23 mo) C57BL/6 NIA mice were sleep-deprived for 5 h by gentle handling. Immediately afterward, hippocampal tissue was dissected and analyzed by quantitative real-time RT-PCR for the expression of several genes that had previously been shown to be up- or down-regulated by SD in the hippocampus of young mice (Vecsey et al. 2012). (A) Up-regulated genes. There was an overall effect of SD across both age groups on each gene. No significant effects of age were observed. The exception was Hspb1, for which there was a significant interaction between age and SD, indicating that SD caused a smaller up-regulation in aged animals than in young animals. (B) Down-regulated genes. There was an overall effect of SD across both age groups in each gene. For Usp2 and Rbm3, aged animals had significantly higher levels of gene expression, although there was no interaction between SD and aging. For a given gene, (#) a significant overall effect of SD relative to NSD, (+) a significant overall effect of age, and (*) a significant post hoc comparison following a significant interaction between SD and age (P ≤ 0.05 was considered significant). Data are graphed as the mean ± SEM, and n = 8 for each group.
In contrast to the widespread effects of SD, the only significant effect of aging was a slight increase in the expression of Rbm3 and Usp2 (two-way ANOVA, P < 0.05). For both of these genes, SD caused a similar decrease in their expression, even with increased baseline expression in aged animals. An interaction between aging and SD was found only for Hspb1 (two-way ANOVA, P = 0.007). Thus, across the eight genes tested, the effects of SD on gene expression were similar in both young and aged mice, and age itself had only minor effects. For example, Hspb1, Rbm3, and Usp2 had small, but significant, increases in hippocampal expression due to aging, but all experienced similar responses to SD. Combined with the behavioral findings in Figures 1, 2 showing that SD impaired memory at the 1-d test in both young and aged animals, these findings support the conclusion that SD has a similar impact on hippocampal function in young and aged animals. It is likely that the SD-induced impairment in remote memory in aged animals is mediated by a different set of genes and/or by a different brain area other than the hippocampus.
One of the brain areas thought to be key for systems memory consolidation is the anterior cingulate cortex (ACC) (Frankland et al. 2004, 2006; Teixeira et al. 2006; Goshen et al. 2011). It is possible that aging negatively affects plasticity mechanisms within this area, or limits the ability of the hippocampus to pass information to the ACC. One study (Miller et al. 2010) found that expression of calcineurin, a negative regulator of plasticity and memory (Mansuy 2003), was reduced in the dorsomedial prefrontal cortex (a region that includes the ACC) 30 d after CFC training, an effect that was correlated with hypermethylation of the gene locus. There are discrete time windows following learning when gene expression and protein synthesis events occur that mediate the initial phase of memory consolidation (Bourtchouladze et al. 1998; Igaz et al. 2002), but this is not the case for systems memory consolidation, which seems to happen piecemeal over the course of weeks. Screens for mutants with selective deficits in systems memory consolidation will be necessary to identify the genes involved in this process. It will also be important to identify the subset of these genes that may be affected by aging. In fact, no broad assessment of aging on ACC gene expression has been performed. Thus, it would be of interest to perform an experiment in which FC is performed in young and aged mice, and gene expression is broadly analyzed 30 d following training in the ACC versus hippocampus.
Studies in humans have observed several sleep disturbances with healthy aging, including reduced slow wave and REM sleep density as well as alterations in circadian rhythms (Pace-Schott and Spencer 2014). This is very similar to the effects of age on sleep in rodents (Welsh et al. 1986; Stone 1989; Hasan et al. 2012; Wimmer et al. 2013). It has been suggested that these changes in sleep contribute to age-related cognitive decline, including deficits in the consolidation of episodic, hippocampus-dependent memories (Pace-Schott and Spencer 2014).
The data presented here show that aged mice do not have impaired acquisition or initial consolidation of long-term contextual fear memory, demonstrating robust memory when tested a day after training. This suggests that ongoing age-related sleep abnormalities do not significantly impair memory consolidation in this task. In contrast, young and old mice both showed clear deficits in memory due to acute post-training SD. Although old mice tend to have reductions in sleep compared with young mice, they still obtain substantial amounts of sleep, whereas gentle handling SD has been shown to be highly effective at eliminating all sleep during the SD period (Hasan et al. 2012). Consistent with this view, a study in humans found that episodic memory in both young and old subjects was better preserved across a period of sleep than a period of wakefulness, demonstrating that the sleep older people obtain is still beneficial for memory (Aly and Moscovitch 2010). One might have expected that young mice, presumably achieving greater amounts of more consolidated sleep than aged mice, would have demonstrated better initial memory consolidation, but they did not, either in terms of total freezing or the specificity of memory for the trained context. A possible explanation is that there may be a threshold quality of sleep that is satisfactory for normal initial consolidation, but which is not enough to support gradual systems consolidation.
Not only did aged mice not show a deficit in memory during the Recent memory test, they showed significantly better memory specificity than young mice at the Recent test (F(1,76) = 4.04, P = 0.048), with no significant interaction between age and whether the mice were sleep-deprived (F(1,76) = 0.18, P = 0.67). In contrast, the trend had completely reversed by the Remote test, with younger mice showing greater memory specificity (F(1,86) = 5.35, P = 0.023), again with no interaction between age and SD treatment (F(1,86) = 1.09, P = 0.30). The finding that aged mice performed better than young mice during the Recent test was unexpected. However, this may be a consequence of a limitation of our study, which was that young mice were trained and tested in separate experimental cohorts from aged mice. Thus, it is possible that environmental conditions were not identical between age groups, which could have created an initial difference in the overall freezing levels between the two age groups. In future studies, it would be ideal to run all age groups intermixed within each cohort to prevent issues of batch effects. The fact that memory in aged mice became worse than in young mice by the Remote test, on the other hand, was not surprising. Our data showed that memory in young mice improves over a month-long interval, whereas it decays in aged mice, consistent with previous reports (Oler and Markus 1998; Houston et al. 1999; Pickens et al. 2009). This deficit in long-term retention in aged animals may be a result of issues with memory storage in the hippocampus over the time-course of weeks, or because of problems with transferring information to cortical regions such as the ACC (Frankland et al. 2004; Frankland and Bontempi 2005). Although SD after training impairs initial memory consolidation, our findings suggest that the portion of the memory that is formed can be maintained effectively and eventually transferred into permanent storage. This indicates that changes in memory over prolonged delays after learning may be independent of the influence of SD occurring during initial memory consolidation.
In the current study, altered context tests were always run 24 h after the trained context test. Thus, reactivation of the fear memory during the trained context test could have had an impact on the fear response during the subsequent altered context test through extinction or a reconsolidation effect (Debiec et al. 2002). However, the design of the experiment allowed us to analyze freezing in the trained context alone (see Figs. 1B, 2A), which is free of confounds of prior reactivation. The patterns of the effects of age and SD on memory in the trained context were very similar to those for memory specificity, in which freezing in the altered context was subtracted from freezing in the trained context. Thus, we do not believe that the order of testing affected our conclusions.
In general, our findings suggest that aging and acute SD disrupt episodic memory via different mechanisms, and that acute SD has similar effects on initial, hippocampus-dependent memory consolidation, and hippocampal gene expression in young and aged animals. It will be of future interest to explore the cellular and molecular nature of the deficits in remote memory seen in aged animals, and to determine if aging-related sleep abnormalities do in fact contribute to worsening in other areas of cognitive function or general health.
Acknowledgments
This research was supported by Systems and Integrative Biology Training grant GM07517 to C.G.V., M. Nusbaum PI, NIH training grant HL07953 to C.G.V., A.I. Pack PI, and the National Institutes of Health, P50 AG 017628 to T.A., A. I. Pack PI and RO1 MH 099544 to T.A.
Author contributions: T.A. and C.V. conceived and designed the study. N.K., A.P., and C.V. acquired data. N.K., A.P., C.V., and T.A. analyzed and interpreted data. C.V., N.K., and A.P. drafted the manuscript. C.V., A.P., and T.A. critically revised the manuscript. N.K., A.P., and C.V. performed statistical analysis.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.036590.114.
References
- Abel T, Lattal KM 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11: 180–187. [DOI] [PubMed] [Google Scholar]
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615–626. [DOI] [PubMed] [Google Scholar]
- Aly M, Moscovitch M 2010. The effects of sleep on episodic memory in older and younger adults. Memory 18: 327–334. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS 1999. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci 19: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroldi E, Zollo O, Bastianelli A, Marchetti C, Agostini M, Di Virgilio R, Riccardi C 2007. GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling. J Clin Invest 117: 1605–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL 1985. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci 99: 1040–1048. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC 1969. Crouching as an index of fear. J Comp Physiol Psychol 67: 370–375. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL 1989. Sleep loss in aging. Clin Geriatr Med 5: 405–420. [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R 1999. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 400: 671–675. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER 1998. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem 5: 365–374. [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA 2006. Neural plasticity in the ageing brain. Nat Rev Neurosci 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A 2006. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int 23: 461–474. [DOI] [PubMed] [Google Scholar]
- Carling D 2005. AMP-activated protein kinase: balancing the scales. Biochimie 87: 87–91. [DOI] [PubMed] [Google Scholar]
- Chikahisa S, Fujiki N, Kitaoka K, Shimizu N, Séi H 2009. Central AMPK contributes to sleep homeostasis in mice. Neuropharmacology 57: 369–374. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K 2002. Cellular and systems reconsolidation in the hippocampus. Neuron 36: 527–538. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R 2010. Sleep and brain energy levels: ATP changes during sleep. J Neurosci 30: 9007–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H 1999. The hippocampus and mechanisms of declarative memory. Behav Brain Res 103: 123–133. [DOI] [PubMed] [Google Scholar]
- Fanselow MS 1980. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15: 177–182. [DOI] [PubMed] [Google Scholar]
- Foster TC, Defazio RA, Bizon JL 2012. Characterizing cognitive aging of spatial and contextual memory in animal models. Front Aging Neurosci 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B 2005. The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ 1998. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci 112: 863–874. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ 2004. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304: 881–883. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ 2006. Stability of recent and remote contextual fear memory. Learn Mem 13: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething MJ 1999. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol 10: 465–472. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K 2011. Dynamics of retrieval strategies for remote memories. Cell 147: 678–689. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T 2003. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem 10: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Peng X, Fang J 2004. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res 1018: 38–47. [DOI] [PubMed] [Google Scholar]
- Gusev NB, Bogatcheva NV, Marston SB 2002. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry (Mosc) 67: 511–519. [DOI] [PubMed] [Google Scholar]
- Hasan S, Dauvilliers Y, Mongrain V, Franken P, Tafti M 2012. Age-related changes in sleep in inbred mice are genotype dependent. Neurobiol Aging 33: 195.e13–195.e26. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Danker-Hopfe H, Heuser I 2005. Age-related changes in sleep and memory: commonalities and interrelationships. Exp Gerontol 40: 279–285. [DOI] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA 1999. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem 6: 111–119. [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Vianna MR, Medina JH, Izquierdo I 2002. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci 22: 6781–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Tomozoe Y, Akagi T, Kamei D, Ochiai M, Yamada M 2002. Identification of the nucleocytoplasmic shuttling sequence of heterogeneous nuclear ribonucleoprotein D-like protein JKTBP and its interaction with mRNA. J Biol Chem 277: 2732–2739. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS 1992. Modality-specific retrograde amnesia of fear. Science 256: 675–677. [DOI] [PubMed] [Google Scholar]
- Luyster FS, Strollo PJ Jr, Zee PC, Walsh JK; Boards of Directors of the American Academy of Sleep Medicine and the Sleep Research Society. 2012. Sleep: a health imperative. Sleep 35: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy IM 2003. Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun 311: 1195–1208. [DOI] [PubMed] [Google Scholar]
- Metzig M, Nickles D, Falschlehner C, Lehmann-Koch J, Straub BK, Roth W, Boutros M 2011. An RNAi screen identifies USP2 as a factor required for TNF-α-induced NF-κB signaling. Int J Cancer 129: 607–618. [DOI] [PubMed] [Google Scholar]
- Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G, Sweatt JD 2010. Cortical DNA methylation maintains remote memory. Nat Neurosci 13: 664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G, McLachlan D 1986. Memory as assessed by recognition and reading time in normal and memory-impaired people with Alzheimer's disease and other neurological disorders. J Exp Psychol Gen 115: 331–347. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, et al. 2005. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat 207: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N, Giang W, Galante RJ, Pack AI 2005. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem 92: 1150–1157. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Casiano V, Cater J, Zimmerman J, Pack AI 2007. A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis. Sleep 30: 557–565. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M 2008. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol Aging 23: 104–118. [DOI] [PubMed] [Google Scholar]
- Oler JA, Markus EJ 1998. Age-related deficits on the radial maze and in fear conditioning: hippocampal processing and consolidation. Hippocampus 8: 402–415. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM 2014. Sleep-dependent memory consolidation in healthy aging and mild cognitive impairment. Curr Top Behav Neurosci. 10.1007/7854_2014_300. [DOI] [PubMed] [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW 1994. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci 108: 810–817. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y 2009. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry 65: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H 2008. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci 28: 8945–8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ 2004. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci 19: 3121–3124. [DOI] [PubMed] [Google Scholar]
- Scoma HD, Humby M, Yadav G, Zhang Q, Fogerty J, Besharse JC 2011. The de-ubiquitinylating enzyme, USP2, is associated with the circadian clockwork and regulates its sensitivity to light. PLoS One 6: e25382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B 1957. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, Vanderklish PW 2007. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem 101: 1367–1379. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N 1995. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging 10: 527–539. [DOI] [PubMed] [Google Scholar]
- Stone WS 1989. Sleep and aging in animals. Relationships with circadian rhythms and memory. Clin Geriatr Med 5: 363–379. [PubMed] [Google Scholar]
- Sutherland RJ, Lehmann H 2011. Alternative conceptions of memory consolidation and the role of the hippocampus at the systems level in rodents. Curr Opin Neurobiol 21: 446–451. [DOI] [PubMed] [Google Scholar]
- Tanila H, Shapiro M, Gallagher M, Eichenbaum H 1997. Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci 17: 5155–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW 2006. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci 26: 7555–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA 2006. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron 52: 403–407. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, et al. 2009. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature 461: 1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S, et al. 2012. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics 44: 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Wimmer ME, Havekes R, Park AJ, Perron IJ, Meerlo P, Abel T 2013. Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in mice. Sleep 36: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Richardson GS, Dement WC 1986. Effect of age on the circadian pattern of sleep and wakefulness in the mouse. J Gerontol 41: 579–586. [DOI] [PubMed] [Google Scholar]
- Wimmer ME, Rising J, Galante RJ, Wyner A, Pack AI, Abel T 2013. Aging in mice reduces the ability to sustain sleep/wake states. PLoS One 8: e81880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G 1988. Long-term memory loss in senescent rats: neuropsychological analysis of interference and context effects. Psychol Aging 3: 273–279. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B 2010. Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia 48: 2339–2356. [DOI] [PubMed] [Google Scholar]
- Yang Y, Duguay D, Bédard N, Rachalski A, Baquiran G, Na CH, Fahrenkrug J, Storch KF, Peng J, Wing SS, et al. 2012. Regulation of behavioral circadian rhythms and clock protein PER1 by the deubiquitinating enzyme USP2. Biol Open 1: 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]