Abstract
The common gamma chain (γc) is the central signaling unit for a number of cytokine receptors collectively known as the γc cytokine receptor family. γc is critical for ligand binding and signaling by γc cytokines. γc cytokine signaling had been thought to be mainly regulated by cytokine-specific receptor α chain expression levels with little or no effect by γc surface levels because γc expression was presumed to remain unchanged during T-cell activation and development. The extent of γc cytokine responses is thought to be regulated by cytokine specific receptor subunits and not by the γc receptor. In contrast to this prevailing view, we have recently reported that γc itself actively regulates γc cytokine responses. Interestingly, γc exerted its regulatory effects not only as a conventional membrane receptor protein but also as a secreted protein whose expression was upregulated upon T-cell stimulation. Here we will review how a soluble form of γc, which is generated by alternative splicing, regulates γc cytokine signaling and plays a role in controlling immune activation related to autoimmune disease.
Keywords: Common gamma chain, Autoimmune disease, Cytokine, Soluble receptors
Introduction
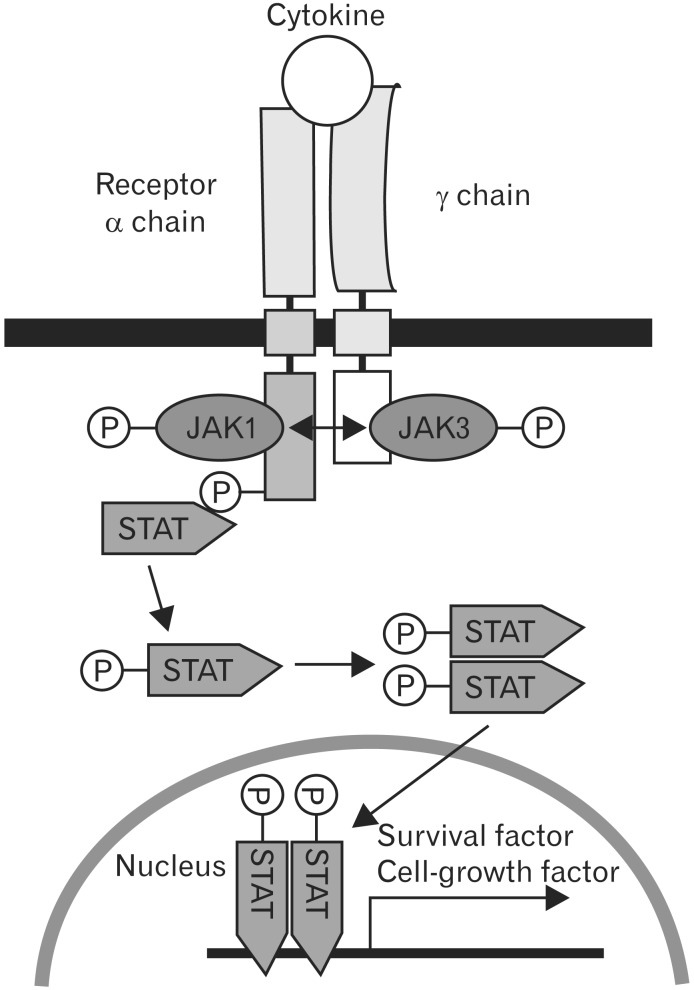
Cytokines are a class of soluble signaling molecules that regulate the activation and differentiated functions of immune cells through their interaction with receptors. Several cytokine receptors are multimeric complexes composed up to two or more different subunit proteins, which are shared between multiple cytokine receptors. It has been shown that the common gamma chain (γc) is shared as the essential signal transducing subunit between the receptors for interleukin (IL)-2, IL-4, IL-7, IL-9, IL-15, and IL-21 [1]. All γc family cytokines similarly activates the Janus kinase (JAK)-family protein tyrosine kinases JAK1 and JAK3, with JAK1 binding a unique α or β chain and JAK3 binding γc [2]. They exert its effect through interaction with γc cytokine receptor complex, which is composed of a unique receptor chain and γc. γc cytokines binding to their specific receptor triggers transphosphorylation of JAK1 and JAK3 and phosphorylated JAK activates the signal transducers and activators of transcription (STAT) of JAK/STAT pathway (Fig. 1) [3]. Interestingly, IL-2, IL-7, IL-9, and IL-15 mainly activate STAT5 (STAT5A and STAT5B) [4], while IL-4 mainly activates STAT6 [5, 6] and IL-21 mainly activates STAT3 [7, 8]. The JAK-STAT pathway has been implicated in immune cell-growth control and survival. Natural defects in γc are responsible for X-linked severe combined immunodeficiency disease in humans, characterized by a complete absence of T and natural killer cells, while B cells are present [9]. Genetic defects of γc in mice severely impair the development of T and B cells. These findings show the critical role of γc-dependent signals in lymphopoiesis [10]. Despite its critical role, the cellular signals and molecular mechanisms that regulate γc expression are still poorly characterized.
Fig. 1. Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling pathway of gamma chain (γc) family cytokines. The trans-activation of JAKs after cytokine stimulation results in the phosphorylation of STATs, which then dimerize and translocate to the nucleus to activate gene transcription.
The extent and magnitude of cytokine signaling must be tightly controlled since excessive cytokine signaling can lead to inflammation, autoimmunity, and cancer while diminished cytokine signaling can result in immune deficiency and lymphopenia. Consequently, the immune system employs various ways to precisely tune both the strength and duration of cytokine signaling in individual cells. One of the mechanisms that control cytokine signaling is the generation of soluble cytokine receptors [11], which are present as immunomodulatory molecules in body fluid of human and mice [12]. Soluble cytokine receptors has two major functions: inhibitors of their membrane-bound counterparts by competing for ligand to prevent signaling or inducers of relevant cytokine responsiveness by serving as binding proteins to stabilize ligand or trans-signaling of cytokine-binding soluble cytokine receptor complex [13]. The molecular mechanisms that generate soluble cytokine receptors include proteolytic cleavage of transmembrane receptors catalyzed by membrane proteases, alternative splicing of mRNA transcripts, and transcription of distinct genes that encode soluble cytokine receptors [14].
A murine soluble γc (sγc) was present in sera of mice and identified as a negative and selective regulator of cytokine responses [15]. However, what mechanisms are responsible for the production of sγc, what is the biological functions of sγc, and how sγc are involved in regulating key inflammatory and immune response are unclear. Recent publications demonstrated that soluble IL-7Rα closely related with rheumatoid arthritis and multiple sclerosis autoimmune disease [16]. So relationship between sγc and disease needs to be elucidated.
Regulation of γc Expression during T-Cell Development
It has been understood that γc expression is consistent in immune responses, while IL-7Rα expression is dynamically regulated during T-cell development [17]. Previous studies described that pre-selection double positive thymocytes should be unsignaled by prosurvival cytokines such as IL-7 to permit thymocytes with appropriate T-cell receptor specificities to survive and to differentiate into functional mature T cells [18]. Indeed, in the presence of exogenous IL-7, cytokine-responsive DP thymocytes differentiated into CD8+ T cells with T-cell receptor (TCR)-independent manner [19]. Several mechanisms to avoid cytokine signaling have been suggested; DP thymocytes express uniquely high levels of the suppressor of cytokine signaling-1 (SOCS1) [20] and are deficient in IL-7Rα expression [21]. We consider the low surface γc levels in DP thymocytes as active regulatory event with potential biological significance which is providing a novel additional mechanism for avoiding cytokine induction of prosurvival factors in DP thymocytes. Low surface γc expression in DP thymocytes might have been resulted from a novel post-transcriptional mechanism. Of special interest, DP thymocytes expressed high levels of an alternative mRNA splice variant of γc that failed to produce a full length γc protein. Thus, immature DP thymocytes fail to express high levels of membrane γc-chain presumably because a significant fraction of their γc-chain transcripts are translated into a secreted isoform in the expense of membrane γc. However, the evaluation of the exact regulatory mechanisms for γc expression awaits further studies.
sγc Generation by Alternative Splicing
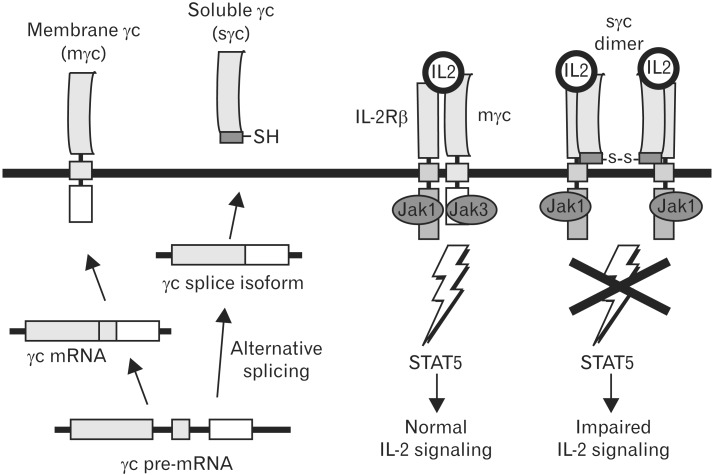
It has been already well documented that soluble cytokine receptors, which mediate agonistic or antagonistic effect in cytokine signaling, are important regulators of inflammation and immunity [22, 23, 24, 25, 26]. It has been reported that soluble form of IL-1R, IL-2Rα, IL-4Rα, IL-5Rα, IL-6Rα, IL-7Rα, IL-9Rα, IL-15Rα, IL-17Rα, GM-CSFRα, TNFRα, and gp130 are present in body fluids [27]. Several cytokine receptors, including TNFR, IL-1R, IL-4Rα, and IL-6Rα, are generated by both mechanisms in the same cell [28, 29, 30, 31, 32, 33, 34, 35]. In contrast to previous report, several considerations and observations make "alternative splicing", but not "shedding", the only mechanism responsible for sγc generation [12, 13, 22, 25]. Alternative spliced massage generates soluble receptors with new C-terminal peptide sequences that are unique to these splice isoforms (Fig. 1). Both expression of sγc and mγc was upregulated in activated T cells [36]. No inhibition of sγc production was observed with a number of protease inhibitors. Furthermore, we specifically detected alternative splicing form of γc using antibodies against the unique 9-amino acid C-terminal epitope of sγc. To test directly whether sγc is generated by alternative splicing, we introduced a full-length mouse γc cDNA into γc-/- background. Because no expression of sγc in γc-/-γcTg mice was detected, shedding mechanism could be excluded as being involved in sγc generation in vivo [36].
Regulatory Function of sγc in Cytokine Signaling
It has been thought that cytokine signals are controlled by regulation of their relevant receptor and SOCS molecules [20]. IL-2 signaling induces upregulation of IL-2 receptor expression which is critical for initiating the autocrine expression of IL-2 in activated T cells [37, 38]. On the other hand, IL-7 signaling downregulates expression of its own receptor, and using transgenic mice, we showed that the failure to downregulate IL-7 receptor results in impaired T-cell development and homeostasis [39]. TCR signals can also desensitize cytokine receptors but the molecular pathway resulting in impaired cytokine signaling is not fully understood. We think that γc plays a critical role in limit of cytokine signal upon TCR stimulation. Indeed, our unpublished data and previous report showed that increased surface and sγc expression inhibits γc family cytokine signaling [36]. Major sources of the sγc in the mouse sera are T cells. JAK3 is closely associated in regulation of cytokine responsiveness by γc, since JAK3 is γc-associated and is involved in cytokine signal transduction [40]. JAK3 expression is limited even upon TCR stimulation (unpublished data), indicating that increased γc level results in impaired γc cytokine signaling. In the same manner, enhanced sγc compete with mγc and thus JAK3-unassociated sγc give rise to a reduction of cytokine singling (Fig. 2). Upregulation of mγc and sγc in activated T cells might be a novel new mechanism in which providing large amount of cytokine produced by activating immune cells for control signaling formed.
Fig. 2. Inhibitory function of soluble gamma chain (sγc) generated by alternative splicing in cytokine signaling. Activated T cells upregulate expression of an alternatively spliced form of gamma chain (γc) mRNA. γc splice isoform expression results in secretion of the γc extracellular domain. Soluble γc binds directly to surface interleukin (IL)-2Rβ and inhibits IL-2 signaling.
sγc level is closely associated with T-cell activity, supporting that high level of sγc expression in CTLA4-/- mice was restored to normal level in CTLA4-/-CD28-/- mice [36]. mγc and sγc was dynamically regulated in vivo and in vitro by TCR stimulation. Our unpublished data and previous report showed that increased surface and sγc expression affects γc family cytokine signaling. Together these findings indicate that regulation of mγc and sγc in activated T cells affect their differentiation through control of γc family cytokine signaling, because IL-4 is important in Th2 differentiation [1] and IL-2 suppresses Th17 differentiation [41] but induces Treg development [42]. However, the role of increased γc expression in activated T cells has currently been unknown to us. It is also not known whether such γc upregulation is of importance for mounting a proper T-cell response. Using sγcTg mice that are specifically overexpressed in T cells, we studied the role of increased sγc expression in T-cell development, homeostasis, and immune responses. Thymocytes number was reduced sγc-level dependently in different sγcTg line, while peripheral lymph node cell number was not changed. As we previously demonstrated that IL-7 is required to develop CD8+ T cells, these data supported that the other γc family cytokines are involved in T-cell development. However, inhibitory function of sγc was not sufficient in homeostasis of peripheral lymphocytes [36]. We found interestingly that memory phenotype CD4 and CD8 T cells are increased in sγc overexpressed mice, suggesting that generation of memory T cells is regulated by sγc [36]. Nevertheless, the elucidation of the exact role of the sγc in memory T cell generation awaits further studies.
Regulatory Function of sγc in Autoimmune Disease
As IL-2 inhibits the differentiation of Th17 cells [41] and high level of sγc was detected in autoimmune disease of mouse, thus we thought that sγc would affect Th differentiation. Exogenously treated sγc permitted the enhanced in vitro differentiation of Th17 cells, while in vitro Th1 and Th2 differentiation was not affected. Enhancement of Th1 and Th17 differentiation was confirmed in sγcTg mice. Thus, we suggest a model in which sγc secreted by activated T cells blocks IL-2 signaling and then leads to enhance the differentiation of Th17 cells. It is remarkable that in vivo effect of sγc in experimental autoimmune encephalomyelitis is highly selective for Th1 and Th17 cells of the effector and memory phenotype. The selectivity of Th1 cells might be explained by the different target of sγc, as sγc can block IL-4 signaling and then lead to suppress the Th2 differentiation. Thus differentiation of Th1 and Th17 involves an extrinsic requirement of sγc in the course of EAE. Furthermore, sγc level is increased in human inflammatory bowel disease (IBD) patients who T cells are highly activated [43] and abundant in synovial fluid of rheumatoid joints where activated T cell-mediated autoimmune response is occurring [44]. In consist to human data, sγc level is elevated in autoimmune mice, in which activated T cells are highly accumulated by lack of Treg cell and IBD is consequently developed [36]. Autoimmune disease is exacerbated as shown by higher clinical score for EAE pathology in sγc transgenic mice. On the contrary, sγc deficient animal resisted the induction of EAE and displayed improvement of inflammatory autoimmune disease [36]. sγc level might be highly correlated to autoimmune disease as shown by human clinical data and our recent report. More studies in human patients are required to concretely indentify how sγc level is involved in autoimmune diseases.
Conclusion
The previously uncovered role of sγc in cytokine signaling and immune responses as discussed here provides initial explanations for novel regulatory mechanism of cytokine signaling. sγc induction impairs naïve T-cell survival and promotes inflammation in a manner of inhibiting IL-7 and IL-2 signaling, representatively. Furthermore, sγc expression is significantly enhanced upon T cell activation. sγc enhances in vitro and in vivo Th17 differentiation through dampening of IL-2 signal, and sγc-overexpressing mice are consequently more susceptible to EAE. Therefore, sγc is a novel immunoregulator that control T cell biology by regulation of γc cytokine signaling.
Acknowledgements
This work was supported by a 2-Year Research Grant of Pusan National University.
References
- 1.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 3.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 4.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, Leonard WJ. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 5.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 6.Quelle FW, Shimoda K, Thierfelder W, Fischer C, Kim A, Ruben SM, Cleveland JL, Pierce JH, Keegan AD, Nelms K, Paul WE, Ihle JN. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995;15:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci U S A. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 9.Fischer A, Hacein-Bey S, Le Deist F, de Saint Basile G, Cavazzana-Calvo M. Gene therapy for human severe combined immunodeficiencies. Isr Med Assoc J. 2002;4:51–54. [PubMed] [Google Scholar]
- 10.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 11.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–5348. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- 12.Heaney ML, Golde DW. Soluble cytokine receptors. Blood. 1996;87:847–857. [PubMed] [Google Scholar]
- 13.Fernandez-Botran R. Soluble cytokine receptors: their role in immunoregulation. FASEB J. 1991;5:2567–2574. doi: 10.1096/fasebj.5.11.1868981. [DOI] [PubMed] [Google Scholar]
- 14.Levine SJ. Molecular mechanisms of soluble cytokine receptor generation. J Biol Chem. 2008;283:14177–14181. doi: 10.1074/jbc.R700052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meissner U, Blum H, Schnare M, Röllinghoff M, Gessner A. A soluble form of the murine common gamma chain is present at high concentrations in vivo and suppresses cytokine signaling. Blood. 2001;97:183–191. doi: 10.1182/blood.v97.1.183. [DOI] [PubMed] [Google Scholar]
- 16.Lundstrom W, Highfill S, Walsh ST, Beq S, Morse E, Kockum I, Alfredsson L, Olsson T, Hillert J, Mackall CL. Soluble IL7Ralpha potentiates IL-7 bioactivity and promotes autoimmunity. Proc Natl Acad Sci U S A. 2013;110:E1761–E1770. doi: 10.1073/pnas.1222303110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong C, Luckey MA, Park JH. Intrathymic IL-7: the where, when, and why of IL-7 signaling during T cell development. Semin Immunol. 2012;24:151–158. doi: 10.1016/j.smim.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adoro S, Erman B, Sarafova SD, Van Laethem F, Park JH, Feigenbaum L, Singer A. Targeting CD4 coreceptor expression to postselection thymocytes reveals that CD4/CD8 lineage choice is neither error-prone nor stochastic. J Immunol. 2008;181:6975–6983. doi: 10.4049/jimmunol.181.10.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. 'Coreceptor tuning': cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 21.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4-versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Botran R, Chilton PM, Ma Y. Soluble cytokine receptors: their roles in immunoregulation, disease, and therapy. Adv Immunol. 1996;63:269–336. doi: 10.1016/s0065-2776(08)60858-5. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Botran R, Vitetta ES. Evidence that natural murine soluble interleukin 4 receptors may act as transport proteins. J Exp Med. 1991;174:673–681. doi: 10.1084/jem.174.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gessner A, Schröppel K, Will A, Enssle KH, Lauffer L, Röllinghoff M. Recombinant soluble interleukin-4 (IL-4) receptor acts as an antagonist of IL-4 in murine cutaneous Leishmaniasis. Infect Immun. 1994;62:4112–4117. doi: 10.1128/iai.62.10.4112-4117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64:135–146. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- 26.Novick D, Shulman LM, Chen L, Revel M. Enhancement of interleukin 6 cytostatic effect on human breast carcinoma cells by soluble IL-6 receptor from urine and reversion by monoclonal antibody. Cytokine. 1992;4:6–11. doi: 10.1016/1043-4666(92)90029-q. [DOI] [PubMed] [Google Scholar]
- 27.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300(Pt 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Althoff K, Reddy P, Voltz N, Rose-John S, Müllberg J. Shedding of interleukin-6 receptor and tumor necrosis factor alpha. Contribution of the stalk sequence to the cleavage pattern of transmembrane proteins. Eur J Biochem. 2000;267:2624–2631. doi: 10.1046/j.1432-1327.2000.01278.x. [DOI] [PubMed] [Google Scholar]
- 29.Hughes DP, Crispe IN. A naturally occurring soluble isoform of murine Fas generated by alternative splicing. J Exp Med. 1995;182:1395–1401. doi: 10.1084/jem.182.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen LE, Muzio M, Mantovani A, Whitehead AS. IL-1 signaling cascade in liver cells and the involvement of a soluble form of the IL-1 receptor accessory protein. J Immunol. 2000;164:5277–5286. doi: 10.4049/jimmunol.164.10.5277. [DOI] [PubMed] [Google Scholar]
- 31.Lust JA, Donovan KA, Kline MP, Greipp PR, Kyle RA, Maihle NJ. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4:96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 32.Michel J, Langstein J, Hofstädter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol. 1998;28:290–295. doi: 10.1002/(SICI)1521-4141(199801)28:01<290::AID-IMMU290>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Mosley B, Beckmann MP, March CJ, Idzerda RL, Gimpel SD, VandenBos T, Friend D, Alpert A, Anderson D, Jackson J, Wignall JM, Smith C, Gallis B, Sims JE, Urdal D, Widmer MB, Cosman D, Park LS. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989;59:335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- 34.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 35.Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem. 2000;275:14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- 36.Hong C, Luckey MA, Ligons DL, Waickman AT, Park JY, Kim GY, Keller HR, Etzensperger R, Tai X, Lazarevic V, Feigenbaum L, Catalfamo M, Walsh ST, Park JH. Activated T cells secrete an alternatively spliced form of common gamma-chain that inhibits cytokine signaling and exacerbates inflammation. Immunity. 2014;40:910–923. doi: 10.1016/j.immuni.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel JP, Sharon M, Smith PL, Leonard WJ. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987;238:75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- 39.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O'Shea JJ. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 41.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen OH, Kirman I, Johnson K, Giedlin M, Ciardelli T. The circulating common gamma chain (CD132) in inflammatory bowel disease. Am J Gastroenterol. 1998;93:323–328. doi: 10.1111/j.1572-0241.1998.00323.x. [DOI] [PubMed] [Google Scholar]
- 44.Nishio J, Kohsaka H, Shimamura T, Hamuro J, Miyasaka N. Abundant expression of common cytokine receptor gamma chain (CD132) in rheumatoid joints. J Rheumatol. 2001;28:240–244. [PubMed] [Google Scholar]