Abstract
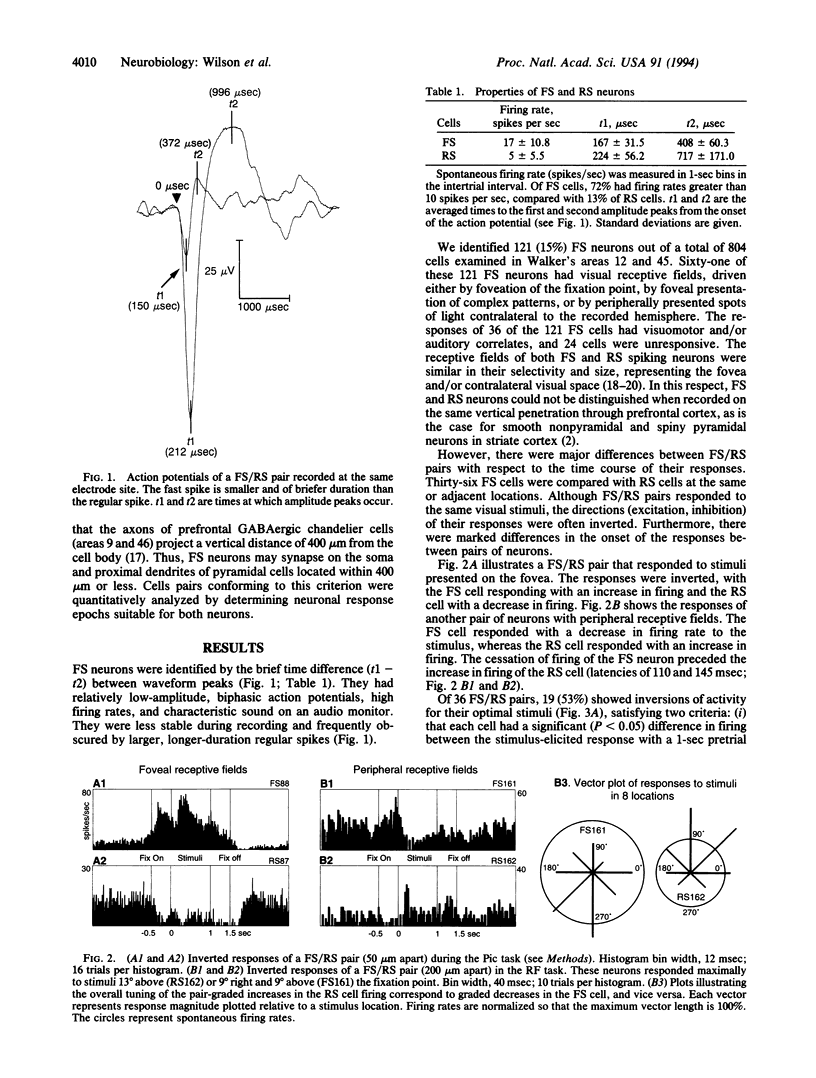
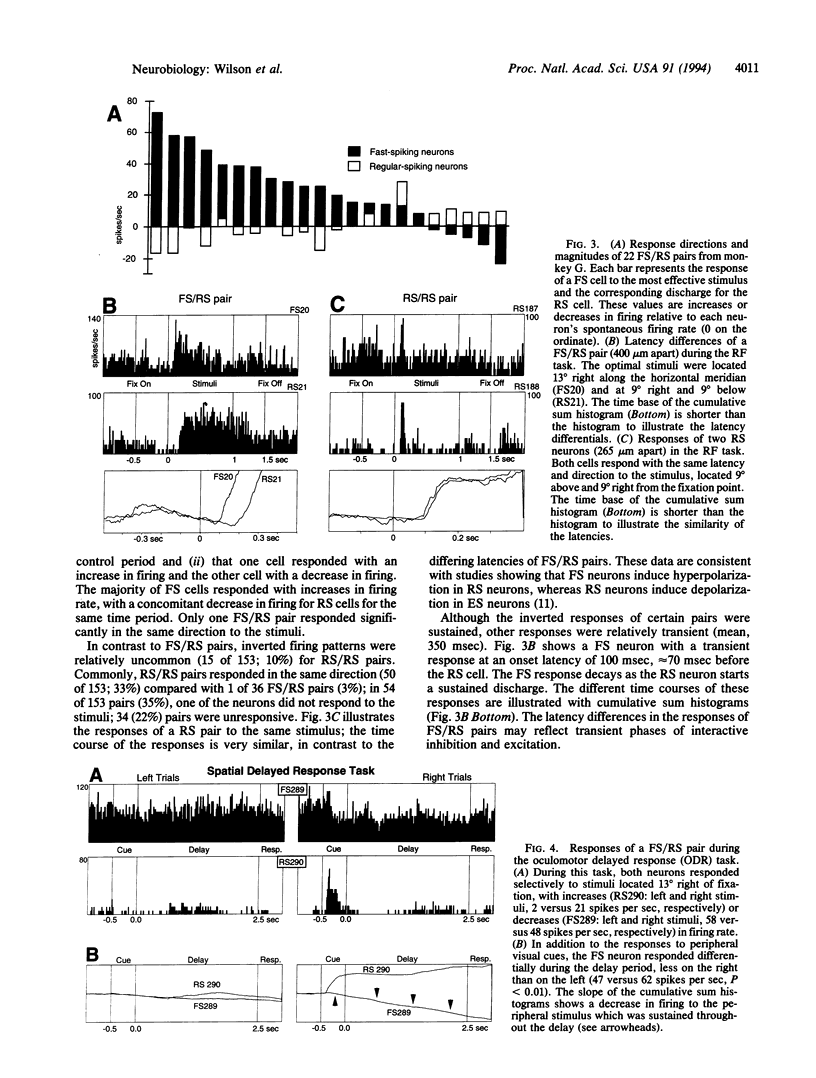
The responses of putative gamma-aminobutyratergic interneurons (fast-spiking) and pyramidal (regular-spiking) cell pairs were compared in monkeys performing visual and memory-guided oculomotor tasks. Both fast- and regular-spiking neurons had similar receptive fields, indicating that gamma-aminobutyratergic interneurons carry a specific informational signal, as opposed to providing nonspecific modulation. However, the responses of the pairs were inverted and the timing of excitatory and inhibitory responses appeared to be phased, a property consistent with gamma-aminobutyrate-mediated shaping of receptive fields. These observations (i) provide evidence that interneurons and pyramidal cells can be differentiated in vivo and (ii) begin to elucidate the role of gamma-aminobutyratergic mechanisms in cognition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988 Oct 15;276(3):313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Bruce C. J., Goldberg M. E. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985 Mar;53(3):603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Cavada C., Goldman-Rakic P. S. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989 Sep 22;287(4):422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Celio M. R. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986 Feb 28;231(4741):995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990 Mar;13(3):99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes R. W., Landry P., Metherate R., Hicks T. P. Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J Neurophysiol. 1984 Dec;52(6):1066–1093. doi: 10.1152/jn.1984.52.6.1066. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Ferino F., Thierry A. M., Saffroy M., Glowinski J. Interhemispheric and subcortical collaterals of medial prefrontal cortical neurons in the rat. Brain Res. 1987 Aug 11;417(2):257–266. doi: 10.1016/0006-8993(87)90450-1. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Martin K. A., Somogyi P., Whitteridge D. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y- type thalamic afferents. II. Identification of postsynaptic targets by GABA immunocytochemistry and Golgi impregnation. J Comp Neurol. 1985 Dec 8;242(2):275–291. doi: 10.1002/cne.902420209. [DOI] [PubMed] [Google Scholar]
- Funahashi S., Bruce C. J., Goldman-Rakic P. S. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989 Feb;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Schwark H. D., Jones E. G., Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987 May;7(5):1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993 Feb;69(2):416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981 Mar;1(3):318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. A., Lund J. S. Heterogeneity of chandelier neurons in monkey neocortex: corticotropin-releasing factor- and parvalbumin-immunoreactive populations. J Comp Neurol. 1990 Mar 22;293(4):599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- Martin K. A. The Wellcome Prize lecture. From single cells to simple circuits in the cerebral cortex. Q J Exp Physiol. 1988 Sep;73(5):637–702. doi: 10.1113/expphysiol.1988.sp003190. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Mikami A., Ito S., Kubota K. Visual response properties of dorsolateral prefrontal neurons during visual fixation task. J Neurophysiol. 1982 Apr;47(4):593–605. doi: 10.1152/jn.1982.47.4.593. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Park T. J., Pollak G. D. GABA shapes a topographic organization of response latency in the mustache bat's inferior colliculus. J Neurosci. 1993 Dec;13(12):5172–5187. doi: 10.1523/JNEUROSCI.13-12-05172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirot S., Godbout R., Mantz J., Tassin J. P., Glowinski J., Thierry A. M. Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: evidence for the involvement of both dopaminergic and GABAergic components. Neuroscience. 1992 Aug;49(4):857–865. doi: 10.1016/0306-4522(92)90362-6. [DOI] [PubMed] [Google Scholar]
- Richardson R. T., DeLong M. R. Context-dependent responses of primate nucleus basalis neurons in a go/no-go task. J Neurosci. 1990 Aug;10(8):2528–2540. doi: 10.1523/JNEUROSCI.10-08-02528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol. 1986 Nov;56(5):1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Zheng D. S., Goldman-Rakic P. S. Periodicity of GABA-containing cells in primate prefrontal cortex. J Neurosci. 1988 Jun;8(6):1962–1970. doi: 10.1523/JNEUROSCI.08-06-01962.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Kunkel D. D. Morphology of identified interneurons in the CA1 regions of guinea pig hippocampus. J Comp Neurol. 1985 Feb 8;232(2):205–218. doi: 10.1002/cne.902320206. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Simons D. J. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978 May;41(3):798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Swadlow H. A. Efferent neurons and suspected interneurons in binocular visual cortex of the awake rabbit: receptive fields and binocular properties. J Neurophysiol. 1988 Apr;59(4):1162–1187. doi: 10.1152/jn.1988.59.4.1162. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Rolls E. T. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Res. 1990 Feb 19;509(2):213–231. doi: 10.1016/0006-8993(90)90546-n. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Scalaidhe S. P., Goldman-Rakic P. S. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993 Jun 25;260(5116):1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]



