Abstract
Aiming to identify new sources of bioactive secondary metabolites, we isolated 82 endophytic fungi from stems and barks of the native Brazilian tree Caesalpinia echinata Lam. (Fabaceae). We tested their ethyl acetate extracts in several in vitro assays. The organic extracts from three isolates showed antibacterial activity against Staphylococcus aureus and Escherichia coli [minimal inhibitory concentration (MIC) 32-64 μg/mL]. One isolate inhibited the growth of Salmonella typhimurium (MIC 64 μg/mL) and two isolates inhibited the growth of Klebsiella oxytoca (MIC 64 μg/mL), Candida albicans and Candida tropicalis (MIC 64-128 μg/mL). Fourteen extracts at a concentration of 20 μg/mL showed antitumour activities against human breast cancer and human renal cancer cells, while two isolates showed anti-tumour activities against human melanoma cancer cells. Six extracts were able to reduce the proliferation of human peripheral blood mononuclear cells, indicating some degree of selective toxicity. Four isolates were able to inhibit Leishmania (Leishmania) amazonensis and one isolate inhibited Trypanosoma cruzi by at least 40% at 20 μg/mL. The trypanocidal extract obtained from Fusarium sp. [KF611679] culture was subjected to bioguided fractionation, which revealed beauvericin as the compound responsible for the observed toxicity of Fusarium sp. to T. cruzi. This depsipeptide showed a half maximal inhibitory concentration of 1.9 μg/mL (2.43 μM) in a T. cruzi cellular culture assay.
Keywords: endophytic fungi, bioactive, Caesalpinia echinata Lam., Fabaceae, Trypanosoma cruzi, beauvericin
Neglected tropical diseases (NTDs) and cancer are disorders that generate a high global burden and novel therapies for these disorders are needed (Ehrenberg & Ault 2005, Farmer et al. 2010). Although a large number of antibiotics have saved hundreds of millions of lives over the last few years, the increase of opportunistic infections and antimicrobial resistance to drugs used in the clinic have contributed to the challenges faced by medicine in curing infectious diseases (Fauci & Morens 2012). The treatment of some cancers is palliative and this is not only a problem for the developed world (Farmer et al. 2010). The influence of small-molecules approved as drugs between 1981-2010, as natural products (N), and small-molecules directly derived from N (ND), is quite marked in the treatment of cancers (N = 11.1%, ND = 32.3%) and infectious diseases (N = 6.2%, ND = 40.9%) (Newman & Cragg 2012). NTDs, especially Chagas disease and leishmaniasis, affect poor and vulnerable groups and working to discover new medicines is not an attractive endeavour for pharmaceutical companies (Ehrenberg & Ault 2005). Several challenges in the treatment of these diseases, such as the ability of the drugs used clinically to cause toxic effects and their effectiveness at chronic stages, have not been overcome (Feasey et al. 2010).
The Caesalpinia genus (Leguminosae, Caesalpinioideae) includes approximately 130 species occurring in the tropics (Larsen et al. 1980, Lewis 1998). Caesalpinia echinata Lam. (Fabaceae) is an endangered species occurring in a highly threatened ecosystem. C. echinata is a native tree from Brazil that was the main source of red pigment in the XVI century during colonisation by Portugal and its popular name was given to the new land discovered when Portuguese navigators arrived in South America (Oliveira et al. 2002).
Endophytic fungi colonise all plant tissues (Petrini et al. 1992, Rodriguez et al. 2009, Vaz et al. 2009, Campos et al. 2011) and based on estimates, many fungal species and their secondary metabolites have not yet been described (Hyde & Soytong 2007, Hyde et al. 2007). The analysis of data recorded by PubMed and SciFinder in the last five years has revealed promising drug candidates from endophytic fungi that could be useful for different therapeutic applications.
Considering that only a small proportion of the existing endophytic fungi have been studied, especially those growing in tropical plants from Brazil, this paper focused on the investigation of the endophytic fungi living in the tissues of C. echinata as sources of bioactive natural products that could be used against some neglected diseases. In this work, we describe the taxonomic identification of the fungal isolates that produced biologically active extracts and the identification of beauvericin as the trypanocidal component of Fusarium sp. extract.
subjects, Materials and Methods
Plant material - Healthy stems and barks of C. echinata were collected in Zoo-Botanical Foundation, Belo Horizonte (FZB-BH), state of Minas Gerais, Brazil, in March 2008. A voucher specimen was deposited at the FZB-BH Herbarium under the code BHZB-6458.
Endophytic fungi isolation and storage - Plant samples were collected in plastic bags and taken to the laboratory for processing. Plant material was washed in tap water, allowed to dry at room temperature (RT) and cut into pieces of approximately 1 × 1 cm. The surface of the fragments were sterilised by immersion in 70% ethanol (1 min) and 2% sodium hypochlorite (3 min), followed by one wash with sterile distilled water (2 min) (Collado et al. 1996). The fragments were plated onto potato dextrose agar (PDA) (Difco, USA) plates (Merck) containing 0.1 g/L chloramphenicol (Sigma, USA). The plates were incubated for up to 60 days at 25ºC and individual colonies were transferred to PDA. After complete growth, these colonies were photographed. Stock fungal cultures were deposited in the Culture Collection of Microorganisms and Cells of the Federal University of Minas Gerais. Fungal mycelial pieces were preserved at RT in sterile distilled water containing 30% v/v of glycerol.
Cultivation and extraction of the fungal cultures - Pieces of fungi mycelia (5 mm diameter) were transferred to five Petri dishes containing 20 mL of malt extract agar (PDA, Difco) medium (malt extract 1%, glucose 1%, peptone 0.1% and agar 1% in 1 L of purified water) and were cultured for 14 days at 28ºC. The biomass of the fungi mycelia were extracted by maceration with ethyl acetate for 48 h at RT. After passing through filter paper, the solvents were evaporated under reduced pressure using a rotary evaporator at 45ºC. Residual solvent in the extracts was eliminated in a vacuum centrifuge at 40ºC.
Antimicrobial activity assays - Antimicrobial activity was evaluated using the following microorganisms from the American Type Culture Collection (ATCC) (USA): Staphylococcus aureus ATCC 25295, Escherichia coli ATCC 18804, Bacillus cereus ATCC 11778, Klebsiella oxytoca ATCC 49131, Salmonella typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 18804 and Candida tropicalis ATCC 750.
Bacterial strains were maintained on brain heart infusion agar (Difco). Yeast strains were maintained on Sabouraud dextrose agar (Oxoid, UK).
Culture media and inocula - Mueller Hinton Broth (Himedia, India) was prepared in accordance with the Clinical and Laboratory Standards Institute (CLSI) document M7-A6 for minimal inhibitory concentration (MIC) bacterial assays (NCCLS 2003). Inocula of all bacteria were obtained using the spectrophotometric method prescribed by CLSI M7-A6, with final concentrations of 5 x 105 colony-forming unit (CFU)/mL. Candida cultures were grown at 35ºC and their inocula were prepared from fresh cultures according to the CLSI document M27-A2 (CLSI 2008). For the susceptibility tests, the final concentration was 1.5 x 103 CFU/mL. After homogenisation by vortexing, the transmittance was measured at 520 nm and was adjusted to 69-70%.
Susceptibility test - The broth microdilution tests for bacteria and yeast were performed following the CLSI guidelines of M7-A6 and M27-A2, respectively. Susceptibility to antimicrobial agents was determined by the microbroth dilution method performed in sterile flat-bottom 96-well microplates (Difco). Fungal extracts were dissolved in dimethyl sulphoxide (DMSO) followed by the addition of Mueller Hinton Broth for bacterial assays and RPMI for yeast assays. Eight serial dilutions (2-256 µg/mL) were prepared using the corresponding media as the diluents and maintaining a constant volume of 1 mL in each tube. For each dilution, aliquots of 0.1 mL were distributed in the microplates. For growth and sterility control, media alone was used without the addition of extract and solvent. As a control for toxicity of the solvent, culture with DMSO was used. Chloramphenicol (Sigma-Aldrich) (0.78-100 µg/mL) was used as the positive antibacterial control and amphotericin B (AMB) (Sigma-Aldrich) (0.03-15 µg/mL) was used as the positive antifungal control. After the assembly of the plates, each bacterial and fungal strain was inoculated and the plates were incubated at 37ºC for 24 h for bacteria and 48 h for Candida species. Endpoints were determined visually by comparing the growth in the sample wells to the growth in drug-free control wells. MIC measurements were defined as the lowest sample concentration for which the well was optically clear and were expressed in µg/mL.
Cytotoxicity assays with human cancer cell lines - The assays were performed using the following tumour cell lines purchased from the National Cancer Institute (NCI) (USA): UACC-62 (human melanoma cancer), MCF-7 (human breast cancer) and TK-10 (human renal cancer). The cell toxicity assays were run according to the protocols established at NCI using the sulforhodamine colorimetric assay (Monks et al. 1991). Briefly, the cells were inoculated in 96-well plates and incubated at 37ºC for 24 h in a 5% CO2 atmosphere. The solutions of the test samples were added to the culture wells to attain the desired concentrations and the plates were incubated for another 48 h. Trichloroacetic acid was added to each well to precipitate the proteins, which were stained with sulforhodamine B. After washing out the unbound dye, the stained protein was dissolved in 10 mM Tris and absorbance was measured at the wavelength of 515 nm. The results were calculated using the absorbance measured in the test wells (T) in comparison with that of the control wells for the initial cell inoculum (Ti) and cells grown for 48 h without drug (Tf), using the following formula: [(T-Ti)/(Tf-Ti)] x 100. The results were expressed in terms of the growth inhibition percentage where the sample tested was considered cytostatic from 0-99% and cytocidal from 100-200%. Etoposide (ETO) at 1.6 µg/mL, culture medium without samples and culture medium with DMSO 1% (v/v) were used as controls.
In vitro assay with human peripheral blood mononuclear cells (PBMC) - PBMC isolation from venous blood - Venous blood from healthy adult volunteers was collected in heparinised tubes and centrifuged over a Ficoll-Hypaque cushion (Histopaque, Sigma). PBMC were collected from the Ficoll-Hypaque interphase and washed three times with RPMI-1640 medium (Gibco, USA). An aliquot of the cells was incubated with trypan blue (0.4% in NaCl 0.9%) and the viability of the cells was evaluated by visual inspection under a microscope. The cell suspensions were adjusted to 1.5 x 106 cell/mL and cultured in RPMI-1640 medium supplemented with 5% (v/v) heat-inactivated, pooled human sera type AB (Flow Laboratories, Royaune-UNI) and L-glutamine (2 mM) (Gibco). An antibiotic/antimycotic solution containing 1 mg/mL penicillin, 1 mg/mL streptomycin and 25 µg/mL fungisone (Sigma) was added to control for fungal and bacterial contamination. In vitro cellular proliferation (blastogenesis) was assessed as previously described (Gazzinelli et al. 1983). Briefly, 1.5 x 105 cells were cultured in complete RPMI-1640 in flat-bottomed microtitre plates (Costar, tissue culture treated polystyrene # 3512, Sigma). The cultures were stimulated with 2.5 µg/mL of lectin from Phaseolus vulgaris phytohaemagglutinin (PHA) (Sigma) and incubated for 72 h at 37°C in a humidified atmosphere containing 5% CO2. Cell proliferation was determined using Alamar Blue according to the manufacturer's recommendations (Invitrogen, cat. DAL1100). The experiments were repeated three times using different samples of blood. Allopurinol and dexamethasone at 20 µg/mL were used as controls on PHA stimulated PBMC cultures. The results were expressed as percent inhibition of the PHA stimulated lymphocyte proliferation in relation to the control (no extracts added).
In vitro assay with human PBMC - The assay was performed as above, except that the culture was not stimulated with PHA. Cell toxicity was determined using Alamar Blue following the manufacturer's protocol (Invitrogen, cat. DAL1100). ETO at 20 µg/mL was used as the positive (toxic) control. The cytotoxic activity was evaluated by comparing the PBMC cultures with and without fungi extracts.
Assays with Leishmania (Leishmania) amazonensis amastigotes-like forms - Leishmanicidal activity was determined against amastigote-like forms of the parasite, which were obtained as previously described (Callahan et al. 1997). Briefly, promastigotes of L. (L.) amazonensis (strain IFLA/BR/196/PH-8) were obtained from lesions of infected hamsters. The parasites were grown at 26ºC in Schneider's medium (pH 7.2) and then stimulated to differentiate into amastigote forms by raising the temperature (32ºC) and lowering the pH (6.0) of the Schneider's medium. After seven days under these conditions, 90% of the promastigotes were transformed into amastigote-like forms, verified by microscopy and they were then used in the bioassays. The amastigote density was adjusted to 1 x 108 parasites per mL and 90 μL was added to each well of the 96-well plates. Solutions of the test samples at 200 μg/mL containing DMSO 1% (v/v) in water were performed for each sample and then, 10 μL of the solution were added to each well of the 96-well plates. The plates were incubated at 32ºC for 72 h and then, cell viability was determined using the methyl thiazolyl tetrazolium assay (Teixeira et al. 2002). The results were calculated from the measured absorbencies using the formula [1-(Abs exp/Abs contr) x 100], which expresses the percentage of parasite death in relation to the controls without drug. AMB at 0.02 μg/mL (Fungison(r), Bristol-Myers Squibb, Brazil) was used as a positive drug control.
Assays with Trypanosoma cruzi amastigote and trypomastigote forms - This assay was performed using T. cruzi (Tulahuen strain) expressing E. coli β-galactosidase as a reporter gene (Buckner et al. 1996, Romanha et al. 2010). Infective trypomastigote forms were obtained by monolayer culture of mouse L929 fibroblasts in RPMI-1640 medium (pH 7.2-7.4) without phenol red (Gibco) and with 10% foetal bovine serum and 2 mM glutamine. For the bioassay, 4,000 L929 cells in 80 μL of supplemented medium were added to each well of a 96-well microtitre plate. After an overnight incubation, 40,000 trypomastigotes in 20 μL were added to the cells and incubated for 2 h. The medium containing extracellular parasites was replaced with 200 μL of fresh medium and the plate was incubated for an additional 48 h to establish the infection. The medium was then replaced with solutions of the test samples at a concentration of 20 μg/mL in DMSO (< 1% in aqueous RPMI-1640 medium) and the plate was incubated for 96 h. To determine the half maximal inhibitory concentration (IC50) values, the cells were exposed to active samples at serial decreasing dilutions starting at 20 μg/mL and the IC50 values were calculated by linear interpolation. After this period, 50 μL of 500 μm chlorophenol red β-D-galactopyranoside in 0.5% Nonidet P-40 was added to each well and the plate was incubated for 16-20 h, after which the absorbance at 570 nm was measured. Controls with uninfected cells, untreated infected cells, infected cells treated with benznidazole (BNZ) at 1 μg/mL (positive control) and cells treated with DMSO 1% were used. Tetraplicates were run in the same plate and the experiments were repeated at least once.
The cytotoxicities of beauvericin and BNZ on uninfected mouse L929 fibroblasts were obtained. The IC50 values were calculated by linear interpolation and the selectivity index (SI) values were determined based on the ratio of the IC50 value in the host cell divided by the IC50 value of the parasite (Romanha et al. 2010).
Statistical analysis - The samples were tested in quadruplicate in the T. cruzi assays and in triplicate in the other assays. At least two independent experiments were performed. Values represent the mean ± variation coefficient.
Molecular identification of endophytic fungi - The extracts of 14 fungi showed positive results in at least one bioassay and thus were selected for molecular taxonomy. The DNA was extracted according to the procedure previously described (Rosa et al. 2009). The identification was based on the internal transcribed spacer-ribosomal DNA (ITS-rDNA) sequences. The pair of primers ITS1 (sequence: 5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') was used for ITS-rDNA amplification (White et al. 1990). The sequences were generated using MEGABACE (Amersham Biosciences, USA), which were used to feed PHRED-PHRAP software to find the consensus sequence. The sequence was then compared with those deposited in GenBank using BLASTN software to identify the isolate down to the genus level. All fungal ITS-rDNA sequences obtained in this work were deposited in the GenBank with accessions KF611676-KF611689 (Table I).
Table I Identification of endophytic fungi isolated from Caesalpinia echinata Lam. (Fabaceae) using primers internal transcribed spacer (ITS)1 and ITS4.
| WC | Closest related species | Similarity(%) | Base pairs analysed(n) | Identification and GenBankaccessions |
|---|---|---|---|---|
| 25 | Aspergillus sp. [KF367538.1] | 100 | 523 | Aspergillus sp. [KF611682] |
| 45 | Epicoccum sorghi [KC106717.1] | 100 | 461 | E. sorghi [KF611685] |
| 46 | E. sorghi [KC106698.1] | 100 | 509 | E. sorghi [KF611686] |
| 9 | Fusarium sp. [JQ905668.1] | 100 | 357 | Fusarium sp. [KF611679] |
| 58 | Fusarium sp. [HM631978.1] | 99 | 481 | Fusarium sp. [KF611688] |
| 2 | Nectria pseudotrichia [JN995626.1] | 100 | 495 | N. pseudotrichia [KF611677] |
| 6 | N. pseudotrichia [JF832647.1] | 100 | 360 | N. pseudotrichia [KF611678] |
| 33 | N. pseudotrichia [JN995626.1] | 100 | 509 | N. pseudotrichia [KF611683] |
| 24 | Talaromyces sp. [JX898040.1] | 100 | 566 | Taralomyces sp. [KF611681] |
| 1 | Xylaria arbuscula [JN601145.1] | 99 | 497 | X. arbuscula [KF611676] |
| 11 | Xylaria sp. [DQ322134.1] | 96 | 515 | Xylaria sp. [KF611680] |
| 41 | Xylaria berteri [JQ936300.1] | 100 | 411 | X. berteri [KF611684] |
| 55 | Xylaria sp. [JQ862693.1] | 100 | 473 | Xylaria sp. [KF611687] |
| 84 | Xylaria sp. [KC507252.1] | 99 | 516 | Xylaria sp. [KF611689] |
WC: working code.
Mass cultivation and extraction of Fusarium sp. [working code (WC) 9] - Pieces of mycelia (5 mm diameter) were transferred to 30 Petri dishes containing 20 mL of PDA and were cultured for 14 days at 28ºC. The fungal biomass was placed in Erlenmeyer flasks and extracted by maceration with ethyl acetate for 48 h. The suspensions were filtered through filter paper and the filtrate evaporated to dryness under reduced pressure using a rotary evaporator at 45ºC. The residue was transferred to 20 mL flasks and the residual solvent was removed in a vacuum centrifuge at 40ºC for 18 h.
Bioassay-guided fractionation of the Fusarium sp. extract - The extract obtained (110 mg) was dissolved in 1 mL of a mixture of methanol (MeOH) and water (MeOH: H2O, 75:25 v/v). After centrifugation, the solution was injected into a semi-preparative reverse phase high-performance liquid chromatographic (RP-HPLC) column [250 mm × 4.6 mm internal dimension (i.d.)], 5 μm particle diameter) using a Shimadzu chromatograph (Shimadzu Corp, Japan) equipped with a LC6AD pump and manual injection valve (RheodyneTM 7125, Rheodyne Co, USA) using a fixed 1.000 µL sample loop and a dual-wavelength detector (SPD M10A) controlled by LCsolution software v.1.25 (Shimadzu Corp). The sample was purified with a linear gradient of water (A) and MeOH (B) using 10%B-100%B for 50 min and 100%B for 10 min. The eluent was pumped at 7 mL/min and the effluent absorption measured at λ 220 nm and 254 nm. Ten fractions corresponding to different peaks were collected and tested in the T. cruzi assay. Fraction (Fr)-5 (17 mg, 95% pure by HPLC) was the most active.
Spectral data of the trypanocidal Fr-5 - Proton (1H) and carbon nuclear magnetic resonance (NMR) spectra, distortionless enhancement by polarisation transfer, heteronuclear single quantum coherence and heteronuclear multiple bond coherence (HMBC) experiments were performed on a Brucker DRX 400 spectrometer using the pulse programs provided by the manufacturer. The substance was dissolved in perdeuterated solvents doped with 0.1% tetramethyl silane as the internal standard.
Liquid chromatography-diode-array detection-mass spectrometry (LC-DAD-MS) analysis of Fr-5 was performed in a Thermo Surveyor Plus (Thermo Fisher Scientific, USA) chromatograph equipped with a Finnigan Surveyer PDA Plus diode-array detector and C18 column (Atlantis C18, Waters, USA) (3 μm particle diameter, 150 mm × 2.1 mm i.d.). A flow rate of 200 μL/min was used and the effluent entirely directed the Bruker ETD-maXis quadrupole TOF (Bruker Daltonics, Germany) for electrospray ionisation (ESI) in the positive ion mode. The LC-DAD-MS was conducted in a gradient system using a mixture of water (A) and MeOH (B) with 0.1% formic acid, 1%B-100%B for 13 min, 100%B for 4 min, 100%B-1%B for 0.5 min, 1%B for 11.5 min.
The mass detector was set to the mass-to-charge ratio (m/z) range of 50-1500 atomic mass units. The instrument was operated under the following conditions: end plate offset, -500 voltage (V); capillary V, 4.500 V; nebuliser pressure, 0.4 bar; dry gas (nitrogen) flow rate, 4.0 L/min; dry temperature, 180ºC; collision-induced dissociation energy, 25 eV; collision energy, 7 eV; ion cooler radio-frequency, 25 excitation V; transfer time, 45 μs.
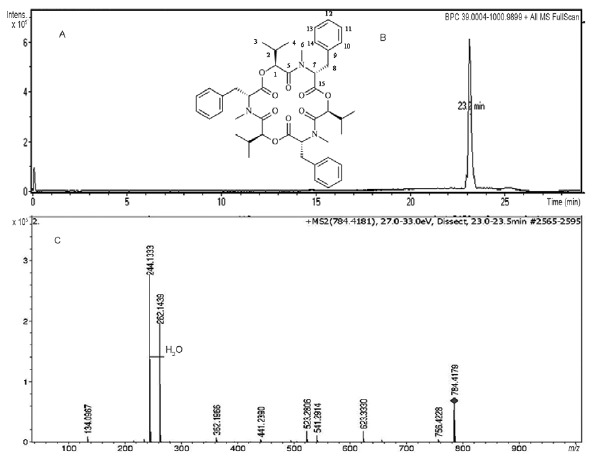
Fr-5 (beauvericin): white powder; specific optical rotation = + 47 [concentration at g/100 mL (c) 0.8, MeOH]; ultraviolet (UV) (DAD, MeOH) maximum wavelength (λmax) 202 nm. 1H NMR [deuterated MeOH (CD3OD), 400 megahertz (MHz)] chemical dislocation in ppm (δ)H 7.26-7.24 [12H, multiplet (m), H-10/H-11/H-13/H-14], 7.17 (3H, m, H-12), 5.45 [3H, doublet of doublets (dd), coupling constant (J) = 10.9 and 3.9, Hz, H-7], 4.92 [3H, dublet (d), J = 8.6 Hz, H-1], 3.36 (3H, dd, J = 14.5 and 5.0 Hz, H-8b), 2.99 [9H, singlet (s), H-6], 2.97 (3H, dd, J = 11.8 Hz, H-8a), 2.01 (1H, td, J = 21.2, 6.8 and 6.7 Hz, H-2), 0.78 (9H, d, J = 6.6 Hz, H-3), 0.43 (9H, d, J = 6.7 Hz, H-4). Carbon-13 (13C) NMR (CD3OD, 100 MHz): δ C 170.2 (C, C-5), 169.7 (C, C-15), 136.9 (C, C-9), 129.1 and 128.8 (CH, C-10/C-11/C-13/C-14), 127.0 (CH, C-12), 75.7 (CH, C-1), 57.6 (CH, C-7), 35.0 (CH2, C-8), 32.6 (CH3, C-6), 29.9 (CH, C-2), 18.5 (CH3, C-3), 17.7 (CH3, C-4); high resolution-ESI-MS m/z 784.4180 [M + H]+ (calcd. for C45H57N3O9, 783.4095).
High performance LC coupled to an UV detector (HPLC-DAD) analysis - The ethyl acetate extract from Fusarium sp. (WC 9) and Fr-5 (beauvericin) were analysed by HPLC-DAD in a Shimadzu chromatograph (Shimadzu Corp) equipped with a LC10AD pump and manual injection valve (RheodyneTM 7725i, Rheodyne Co) using a fixed 20 µL sample loop, a CTO-20A thermostat-controlled oven compartment and a SPD-M20A diode array detector (190-800 nm) controlled by LCsolution software. A Shim-pack(r) C18 column (5 μm, 250 mm × 4.6 mm i.d.) maintained at 40ºC was used in the chromatographic analysis. The separations were conducted in a gradient system, using a mixture of water (A) and acetonitrile (ACN) (B) with 0.1% trifluoroacetic acid (TFA), 10%B-100%B for 30 min and 100%B for 10 min as the mobile phase, at a flow rate of 1.0 mL/min. The ethyl acetate extract (5 mg/mL) and Fr-5 (beauvericin) (200 µg/mL) were dissolved in MeOH. The particulates were removed by centrifugation and the sample injection volume was 20 μL for each sample.
Results
Isolation and molecular identification of endophytic fungi from C. echinata Lam. - Eighty-two endophytic fungal strains were isolated from plant bark (10 samples) and stems (13 samples) (Table II). Fourteen fungal isolates yielded extracts that were active in vitro at 20 μg/mL in at least one biological assay. Based on the results of their ITS1-5.8S-ITS4 partial sequences, these isolates were submitted to the GenBank to obtain their accession numbers and the closest related species were achieved by BLAST analysis. The results (Table I) show that all sequences had more than 96% similarity with the species in GenBank. Most sequences presented 99% (n = 3) or 100% (n = 10) similarity to the closest related species in GenBank.
Table II Number of isolates from different plant parts.
| Plant part | Samples(n) | Fungi isolated(n) | Morphotypes(n) |
|---|---|---|---|
| Barks | 10 | 34 | 14 |
| Stems | 13 | 48 | 10 |
| Total | - | 82 | - |
All 14 isolates belong to the Ascomycota phylum, with 10 from the Sordariomycetes class, two from the Eurotiomycetes class and two from the Dothideomycetes class. Among the fungi that produced active extracts, five were from the Xylaria genus (35%) and three were from the Nectria genus (21%). Fusarium and Epicoccum afforded two active extracts each (14%) and Taralomyces and Aspergillus afforded one active extract each (7%) (Tables III, IV).
Table III In vitro antimicrobial activities of extracts from endophytic fungi of Caesalpinia echinata Lam. (Fabaceae).
| Fungal isolate (WC) | Microorganisms | |||||||
|---|---|---|---|---|---|---|---|---|
| Minimal inhibitory concentration (MIC) (µg/mL) | ||||||||
| SA | EC | BC | ST | PA | KO | CA | CT | |
| Aspergillus sp. (25) | 32 | 64 | > 256 | 64 | > 256 | 64 | > 256 | > 256 |
| Epicoccum sorghi (45) | 64 | 32 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| E. sorghi (46) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| Fusarium sp. (9) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | 64 | 128 |
| Fusarium sp. (58) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| Nectria pseudotrichia (2) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| N. pseudotrichia (6) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| N. pseudotrichia (33) | 64 | > 256 | > 256 | > 256 | > 256 | > 256 | 128 | 128 |
| Talaromyces sp. (24) | 32 | 64 | > 256 | > 256 | > 256 | 64 | > 256 | > 256 |
| Xylaria arbuscula (1) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| Xylaria sp. (11) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| Xylaria berteri (41) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| Xylaria sp.(55) | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| Xylaria sp.(84) | 64 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 | > 256 |
| Controls | ||||||||
| Amphotericin B | NT | NT | NT | NT | NT | NT | 0.12 | 1.2 |
| Chloramphenicol | 16 | 8 | 16 | 16 | 8 | 8 | NT | NT |
BC: Bacillus cereus; CA: Candida albicans; CT: Candida tropicalis; EC: Escherichia coli; KO: Klebsiella oxytoca; NT: not tested; PA: Pseudomonas aeruginosa; SA: Staphylococcus aureus; ST: Salmonella typhimurium; WC: working code. Values in bold mean extracts with MIC values = 128 µg/mL.
Table IV In vitro antiprotozoan, cytotoxic and antiproliferative activities of extracts from endophytic fungi of Caesalpinia echinata Lam. (Fabaceae).
| Fungal isolate(WC) | Tumour cell lineages(%) | PBMC(%) | Protozoan(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| UACC-62 | TK-10 | MCF-7 | Mortality | Proliferation decreased | LA | TC | |||
| Aspergillus sp.(25) | - | 103 ± 9 | - | - | - | - | - | ||
| Epicoccum sorghi (45) | 75 ± 7 | 47 ± 10 | 67 ± 9 | - | - | - | - | ||
| E. sorghi (46) | - | 97 ± 5 | 60 ± 17 | - | 15 ± 7 | - | - | ||
| Fusarium sp. (9) | - | 98 ± 5 | 88 ± 4 | - | 15 ± 8 | 45 ± 0 | 92 ± 4 | ||
| Fusarium sp.(58) | - | - | 48 ± 10 | 49 ± 26 | - | 45± 4 | - | ||
| Nectria pseudotrichia (2) | - | 54 ± 14 | 68 ± 4 | NT | NT | - | - | ||
| N. pseudotrichia (6) | - | 95 ± 7 | 93 ± 0 | NT | NT | - | - | ||
| N. pseudotrichia (33) | 102 ± 7 | 96 ± 15 | 60 ± 11 | - | 37 ± 15 | 77 ± 3 | - | ||
| Talaromyces sp. (24) | - | 95 ± 9 | 60 ± 7 | - | 20 ± 13 | - | - | ||
| Xylaria arbuscula (1) | - | 60 ± 1 | 57 ± 9 | - | - | - | - | ||
| Xylaria sp. (11) | - | 113 ± 3 | 47 ± 8 | NT | NT | 51 ± 1 | - | ||
| Xylaria berteri (41) | - | 43± 6 | - | - | 20 ± 15 | - | - | ||
| Xylaria sp.(55) | - | 92 ± 4 | 58 ± 0 | - | - | - | - | ||
| Xylaria sp.(84) | - | 51±9 | 48 ± 8 | NT | NT | - | - | ||
| Controls | |||||||||
| AMB | NT | NT | NT | NT | NT | 82 ± 3 | NT | ||
| BNZ | NT | NT | NT | NT | NT | NT | 86 ± 8 | ||
| ETO | 176 ± 9 | 185 ± 9 | 100 ± 5 | 33 ± 14 | NT | NT | NT | ||
| DEX | NT | NT | NT | 18 ± 13 | - | NT | NT | ||
| ALL | NT | NT | NT | - | 21 ± 14 | NT | NT | ||
all extracts were tested at 20 µg/mL. Results were expressed in terms of percentage of the inhibition. ALL: allopurinol tested at 20 µg/mL; AMB: amphotericin B tested at 0.02 µg/mL; BNZ: benznidazole tested at 1.0 µg/mL = 3.8 µM; DEX: dexamethasone tested at 20 µg/mL; ETO: etoposide tested at 1.6 µg/mL in tumour cell lineages and at 20 µg/mL in human peripheral blood mononuclear cell (PBMC); LA: amastigotes forms of Leishmania (Leishmania) amazonensis; MCF-7: human breast cancer; NT: not tested; TC: amastigote and trypomastigote forms of Trypanosoma cruzi; TK-10: human renal cancer; UACC-62: human melanoma cancer; WC: working code; -: inactive;
Biological activities of extracts from endophytic fungi isolated of C. echinata Lam. - The ethyl acetate extracts of 82 fungal isolates from C. echinata Lam. were tested in in vitro biological assays to predict their leishmanicidal, trypanocidal, cytotoxic and antimicrobial activities. Forty-four fungal isolates were considered active (≥ 40% of the inhibition of growth) in at least one biological assay (Tables III, IV).
The extracts from Talaromyces sp. (WC 24), Aspergillus sp. (WC 25) and Epicoccum sorghi (WC 45) showed antibacterial activity against Gram-positive and Gram-negative bacterial species (S. aureus and E. coli) with MIC values ranging from 32-64 μg/mL. Aspergillus sp. extract (WC 25) showed an MIC value of 64 μg/mL against S. typhimurium and K. oxytoca. Talaromyces sp. extract (WC 24) showed an MIC of 64 μg/mL against K. oxytoca. Antifungal activity was only observed for the extracts from Fusarium sp. (WC 9) and Nectria pseudotrichia (WC 33), which inhibited C. albicans and C. tropicalis at concentrations ranging from 64-128 μg/mL (Table III).
Four isolates, Fusarium sp. (WC 9), Xylaria sp. (WC 11), N. pseudotrichia (WC 33) and Fusarium sp. (WC 58), were able to inhibit the growth (45-77%) of the amastigote forms of L. (L.) amazonensis. However, only Fusarium sp. (WC 9) inhibited (92%) the amastigote and trypomastigote forms of T. cruzi when tested at 20 μg/mL (Table IV).
Fourteen fungal isolates exhibited cytotoxicity toward MCF-7 and TK-10 cell lineages, inhibiting their growth by at least 40%. In addition, the extracts of N. pseudotrichia (WC 33) and E. sorghi (WC 45) inhibited the growth of UACC-62. The extracts of tree fungi (Xylaria sp., WC 11; Aspergillus sp. WC 25 and N. pseudotrichia, WC 33) displayed cytocidal activity at 20 μg/mL; in other words, the number of viable cells was less than in the initial inoculum (Table IV). Although all 14 isolates showed some degree of cytotoxicity against three tumour cell lineages at 20 μg/mL, only one was cytotoxic to human PBMCs and six were able to reduce the PHA stimulated proliferation of PBMCs (Table IV).
Chemical characterisation of the trypanocidal compound of the Fusarium sp. extract and HPLC-DAD analysis - To identify the trypanocidal component of Fusa- rium sp. (WC 9), the ethyl acetate extract from this strain was produced at a larger amount and was submitted to semi-preparative RP-HPLC fractionation. The fractions were tested in the intracellular T. cruzi assay and only Fr-5 was able to kill 100% of the T. cruzi amastigote and trypomastigote forms at a concentration of 5 μg/mL. The HPLC chromatogram of Fr-5 showed a single peak (Fig. 1A) with UV purity index of approximately 95%. The spectral data of Fr-5 were in full agreement with those reported for beauvericin (Fig. 1B) (Newman 2008, Hu & Rychlik 2012). The UV spectra exhibited absorptions between 196-230 nm (λmax 202 nm), which is consistent with previous reports (Monti et al. 2000, Mahnine et al. 2011). The HRMS of Fr-5 showed a quasi-molecular [M + H]+ ion peak at m/z 784.4180, which is consistent with the molecular formula of: C45H57N3O9 (calcd. 783.4095) (Fig. 1C). The 13C NMR spectrum showed 15 carbon signals, which together with the mass spectra analysis suggested a symmetrical structure for Fr-5. The 1H and 13C NMR spectra of Fr-5 showed signals of aromatic carbons and 1H (δH 7.26-7.17, δC 127.0-129.1) from a benzyl moiety (δH 3.36, dd, J = 14.5 and 5.0 Hz/δC 35.0) due to the phenylalanine residues. The ESI-(+)-LC-MS/MS spectra of Fr-5 (Fig. 1C) showed fragment ions at m/z 262.1439 and 244.1324 produced by the cleavage of the phenylalanine amide bond followed by loss of H2O, as observed in beauvericin (Sewram et al. 1999, Hu & Rychlik 2012). The hydroxy-isovaleryl moiety showed 1H signals at δH 4.92 (1H, d, J = 8.6 Hz/ δC 75.7), δH 2.01 (1H, td, J = 21.2, 6.8 and 6.7 Hz/ δC 29.9), δH 0.78 (3H, d, J = 6.6 Hz/δC 18.5) and δH 0.43 (3H, d, J = 6.7 Hz/δC 17.7). In addition, the N-methylamino acid moiety of beauvericin matches the signals of Fr-5 at δ 2.99 (3H, s, δC 32.6, N-CH3).
Fig. 1. total-ion chromatogram (A) of beauvericin (B). Column RP-18, 150 mm × 2.1 mm i.d.; mobile phase [A: H2O; B: methanol) with 0.1% formic acid; 1%B-100%B in 13 min, 100%B in 4 min, 100%B-1%B in 0.5 min, 1%B in 11.5 min flow rate 200 µL/min-1. Electrospray ionisation-(+)-MS/MS of (B) (precursor m/z 784.4179 [M + H]+) and main fragments (C).
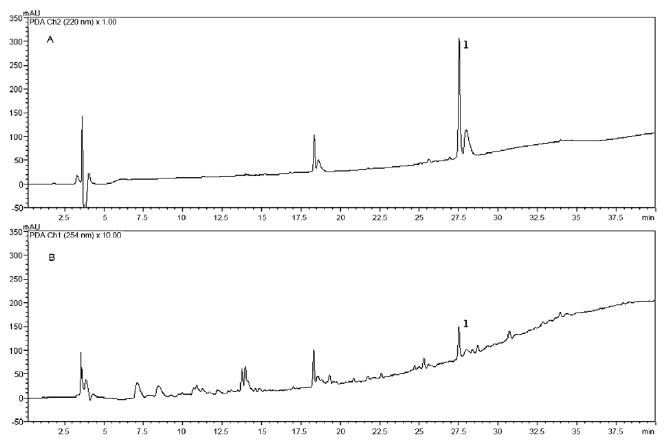
The ethyl acetate extract from Fusarium sp. (WC 9) was analysed using RP-HPLC-DAD and beauvericin (1) eluted after 27.5 min (Fig. 2) using a mixture of ACN and water with 0.1% TFA.
Fig. 2. high performance liquid chromatographic coupled to an ultraviolet (UV) detector profile of ethyl acetate Fusarium sp. (working code 9). UV detection at 220 nm (A) and 254 nm (B). Column RP-18, 250 mm × 4.6 mm i.d.; mobile phase (A: H2O; B: acetonitrile) with 0.1% trifluoroacetic acid; 10%B-100%B in 30 min, 100%B in 10 min, flow rate of 1.0 mL/min. The ethyl acetate extract (5 mg/mL) and beauvericin (1,200 µg/mL).
Trypanocidal and cytotoxicity activities of the beauvericin - While the crude extract of Fusarium sp. WC 9 showed an IC50 of 30 μg/mL in the assay with T. cruzi forms expressing the β-galactosidase gene, Fr-5 (beauvericin) showed an IC50 value 15 times smaller (1.9 μg/mL, 2.43 μM). This compound showed cytotoxic activity against the host cell (mouse L929 fibroblasts) used in the T. cruzi assay, showing an IC50 of 5 μg/mL (6.38 μM). Thus, under our assay conditions, beauvericin showed an SI of only 2.7. BNZ was used as the standard and showed an IC50 value of 3.8 μM and an SI value of 625 against mouse L929 fibroblasts.
Discussion
In our previous work (Cota et al. 2011), we found that the crude ethanol extract of C. echinata Lam. kills 90% of the amastigote-like forms of L. amazonensis at a concentration of 20 µg/mL. This promising result prompted us to continue to study C. echinata as a host plant of potential bioactive endophytic fungi. Previous work by other groups identified 43 taxa belonging to Hyphomycetes and three belonging to Coelomycetes in leaf litter of C. echinata Lam. (Grandi & Silva 2003, 2006). They reported the presence of Epicoccum nigrum as an anamorphic fungi (Grandi & Silva 2006). In the present study, we identified two isolates (E. sorghi; WC 45 and 46) of the same genus as bioactive endophytic fungi.
Few reports are available on the biological activities of fungi growing in C. echinata. Machado (2009) isolated Botryosphaeria rhodina, Xylaria multiplex and Pestalotiopsis sp. as endophytic fungi from the leaves and stems of C. echinata. Although none of these isolates were active against Enterococcus faecalis, P. aeruginosa or S. aureus by agar diffusion assay (100 μg and 1,000 μg), they were able to inhibit the growth of the phytopathogens Pythium debaryanum and Phytoththora palmivora (Machado 2009).
Most of the fungi identified in the present work have been previously reported as endophytic in other plants. Xylaria, Nectria and Aspergillus genera are found in Piper aduncum (Piperaceae) and many other plants in Brazilian savannas (Martínez-Luis et al. 2011, Vaz et al. 2012). In addition, Fusarium species are the most frequent endophytes (Liang et al. 2012). The other two genera described in this paper, Taralomyces and Epicoccum, were recently found to be endophytic (Fávaro et al. 2012, Bara et al. 2013).
Our results show that, overall, approximately 17% of our fungal isolates were active in at least one of the four bioassays performed. Recently, Higginbotham et al. (2013) showed that fungi isolated from the plant family Fabaceae (Fabales) had a high percentage of highly active genotypes and were associated with moderate activity against Plasmodium falciparum and MCF-7 cells (breast cancer cell line) when tested at a concentration of 10 µg/mL. Moreover, extracts from fungi of Aspergillus and Xylaria genera are the most active endophytic fungi according to the results of in vitro assays of these fungi against Leishmania donovani, T. cruzi and MCF-7 cells (Higginbotham et al. 2013). The results of our biological assays lead us believe that all isolates tested, except for the isolate Fusarium sp. (WC 58), which showed high toxicity to the PBMC in vitro, are potential sources of compounds useful in the development of drugs against infectious agents and immunomodulatory metabolites.
In the present study, the fungi Fusarium sp. [KF611679] was the only one that showed activity against T. cruzi amastigote and trypomastigote forms and exhibited the best MIC values against C. albicans and C. tropicalis. Several Fusarium species isolated from plants are known to produce secondary metabolites, such as terpenoids, alkaloids and mycotoxins, with promising biological activities (Hyde & Soytong 2007, Hyde et al. 2007, Campos et al. 2012).
The trypanocidal activity of the fungi extract [KF611679] was attributed to beauvericin. Beauvericin is a mycotoxin produced by many fungi, including Fusarium spp (Wang & Xu 2012). Beauvericin displays insecticidal (Hamill et al. 1969), antitumour (Cheng et al. 2009), antibacterial, antifungal and antiviral activities (Zhan et al. 2007, Shin et al. 2009, Meca et al. 2010, Xu et al. 2010). Beauvericin was also reported to have leishmanicidal activity (EC50 1.86 μM) against promastigotes of Leishmania braziliensis (Nascimento et al. 2012). To the best of our knowledge, this is the first report on the trypanocidal activity of this cyclic hexadepsipeptide. Our results support those of previous studies (Klarić et al. 2008, Nascimento et al. 2012) that also showed that this compound was cytotoxic; we obtained an IC50 of 5 μg/mL (6.38 μM) against the host cell (mouse L929 fibroblasts) used in a T. cruzi assay. Under our assay conditions, beauvericin showed an SI of only 2.7, a value that, according to current guidelines (Romanha et al. 2010), is too low for beauvericin to be considered for pre-clinical studies.
Notwithstanding, according to a recent review (Feudjio et al. 2010), beauvericin-mediated cytotoxicity towards various mammalian and cancer cell lines is only partially understood and involves several cellular targets and molecular mechanisms. Furthermore, only a few studies have addressed the effects of beauvericin in animals and those studies have found only minor acute toxic effects. The authors emphasised that the consequences of chronic exposure and of pharmacologically active doses of beauvericin in humans/animals have not been explored in detail. Therefore, the biological activities of beauvericin on mammalian cancer cells and protozoan parasites suggest that beauvericin is a potential drug candidate for the treatment of cancers and infectious diseases. There is a need for further studies to determine the efficacy and safety of beauvericin in animals infected with T. cruzi.
In conclusion, this work demonstrated the in vitro leishmanicidal, trypanocidal, antimicrobial and cytotoxic activities of crude extracts prepared from endophytic fungi isolated from stems and barks of C. echinata. In addition, the bioassay-guided fractionation of Fusarium sp. (WC 9) extract using the T. cruzi assay allowed us to identify the cyclic hexadepsipeptide mycotoxin beauvericin as the trypanocidal component produced by the fungus.
Acknowledgments
AcknowledgEments
To the PDTIS-Fiocruz, for use of its facilities, to FZB-BH, to provide the plant material, mainly Juliana O Rego, Inês R de Andrade, Albina CO Nogueira and Maria Guadalupe C Fernandes, and to Daniela NB Maia, Priscila AM Ferreira and Markus Kohlhoff, for the technical support.
Footnotes
Financial support: CNPq, FAPEMIG, FIOCRUZ
References
- Bara R, Aly AH, Pretsch A, Wray V, Wang B, Proksch P, Debbab A. Antibiotically active metabolites fromTalaromyces wortmannii, an endophyte ofAloe vera. J Antibiot (Tokyo) 2013;66:491–493. doi: 10.1038/ja.2013.28. [DOI] [PubMed] [Google Scholar]
- Buckner FS, Verlinde CL, La Flamme AC, Van Voorhis WC. Efficient technique for screening drugs for activity againstTrypanosoma cruziusing parasites expressingβ-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan HL, Portal AC, Devereaux R, Grogl M. An axenic amastigote system for drug screening HL. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FF, Johann S, Cota BB, Alves TMA, Rosa LH, Caligiorne RB, Cisalpino PS, Rosa CA, Zani CL. Antifungal activity of trichothecenes fromFusarium sp. against clinical isolates ofParacoccidioides brasiliensis. Mycoses. 2011;54:122–129. doi: 10.1111/j.1439-0507.2009.01854.x. [DOI] [PubMed] [Google Scholar]
- Campos FF, Siqueira EP, Cota BB. Rios FR, Ortega ER. Fusarium:epidemiology, environmental sources and prevention. Nova Science Publishes; New York: 2012. Natural products fromFusarium; pp. p. 1–p.74. [Google Scholar]
- Cheng CK, Chang KC, Lee YJ. Antiproliferative effect of beauvericin on retinoblastoma. Fu-Jen J Med. 2009;7:161–169. [Google Scholar]
- CLSI - Clinical and Laboratory Standards Institute 2008 . Reference method for broth dilution antifungal susceptibility testing of yeast. Approved Standard M27-A3. CLSI; Wayne: 2008. 25 pp [Google Scholar]
- Collado J, Platas G, Peláez F. Fungal endophytes in leaves, twigs and bark ofQuercus ilex from Central Spain. Nova Hedwigia. 1996;63:347–360. [Google Scholar]
- Cota BB, Oliveira DM, Siqueira EP, Souza-Fagundes EM, Pimenta AM, Santos DM, Rabello AL, Zani CL. New cassane diterpenes fromCaesalpinia echinata. Fitoterapia. 2011;82:969–975. doi: 10.1016/j.fitote.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Ehrenberg J, Ault S. Neglected diseases of neglected populations: thinking to reshape the determinants of health in Latin America and the Caribbean. BMC Public Health. 2005;5:119–119. doi: 10.1186/1471-2458-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer P, Frenk J, Knaul FM, Shulman LN, Alleyne G, Armstrong L, Atun R, Blayney D, Chen L, Feachem R, Gospodarowicz M, Gralow J, Gupta S, Langer A, Lob-Levyt J, Neal C, Mbewu A, Mired D, Piot P, Reddy KS, Sachs JD, Sarhan M, Seffrin JR. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376:1186–1193. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366:454–461. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- Fávaro LCL, Sebastianes FLS, Araújo WL. Epicoccum nigrumP16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE. 2012;7: doi: 10.1371/journal.pone.0036826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feasey N, Wansbrough-Jones M, Mabey DCW, Solomon AW. Neglected tropical diseases. Br Med Bull. 2010;93:179–200. doi: 10.1093/bmb/ldp046. [DOI] [PubMed] [Google Scholar]
- Feudjio FT, Dornetshuber R, Lemmens M, Hoffmann O, Lemmens-Gruber R, Berger W. Beauvericin and enniatin: emerging toxins and/or remedies? World Mycotoxin J. 2010;3:415–430. [Google Scholar]
- Gazzinelli G, Katz N, Rocha RS, Colley DG. Immune responses during humanSchistosomiasis mansoni. X. Production and standardization of an antigen-induced mitogenic activity by peripheral blood mononuclear cells from treated, but not active cases of schistosomiasis. J Immunol. 1983;130:2891–2895. [PubMed] [Google Scholar]
- Grandi RAP, Silva TV. Hyphomycetes sobre folhas em decomposição deCaesalpinia echinata Lam.: ocorrências novas para o Brasil. Rev Bras Bot. 2003;26:489–493. [Google Scholar]
- Grandi RAP, Silva TV. Fungos anamorfos decompositores do folhedo deCaesalpinia echinataLam. Rev Bras Bot. 2006;29:275–287. [Google Scholar]
- Hamill RL, Higgens GE, Boaz HE, Gorman M. The structure of beauvericin, a new desipeptide antibiotic toxic toArtemia salina. Tetrahedron Lett. 1969;49:4255–4258. [Google Scholar]
- Higginbotham SJ, Arnold AE, Ibañez A, Spadafora C, Coley PD, Kursar TA. Bioactivity of fungal endophytes as a function of endophyte taxonomy and the taxonomy and distribution of their host plants. PLoS ONE. 2013;8: doi: 10.1371/journal.pone.0073192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Rychlik M. Biosynthesis of 15N3-labeled enniatins and beauvericin and their application to stable isotope dilution assays. J Agric Food Chem. 2012;60:7129–7136. doi: 10.1021/jf3015602. [DOI] [PubMed] [Google Scholar]
- Hyde KD, Bussaban B, Paulus PW, Crous S, Mckenzie EHCL, Photita W, Lumyong S. Diversity of saprobic microfungi. Biodivers Conserv. 2007;16:7–35. [Google Scholar]
- Hyde KD, Soytong K. Understanding microfungal diversity - a critique. CryptogamMycol. 2007;28:281–289. [Google Scholar]
- Klarić MS, Rumora L, Ljubanović D, Pepeljnjak S. Cytotoxicity and apoptosis induced by fumonisin B (1), beauvericin and ochratoxin A in porcine kidney PK15 cells: effects of individual and combined treatment. Arch Toxicol. 2008;82:247–255. doi: 10.1007/s00204-007-0245-y. [DOI] [PubMed] [Google Scholar]
- Larsen K, Larsen S, Vidal JE. Aubréville A, Leroy JF. Flore du Cambodge du Laos et du Vietnam. Vol. 18. Muséum National d'Histoire Naturelle; Paris: 1980. Legumineuses-Caesalpinioides; pp. p. 1–p227. [Google Scholar]
- Lewis GP. Caesalpinia: a revision of the Poincianella - Erythors- temon group. Royal Botanic Gardens; Kew: 1998. 233 pp [Google Scholar]
- Liang H, Xing Y, Chen J, Zhang D, Guo S, Wang C. Antimicrobial activities of endophytic fungi isolated fromOphiopogon japonicus(Liliaceae) BMC Complement Altern Med. 2012;12:238–243. doi: 10.1186/1472-6882-12-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado MABL. Isolamento, caracterização e avaliação da atividade antimicrobiana de fungos endofíticos de Caesalpinia echinata Lam. (Leguminosae-Caesalpinioideae) Universidade Federal de Alagoas; Maceió: 2009. 126 pp PhD Thesis. [Google Scholar]
- Mahnine N, Meca G, Elabidi A, Fekhaoui M, Saoiabi A, Font G, Mañes J, Zinedine A. Further data on the levels of emergingFusariummycotoxins enniatins (A, A1, B, B1), beauvericin and fusaproliferin in breakfast and infant cereals from Morocco. Food Chem. 2011;124:481–485. [Google Scholar]
- Martínez-Luis S, Cherigo L, Higginbotham S, Arnold E, Spadafora C, Ibañez A, Gerwick WH, Cubilla-Rios L. Screening and evaluation of antiparasitic and in vitro anticancer activities of Panamanian endophytic fungi. Int Microbiol. 2011;14:95–102. doi: 10.2436/20.1501.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meca G, Sospedra I, Soriano JM, Ritieni A, Moretti A, Manes J. Antibacterial effect of the bioactive compound beauvericin produced byFusarium proliferatumon solid medium of wheat. Toxicon. 2010;56:349–354. doi: 10.1016/j.toxicon.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor-cell lines. J Nat Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- Monti SM, Fogliano V, Logrieco A, Ferracane R, Ritieni A. Simultaneous determination of beauvericin, enniatins and fusaproliferin by high performance liquid chromatography. J Agric Food Chem. 2000;48:3317–3320. doi: 10.1021/jf990373n. [DOI] [PubMed] [Google Scholar]
- Nascimento AM, Conti R, Turatti ICC, Cavalcanti BC, Costa-Lotufo LV, Pessoa C, Moraes MO, Manfrim V, Toledo JS, Cruz AK, Pupo MT. Bioactive extracts and chemical constituents of two endophytic strains ofFusarium oxysporum. Rev Bras Farmacogn. 2012;22:1276–1281. [Google Scholar]
- NCCLS - National Committee for Clinical Laboratory Standards 2003 . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M7-A6. 6th ed. NCCLS; Wayne: 2003. 53 pp [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman K. Marine-derived fungi: a source for structurally new and bioactive secondary metabolites. Universität Bonn; Bonn: 2008. 153 pp MsD Thesis. [Google Scholar]
- Oliveira LFC, Edwards HGM, Velozo ES, Nesbitt M. Vibrational spectroscopic study of brazilin and brazilein, the main constituents of brazilwood from Brazil. Vib Spectrosc. 2002;28:243–249. [Google Scholar]
- Petrini O, Sieber TN, Toti L, Viret O. Ecology, metabolite production and substrate utilization in endophytic fungi. Nat Toxins. 1992;1:185–196. doi: 10.1002/nt.2620010306. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, White JF, Jr, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- Romanha AJ, de Castro SL, Soeiro MNC, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W, Andrade ZA. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz. 2010;105:233–238. doi: 10.1590/s0074-02762010000200022. [DOI] [PubMed] [Google Scholar]
- Rosa LH, Vaz ABM, Caligiorne RB, Campolina S, Rosa CA. Endophytic fungi associated with the Antarctic grassDeschampsia antarctica(Poaceae) Polar Biol. 2009;32:161–167. [Google Scholar]
- Sewram V, Nieuwoudt TW, Marasas WFO, Shephard GS, Ritieni A. Determination of theFusariummycotoxins, fusaproliferin and beauvericin by high-performance liquid chromatography - electrospray ionization mass spectrometry. J Chromatogr A. 1999;85:175–185. doi: 10.1016/s0021-9673(99)00814-6. [DOI] [PubMed] [Google Scholar]
- Shin CG, An DG, Song HH, Lee C. Beauvericin and enniatins H, I and MK1688 are new potent inhibitors of human immunodeficiency virus type-1 integrase. J Antibiot. 2009;62:687–690. doi: 10.1038/ja.2009.102. [DOI] [PubMed] [Google Scholar]
- Teixeira MC, Santos RJ, Sampaio RB, Pontes-de-Carvalho L, dos Santos WL. A simple and reproducible method to obtain large numbers of axenic amastigotes of differentLeishmaniaspecies. Parasitol Res. 2002;88:963–968. doi: 10.1007/s00436-002-0695-3. [DOI] [PubMed] [Google Scholar]
- Vaz ABM, Brandão LR, Vieira MLA, Pimenta RS, Morais PB, Sobral MEG, Rosa LH, Rosa CA. Diversity and antimicrobial activity of fungal endophyte communities associated with plants of Brazilian savanna ecosystems. Afr J Microbiol Res. 2012;6:3173–3185. [Google Scholar]
- Vaz ABM, Mota RC, Bomfim MRQ, Zani CL, Rosa CA, Rosa LH. Antimicrobial activity of endophytic fungi associated with Orchidaceae in Brazil. Can J Microbiol. 2009;55:1381–1391. doi: 10.1139/W09-101. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu L. Beauvericin, a bioactive compound produced by fungi: a short review. Molecules. 2012;17:2367–2377. doi: 10.3390/molecules17032367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Innis MA, Gelfand DH, Sninsky JJ, White TJ. PCR protocols: a guide to methods and applications. Academic Press; San Diego: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. p. 315–p. 322. [Google Scholar]
- Xu Y, Zhan J, Wijeratne EMK, Burns AM, Gunatilaka AAL, Molnár I. Cytotoxic and antihaptotactic beauvericin analogues from precursor-directed biosynthesis with the insect pathogenBeau- weria bassianaATCC 7159. J Nat Prod. 2010;70:1467–1471. doi: 10.1021/np070262f. [DOI] [PubMed] [Google Scholar]
- Zhan J, Burns AM, Liu MX, Faeth SH, Gunatilaka AAL. Search for cell motility and angiogenesis inhibitors with potential anticancer activity: beauvericin and other constituents of two endophytic strains ofFusarium oxysporum. J Nat Prod. 2007;70:227–232. doi: 10.1021/np060394t. [DOI] [PMC free article] [PubMed] [Google Scholar]


