Abstract
Lipid mediators play an important role in infection- and tissue injury-driven inflammatory responses and in the subsequent inhibition and resolution of the response. Here, we discuss recent findings that substantiate how Mycobacterium tuberculosis promotes its survival in the host by dysregulation of lipid mediator balance. By inhibiting prostaglandin E2 (PGE2) and enhancing lipoxin production, M. tuberculosis induces necrotic death of the macrophage, an environment that favors its growth. These new findings provide opportunities for developing and repurposing therapeutics to modulate lipid mediator balance and enhance M. tuberculosis growth restriction.
Introduction
The recent World Health Organization report on the worldwide incidence of tuberculosis (TB) indicates that in 2012 there were an estimated 8.6 million incident cases of TB and 1.3 million deaths [1]. In addition, it is estimated that one third of the world's population is latently infected with Mycobacterium tuberculosis (Mtb) and is at a 5-10% lifetime risk of reactivation, thereby fueling the cycle of transmission. Clearly, TB remains a global emergency. A significant impediment to TB treatment and global eradication of the disease is a 6-month multi-drug treatment regimen. Prolonging treatment reduces rates of relapse, but the extended period leads to non-compliance and the emergence of drug-resistant mutants. Additionally, despite successful treatment, relapses still occur in 5% of the patients because of bacterial persistence [2]. Furthermore, TB-HIV co-infected patients receiving anti-retroviral treatment and TB chemotherapy are at risk of developing immune reconstitution inflammatory syndrome [3,4]. Other major challenges to TB treatment and significant threats to global health are the worldwide increase in multi-drug-resistant (MDR) TB and the reported presence of extensively drug resistant TB in 92 countries [1]. In the last decade, therefore, a great deal of research effort in TB was invested in the discovery and development of new TB drugs that target the bacteria and in conducting clinical trials to evaluate their efficacy in shortening TB treatment regimens. However, there is high likelihood of also developing resistance against these newly discovered drugs.
Though still incomplete, our understanding of how Mtb evades the host response has advanced significantly. In addition, through the use of in vivo animal models, immune factors that restrict or promote Mtb growth have been identified. This commentary will focus on lipid mediators, since converging findings from several groups point to their significant role in regulating multiple pathways of host control of Mtb. We integrate these studies to highlight how lipid mediators form the central nexus influencing the various macrophage outcomes, including cell apoptosis, necrosis, and tumor necrosis factor (TNF) production, thereby positioning them as strong targets for host-directed therapy (HDT). HDTs have the potential to shorten TB treatment regimens with first-line TB drugs and, importantly, to improve treatment outcomes when used in conjunction with second-line drugs for MDR patients. Another potential advantage of HDT is the ability to restrain inflammatory responses in the lung and thereby ameliorate pulmonary pathology. In this regard, treatment of Mtb-infected mice with ibuprofen alone was found to be sufficient to reduce the number and size of lung lesions [5], and a meta-analysis study of 41 clinical trials found that corticosteroids were effective in reducing mortality in patients with TB [6].
Eicosanoids are a family of bioactive lipid mediators derived from arachidonic acid (AA), which is released from membrane phospholipids by phospholipases. There are three major pathways that are involved in the production of eicosanoids: (i) the cyclooxygenase pathway (COX-1 and COX-2), which produces prostaglandins and thromboxanes; (ii) the lipooxygenase (LOX) pathway (5-LOX, 12-LOX, and 15-LOX), which catalyzes the production of leukotrienes and lipoxins; and (iii) the cytochrome p40 pathway, which generates hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids. In addition, omega-3 essential eicosapentaenoic acid (EPA) and docosahexaenoic acid are substrates for the production of resolvins, protectins, and maresins that are termed as specialized pro-resolving mediators (SPMs) [7–10]. Prostaglandins and leukotrienes are the initial mediators of the inflammatory response and stimulate recruitment of neutrophils and monocytes. Lipoxins and SPMs have anti-inflammatory pro-resolving activities and contribute to the resolution phase of an inflammatory response. Prostaglandin E2 (PGE2) and prostaglandin D2 (PGD2) function as pro-inflammatory mediators during the initial phase of an inflammatory response. However, later, through a process termed “lipid mediator class switching”, these mediators reprogram exudate neutrophils to a pro-resolution phenotype by inducing 15-LOX and lipoxin generation [11]. Lipoxins are also double-edged mediators with anti-inflammatory and pro-resolving activities [12]. Ligation of the lipoxin A4 (LXA4) receptor on neutrophils arrests their migration but in monocytes signals for migration and non-phlogistic responses [13]. Thus, lipid mediators participate in the initiation and resolution of inflammation and aid in the return to homeostasis.
In this commentary, we will first present evidence that eicosanoids regulate host defense against Mtb. Based on fundamental findings, it is well established that TNF is critical to restricting Mtb growth [14] but that inhibition of apoptosis is a virulence strategy that Mtb employs to evade innate defense mechanisms of the host [15]. Furthermore, type I interferons (IFNs) have been shown to impinge on host-protective responses against TB [16]. We will then review the growing body of literature that mechanistically links these three host responses to regulation by lipid mediators. We conclude that these advances are paving the way for lipid mediator manipulation as promising targets for HDT in TB. However, one needs to be mindful of the possibility that these host-targeted therapeutic interventions for TB may have a deleterious effect on the control of coincident viral infections.
Mycobacterium tuberculosis and lipid mediators
There is rapidly accumulating evidence that the eicosanoids leukotrienes, prostaglandins, and lipoxins are critical to determining the outcome of Mtb infection in the host. The first evidence that lipid mediators may serve as druggable host targets came from Mtb infection studies in mice deficient in 5-LOX (Alox5−/−) [17], the enzyme required for both leukotriene and lipoxin biosynthesis. These mice had elevated expression of interleukin-12 (IL-12) and IFNγ, and exhibited significantly lower Mtb burdens in their lungs at 5 weeks following infection, compared with wild-type (WT) mice [17]. The further finding that administration of a lipoxin analog to Mtb-infected Alox5−/− mice reversed the protection [17] indicates that lipoxins, and not leukotrienes, negatively regulate host control of Mtb. In contrast, other studies have reported that inhibition of 5-LOX with the pharmacological inhibitor MK886 abrogated host control of experimental pulmonary TB and that this was associated with a reduction in leukotrienes [18,19]. Despite the discrepant outcomes between Alox-5−/− and MK886-treated mice, the finding that ALOX-5 variants are associated with susceptibility to TB supports that 5-LOX products, lipoxin and leukotrienes, regulate host-protective immune response against Mtb [20].
Tumor necrosis factor and lipid mediators
Findings from a forward genetic screen in zebrafish larvae found that mutations in the Ita4h locus encoding leukotriene A4 hydrolase (LTA4H) conferred hypersusceptibility to Mycobacterium marinum infection [21]. LTA4H catalyzes the conversion of leukotriene A4 to the pro-inflammatory eicosanoid, leukotriene B4 (LTB4). The study found that the phenotype of the mutant was not due to the lack of LTB4 but rather to the deviation of the eicosanoid substrates toward the production of anti-inflammatory lipoxins [21]. The increased levels of lipoxin inhibited TNF production and resulted in increased growth of mycobacteria. Contrary to expectation, modulation of LTA4H expression with anti-sense RNA and morpholinos showed that not only LTA4H-low but also LTA4H-high zebrafish exhibited increased bacterial growth [22]. LTA4H-low animals expressed high levels of LXA4 and low levels of TNF. LTA4H-high animals, on the other hand, expressed enhanced levels of LTB4, increased TNF, and an overall heightened inflammatory state [22]. Additional experimental manipulations of TNF levels in the fish revealed TNF to be the key executioner of increased necrosis and bacterial growth in both states of LTA4H expression [22]. Together, these studies indicate that a fine balance of the eicosanoid mediators is essential to generate the right level of TNF since either too little or too much of the cytokine can be detrimental to host control of Mtb. The finding that optimal TNF levels and control of mycobacterial growth can be achieved by modulation of lipoxin levels with pharmacologic modulators [22] or via overexpression of an enzyme that inactivates LTB4 [23] underscores the potential of targeting lipid mediators for the development of HDTs.
Subsequent work on the LTA4H promoter showed that a functional single-nucleotide polymorphism influenced the level of transcription in macrophages, thereby providing an explanation for why adjunctive therapy with dexamethasone benefitted only a subset of patients with TB meningitis [24]. Reanalysis of study patients for LTA4H polymorphism showed that patients who benefitted from dexamethasone adjunctive treatment had a high LTA4H-expressing genotype but that those who did not benefit had the low-LTA4H-expressing genotype [22]. This finding is paradigm-shifting and indicates that TB treatment with HDT may have to take into consideration the individual's genotype.
Apoptosis and lipid mediators
Modulation of the macrophage cell death pathway is exploited by Mtb as a virulence strategy to survive in the host [25]. Early studies clearly delineated two distinct outcomes following infection of alveolar macrophages with virulent and avirulent Mtb strains. Infection of murine bone marrow-derived macrophages [26] or human monocyte-derived macrophages [27] with avirulent Mtb strains resulted in TNF-dependent apoptosis of the infected cell while virulent Mtb evaded this innate defense mechanism and instead induced macrophage necrosis as the major mode of cell death. Mechanistically, virulent Mtb, via annexin-1 blockade, prevents the complete formation of the apoptotic envelope [28] and inhibits apoptosis induction. Apoptosis is host-protective since it restricts Mtb growth [29,30], enhances antigen presentation by dendritic cells, and induces an early Mtb-specific adaptive Th1 response [31,32]. On the other hand, necrosis results from an irreversible increase in the mitochondrial permeability transition, resulting in mitochondrial damage and enhanced release of cytochrome c [33]. Together, these studies underscore the pivotal role for the necrotic form of macrophage cell death in creating a safe niche for virulent Mtb.
In subsequent studies, the mechanistic basis for the induction of disparate cell death pathways by avirulent and virulent strains of Mtb was found to be at the level of lipid mediators. Avirulent Mtb induced PGE2 production which protected against cellular necrosis by preventing mitochondrial inner membrane damage [34] and by promoting rapid plasma membrane repair [35]. On the other hand, LXA4 was the dominant lipid mediator produced by macrophages infected with virulent Mtb and was responsible for induction of necrosis. LXA4 suppressed COX-2 expression and synthesis of PGE2, thereby deviating the infected macrophage toward a necrotic fate [34]. The ensuing findings that prostaglandin E synthase (Ptges)-deficient mice [34] and mice lacking the prostaglandin receptor EP2 [36] have increased susceptibility to Mtb infection [36] provide strong evidence that PGE2 and the apoptotic death of macrophages are critical to regulating Mtb growth in vivo [34].
In this commentary, the role of apoptosis in Mtb infection is discussed under the rubric of lipid mediators and in this context the literature supports apoptosis as being host-protective. However, we would like to emphasize that the role of apoptosis in Mtb infection is contentious and that several studies have reported that the induction of apoptosis is indeed a virulence strategy of Mtb [37–41]. Clearly, additional extensive studies with Mtb clinical isolates are necessary to determine how the two forms of cell death regulate Mtb growth and pathogenesis in vivo.
Type I interferon and lipid mediators
Several studies have shown that type I IFNs promote Mtb growth. For example, clinical isolates of the Beijing family induce a strong type I IFN response, and in vivo studies have shown that type I IFNs exacerbate disease in Mtb-infected mice [42,43]. Enhancing type I IFN expression in the lungs of WT mice through intranasal poly-IC administration [44] or exposure to influenza A virus [45] also resulted in increased pathology and bacterial load in the lungs in comparison with control mice. Consistent with IFN's deleterious effect on the host, IFNα receptor knockout mice (IFNARKO) mice were found to be more resistant to Mtb infection compared with their WT counterparts [46,47], and poly-IC treatment had no effect on IFNARKO mice [44]. Removal of TPL2, a negative regulator of type I IFN, also led to excess type 1 IFN and exacerbated disease in Mtb-infected mice [48]. Type I IFN downregulates the host immune response by inhibiting the production of IL-1β [49,50], a cytokine critical to protection against Mtb infection [51–54]. Type I IFN also inhibits IL-12 production and IFNγ-mediated killing by macrophages [55]. Findings from a seminal study performed in a cohort of TB patients from London and South Africa showed that active disease was associated with a type I IFN gene signature and that the intensity of the signature correlated with severity of disease [56]. Subsequently, a similar disease-related type I IFN gene signature was reported in South African and Gambian cohorts [57] and in an Indonesian cohort [58].
Despite the growing evidence of the cross-talk between IL-1β and type I IFN and the importance of IL-1β to host protection, the mechanism of how IL-1β promotes resistance remained unclear. In a recent study, Mayer-Barber and colleagues [59] probed this question by first examining the typically studied mediators of protection, including IFNγ, nitric oxide synthase 2 (NOS2), IL-12p40, and TNF, and found that these were not abrogated in the susceptible IL-1R1KO mice. Instead, the authors discovered differential expression levels of eicosanoid mediators between WT and IL-1RKO mice [59]. In comparison with bronchoalveolar lavage fluid of WT mice, that of IL-1R1KO mice had significant reductions in PGE2 and PGF2a and concomitant increases in the levels of LXA4 and LTB4. Using a number of elegant in vitro and in vivo approaches to ablate COX-2 and PGE2 expression, the authors were able to effectively demonstrate that, during Mtb infection, IL-1β induces COX-2 expression which in turn upregulates PGE2 and subsequent Mtb growth restriction. IFNARKO mice that are resistant to Mtb express significantly high levels of IL-1β and PGE2 [59]. Furthermore, removal of IFNAR1 in IL-1R1KO mice reduced their susceptibility compared with IL-1R1KO alone, clearly indicating that type I IFN abrogates host protection [59]. This study definitely shows that the suppression of type 1 IFN by IL-1β-induced PGE2 is a key step in conferring resistance to Mtb. Importantly, the authors were able to confirm their observations in two human cohorts where they found that patients with a lower PGE2-to-LXA4 ratio had worse sputum grade [59]. Mice lacking IL-1α and IL-1β express high type 1 IFN levels [59]. Treatment of these mice with zileuton, a US Food and Drug Administration-approved 5-LOX inhibitor, together with PGE2 led to decreased lung pathology and bacterial burden [59], indicating that lipid mediators are attractive host targets for therapeutic intervention in TB.
Summary and closing remarks
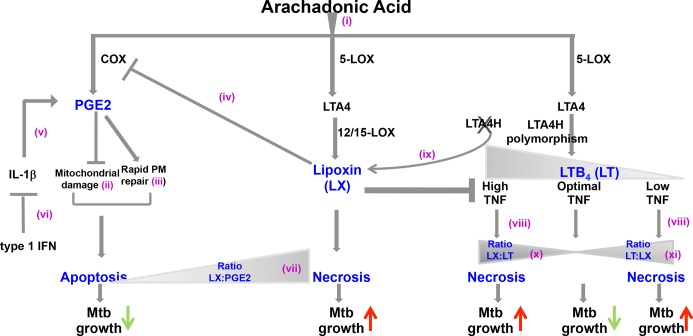
In Figure 1, we summarize the findings that demonstrate the contribution of the three major eicosanoid pathways to the ability of the host to restrict Mtb. AA metabolism leads to the induction of PGE2, lipoxin, and leukotrienes (i). PGE2 promotes apoptosis and prevents necrosis by inhibiting mitochondrial damage (ii) and inducing rapid plasma membrane repair (iii). However, lipoxin can inhibit PGE2 via inhibition of COX-2 expression (iv) and ablate the protective effect of PGE2 in the macrophage, leading to necrosis. IL-1β enhances PGE2 expression (v), but IL-1β expression is inhibited by type I IFN (vi). Thus, in the presence of type I IFN, IL-1β expression is abrogated and consequently PGE2 levels decrease. This results in an overall increased ratio of lipoxin to PGE2 in the host, promoting Mtb growth (vii). LTA4H induces leukotriene which in turn upregulates TNF expression. Low expression of LT4AH leads to reduced TNF (viii) and inability to control bacteria and subsequent necrosis of macrophages. The fact that removal of LTA4H deviates eicosanoid expression toward enhanced lipoxin levels (ix) indicates that an increased ratio of lipoxin to leukotrienes leads to macrophage necrosis (x). High expression of LTA4H is also detrimental since it tips the balance toward high leukotriene expression, excess TNF and macrophage necrosis (xi), and enhanced growth of Mtb. This indicates that the levels of lipoxin and leukotriene should be in equilibrium for optimal macrophage response to Mtb.
Figure 1. Cross-regulation of the eicosanoid pathways in restricting Mtb growth.
Abbreviations: COX, cyclooxygenase; IFN, interferon; IL-1β, interleukin-1-beta; LOX, lipooxygenase; LTA4, leukotriene A4; LTA4H, leukotriene A4 hydrolase; LTB4, leukotriene B4; Mtb, Mycobacterium tuberculosis; PGE2, prostaglandin E2; PM, plasma membrane; TNF, tumor necrosis factor.
Together, the findings demonstrate that striking the proper balance between the levels of lipoxin, PGE2, and leukotrienes is critical for successful control of Mtb. Thus, several drug targets in the eicosanoid pathway can be exploited to modulate the expression of the individual eicosanoids to the right level.
The paradigm that PGE2-mediated acquisition of the apoptotic phenotype by macrophages is host-beneficial is not without caveats. For example, lipid bodies contained in foamy macrophages promote the intracellular survival of mycobacteria [60,61] but are also intracellular sites for PGE2 generation [61]. Also, contrary to expectation, mice treated with ibuprofen, a drug that suppresses PGE2 production, exhibit reduced bacterial load [5]. Of note, this study used the C3HeB/FeJ strain of mice that develop necrotic lung pathology similar to active TB. Clearly, further mechanistic studies are required to determine how ibuprofen treatment and decreased PGE2 can be beneficial in the context of extensive lung pathology. Also, in vivo studies need to examine whether ibuprofen and other non-steroidal anti-inflammatory drugs protect the host against TB by mechanisms that are independent of host lipid mediators.
To understand the role of lipid mediators during influenza infection, lipidomic profiling was performed in mice infected with a low-pathogenicity influenza strain (X31/H3N2) and a high-pathogenicity strain (PR8/H1N1). Comparison of lipid class composition showed that 12/15-LOX mediators were associated with the resolution phase of the X31 infection but that a high percentage of pro-inflammatory 5-LOX mediators correlated with the H1N1 strain [60]. These data suggest that a similar analysis of the composition of lipid mediators induced by different clinical strains of Mtb may yield important data regarding the temporal role of lipid mediators in regulating pulmonary pathogenesis and inform whether the infecting strain of Mtb may have to be considered in HDTs.
As clinical trials with HDTs are implemented, it is important to consider the duality in function of several of the lipid mediators. There is strong evidence that high lipoxin levels abrogate control of Mtb growth. However, lipoxin also reduces airway inflammation [61] and therefore it would be important to investigate whether HDT targeting the lipoxin pathway may have adverse effects of enhanced immunopathology. In this regard, it is worth investigating whether pro-resolving mediators that have no anti-inflammatory effects will provide a new therapeutic opportunity for complete resolution of lung pathology in patients with TB. It may also be important to study the effect of lipoxin on non-hematopoietic cells since it induces bactericidal/permeability-increasing protein in human mucosal epithelia [62]. A recent study reported that influenza virus A uses the PGE2 pathway to enhance its replication in the host [63]. The virus limits the induction of type I IFN and also inhibits cell apoptosis by upregulating the expression of PGE2. Inhibition of PGE2 via genetic or pharmacological means enhanced the ability of the host to control viral replication. This finding suggests that PGE2 that is protective in Mtb infection has an opposing role in influenza virus infection and raises a note of caution regarding lipid mediator-directed HDT against Mtb. Conversely, it is equally important to consider whether inhibition of PGE2 as an HDT against influenza virus infection may lead to a burst in type I IFN and be detrimental if a co-infection with Mtb is present.
Despite these caveats, the exciting findings on how the balance of lipid mediators dictates the ability of macrophages to control Mtb growth will open up novel pathways for developing HDTs. Targeted delivery of the HDTs can be employed to ameliorate effects on viral co-infections.
Abbreviations
- AA
arachidonic acid
- COX
cyclooxygenase
- HDT
host-directed therapy
- IFN
interferon
- IFNARKO
interferon alpha receptor knockout mice
- IL
interleukin
- LOX
lipooxygenase
- LTA4H
leukotriene A4 hydrolase
- LTB4
leukotriene B4
- LXA4
lipoxin A4
- MDR
multi-drug-resistant
- Mtb
Mycobacterium tuberculosis
- PGE2
prostaglandin E2
- SPM
specialized pro-resolving mediator
- TB
tuberculosis
- TNF
tumor necrosis factor
- WT
wild-type
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/7/29
References
- 1.World Health Organization (WHO); 2013. Global tuberculosis report 2013.http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother. 2012;56:2223–30. doi: 10.1128/AAC.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai RP, Nakiwala JK, Meintjes G, Wilkinson RJ. The immunopathogenesis of the HIV tuberculosis immune reconstitution inflammatory syndrome. Eur J Immunol. 2013;43:1995–2002. doi: 10.1002/eji.201343632. [DOI] [PubMed] [Google Scholar]
- 4.Gopalan N, Andrade BB, Swaminathan S. Tuberculosis-immune reconstitution inflammatory syndrome in HIV: from pathogenesis to prediction. Expert Rev Clin Immunol. 2014;10:631–45. doi: 10.1586/1744666X.2014.892828. [DOI] [PubMed] [Google Scholar]
- 5.Vilaplana C, marzo E, Tapia G, Diaz J, Garcia V, Cardona PH. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J Infect Dis. 2013;208:199–202. doi: 10.1093/infdis/jit152. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717998120
- 6.Critchley JA, Young F, Orton L, Garner P. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:223–37. doi: 10.1016/S1473-3099(12)70321-3. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717983496
- 7.Li Y, Dalli J, Chiang N, Baron RM, Quintana C, Serhan CN. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by MicroRNA and proresolving mediators. Immunity. 2013;39:885–98. doi: 10.1016/j.immuni.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718178065
- 8.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:69–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1088475
- 9.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Hong S, Gronert K, colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13277075
- 11.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–87. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 14.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 15.Divangahi M, Behar SM, Remold H. Dying to live: how the death modality of the infected macrophage affects immunity to tuberculosis. Adv Exp Med Biol. 2013;783:103–20. doi: 10.1007/978-1-4614-6111-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry MP, Blankley S, Graham CM, Bloom CI, O’Garra A. Systems approaches to studying the immune response in tuberculosis. Curr Opin Immunol. 2013;25:579–87. doi: 10.1016/j.coi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115:1601–6. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1026193
- 18.Peres CM, de Paula L, Medeiros AI, Sorgi CA, Soares EG, Carlos D, Peters-Golden M, Silva CL, Faccioli LH. Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis. Microbes Infect. 2007;9:483–9. doi: 10.1016/j.micinf.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1070793
- 19.Peres-Buzalaf C, de Paula L, Frantz FG, Soares EM, Medeiros AI, Peters-Golden M, Silva CL, Faccioli LH. Control of experimental pulmonary tuberculosis depends more on immunostimulatory leukotrienes than on the absence of immunosuppressive prostaglandins. Prostaglandins Leukot Essent Fatty Acids. 2011;85:75–81. doi: 10.1016/j.plefa.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725356630
- 20.Herb F, Thye T, Niemann S, Browne EN, Chinbuah MA, Gyapong J, Osei I, Owusu-Dabo E, Werz O, Rüsch-Gerdes S, Horstmann RD, Meyer CG. ALOX5 variants associated with susceptibility to human pulmonary tuberculosis. Hum Mol Genet. 2008;17:1052–60. doi: 10.1093/hmg/ddm378. [DOI] [PubMed] [Google Scholar]
- 21.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, bang ND, Hagge DA, Khadge S, King MC, Hawn TR, Moens CB, Ramakrishnan L. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–30. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/2456973
- 22.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, Vary JC, Hawn TR, Dunstan SJ, Farrar JJ, Thwaites GE, King MC, Serhan CN, Ramakrishnan L. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–46. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/14267376
- 23.Tobin DM, Roca FJ, Ray JP, Ko DC, Ramakrishnan L. An enzyme that inactivates the inflammatory mediator leukotriene b4 restricts mycobacterial infection. PLoS One. 2013;8:e67828. doi: 10.1371/journal.pone.0067828. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/724134161
- 24.Török ME, Nguyen DB, Tran TH, Nguyen TB, Thwaites GE, Hoang TQ, Nguyen HD, Tran TH, Nguyen TC, Hoang HT, Wolbers M, Farrar JJ. Dexamethasone and long-term outcome of tuberculous meningitis in Vietnamese adults and adolescents. PLoS One. 2011;6:e27821. doi: 10.1371/journal.pone.0027821. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13595957
- 25.Spira A, Carroll JD, Liu G, Aziz Z, Shah V, Kornfeld H, Keane J. Apoptosis genes in human alveolar macrophages infected with virulent or attenuated Mycobacterium tuberculosis: a pivotal role for tumor necrosis factor. Am J Respir Cell Mol Biol. 2003;29:545–51. doi: 10.1165/rcmb.2002-0310OC. [DOI] [PubMed] [Google Scholar]
- 26.Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol. 2006;79:80–6. doi: 10.1189/jlb.0505250. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol. 2006;176:3707–16. doi: 10.4049/jimmunol.176.6.3707. [DOI] [PubMed] [Google Scholar]
- 28.Gan H, Lee J, Ren F, Chen M, Kornfeld H, Remold HG. Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat Immunol. 2008;9:1189–97. doi: 10.1038/ni.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1122933
- 29.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–7. [PubMed] [Google Scholar]
- 31.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–46. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1014821
- 32.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–17. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Duan L, Gan H, Golan DE, Remold HG. Critical role of mitochondrial damage in determining outcome of macrophage infection with Mycobacterium tuberculosis. J Immunol. 2002;169:5181–7. doi: 10.4049/jimmunol.169.9.5181. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, Remold HG. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205:2791–801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1124908
- 35.Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, Fortune S, Behar SM, Remold HG. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaul V, Bhattacharya D, Singh Y, Van Kaer L, Peters-Golden M, Bishai WR, Das G. An important role of prostanoid receptor EP2 in host resistance to Mycobacterium tuberculosis infection in mice. J Infect Dis. 2012;206:1816–25. doi: 10.1093/infdis/jis609. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725356631
- 37.Rojas M, Barrera LF, Puzo G, Garcia LF. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J Immunol. 1997;159:1352–61. [PubMed] [Google Scholar]
- 38.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1145173
- 39.Aguiló N, Uranga S, Marinova D, Martín C, Pardo J. Bim is a crucial regulator of apoptosis induced by Mycobacterium tuberculosis. Cell Death Dis. 2014;5:e1343. doi: 10.1038/cddis.2014.313. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718496012
- 40.Aporta A, Arbues A, Aguilo JI, Monzon M, Badiola JJ, de Martino A, Ferrer N, Marinova D, Anel A, Martin C, Pardo J. Attenuated Mycobacterium tuberculosis SO2 vaccine candidate is unable to induce cell death. PLoS One. 2012;7:e45213. doi: 10.1371/journal.pone.0045213. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/724152373
- 41.Aguiló N, Marinova D, Martín C, Pardo J. ESX-1-induced apoptosis during mycobacterial infection: to be or not to be, that is the question. Front Cell Infect Microbiol. 2013;3:88. doi: 10.3389/fcimb.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, Barry C, Kaplan G. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 43.Manca C1, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, 3rd, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci USA. 2001;98:5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonelli LR, Gigliotti Rothfuchs A, Gonçalves R, Roffê E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–82. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725356632
- 45.Redford PS, Mayer-Barber KD, McNab FW, Stavropoulos E, Wack A, Sher A, O’Garra A. Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. J Infect Dis. 2014;209:270–4. doi: 10.1093/infdis/jit424. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718071290
- 46.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–52. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/719468036
- 47.Dorhoi A, Yeremeev V, Nouailles G, Weiner J, 3rd, Jörg S, Heinemann E, Oberbeck-Müller D, Knaul JK, Vogelzang A, Reece ST, Hahnke K, Mollenkopf HJ, Brinkmann V, Kaufmann SH. Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur J Immunol. 2014;44:2380–93. doi: 10.1002/eji.201344219. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718369843
- 48.McNab FW, Ewbank J, Rajsbaum R, Stavropoulos E, Martirosyan A, Redford PS, Wu X, Graham CM, Saraiva M, Tsichlis P, Chaussabel D, Ley SC, O’Garra A. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J Immunol. 2013;191:1732–43. doi: 10.4049/jimmunol.1300146. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718029665
- 49.Novikov A, Cardone M, Thompson R, Shenderov K, Kirschman KD, Mayer-Barber KD, Myers TG, Rabin RL, Trinchieri G, Sher A, Feng CG. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187:2540–7. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/719454537
- 50.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–34. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13985966
- 51.Mayer-Barber KD1, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Núñez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–30. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/2521960
- 52.Juffermans NP, Florquin S, Camoglio L, Verbon A, Kolk AH, Speelman P, van Deventer SJ, van Der Poll T. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis. 2000;182:902–8. doi: 10.1086/315771. [DOI] [PubMed] [Google Scholar]
- 53.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178–89. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 54.Sugawara I, Yamada H, Hua S, Mizuno S. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol Immunol. 2001;45:743–50. doi: 10.1111/j.1348-0421.2001.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 55.McNab FW, Ewbank J, Howes A, Moreira-Teixeira L, Martirosyan A, Ghilardi N, Saraiva M, O’Garra A. Type I IFN Induces IL-10 Production in an IL-27-Independent Manner and Blocks Responsiveness to IFN-gamma for Production of IL-12 and Bacterial Killing in Mycobacterium tuberculosis-Infected Macrophages. J Immunol. 2014;193:3600–12. doi: 10.4049/jimmunol.1401088. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718632517
- 56.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–7. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/4850956
- 57.Maertzdorf J, Ota M, Repsilber D, Mollenkopf HJ, Weiner J, Hill PC, Kaufmann SH. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PLoS One. 2011;6:e26938. doi: 10.1371/journal.pone.0026938. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/724169974
- 58.Ottenhoff TH, Dass RH, Yang N, Zhang MM, Wong HE, Sahiratmadja E, Khor CC, Alisjahbana B, van Crevel R, Marzuki S, Seielstad M, van de Vosse E, Hibberd ML. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS One. 2012;7:e45839. doi: 10.1371/journal.pone.0045839. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/724151980
- 59.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE, 3rd, Sher A. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718478368
- 60.Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, Thomas PG, Dennis EA, Aderem A. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154:213–27. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718685155
- 61.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–23. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1009596
- 62.Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, Colgan SP. Lipid mediator-induced expression of bactericidal/ permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci USA. 2002;99:3902–7. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1005952
- 63.Coulombe F, Jaworska J, Verway M, Tzelepis F, Massoud A, Gillard J, Wong G, Kobinger G, Xing Z, Couture C, Joubert P, Fritz JH, Powell WS, Divangahi M. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–68. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718348579