Abstract
In-group favoritism is the tendency for individuals to cooperate with in-group members more strongly than with out-group members. Similar concepts have been described across different domains, including in-group bias, tag-based cooperation, parochial altruism, and ethnocentrism. Both humans and other animals show this behavior. Here, we review evolutionary mechanisms for explaining this phenomenon by covering recently developed mathematical models. In fact, in-group favoritism is not easily realized on its own in theory, although it can evolve under some conditions. We also discuss the implications of these modeling results in future empirical and theoretical research.
Introduction
Cooperation, when it is not ostensibly lucrative behavior (i.e. in social dilemma situations), is ubiquitously found in human societies and animal kingdoms. We now have a multitude of explanations of evolutionary origins and rationality behind this seemingly irrational behavior. Together with field observations and laboratory experiments, mathematical models have contributed to the elucidation of mechanisms for cooperation, including kin selection, group selection, direct and indirect reciprocity, and network reciprocity [1–3].
In-group favoritism is a related phenomenon repeatedly observed in experiments and real society, both for human and non-human species. Dwelling on human metaphor, we tend to cooperate more with others in the same group than with those in different groups (Figure 1). Groups can be formed exogenously, such as arbitrary allocation by experimenters [4–5], or endogenously, based on visible traits, such as ethnicity and sex. A lot of empirical studies have confirmed in-group favoritism, as covered in review articles [5–12], metareview studies [13–16], and parts of monographs on group processes [17–19].
Figure 1. Schematic of in-group favoritism.
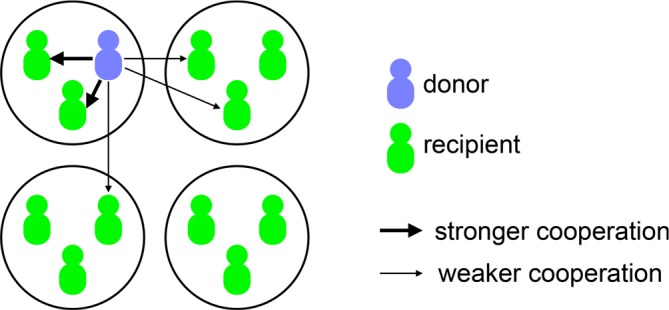
Each circle represents a group. The focal donor in this figure is an in-group cooperator.
What is the mechanism that leads to the evolution of in-group favoritism? The aforementioned reviews and monographs detail experimental findings of and psychological accounts for in-group favoritism. However, they are mute about evolutionary origins or payoff maximization viewpoints of the same behavior. The need for an evolutionary theory of in-group favoritism was also proposed by the psychology community [7,12].
In fact, evolutionary game theory has proposed various mechanisms governing in-group favoritism. In this article, we review these studies, with an emphasis on in-group favoritism by humans. Importantly, in evolutionary biology, review papers on the green-beard effect, which is closely related to in-group favoritism, have been published and provide a theoretical underpinning of this behavior mainly based on inclusive fitness theory [20–22]. Here, we focus on general forms of in-group favoritism. We also discuss the implications of presently available results and future directions.
In-group favoritism, its synonyms, and related concepts
In-group favoritism, or “playing favorites”, has been studied across disciplines, from social psychology to evolutionary biology and anthropology; and from laboratory experiments to mathematical models. Partly for this reason, different names have been used for representing in-group favoritism and related concepts. In this section, we explain them.
Synonyms
As stated earlier, in-group favoritism is the tendency for individuals to cooperate with in-group members more than with out-group members. “In-group bias” is a synonym of in-group favoritism often used in psychology studies. Synonyms used particularly in the context of cultural evolution and anthropology include “parochial altruism” and “ethnocentrism”. Hostility to out-group members, such as belligerence or going to war between tribes, is often emphasized when using these terms [23,24].
“Tag-based cooperation” is a term often used in modeling studies. In models of tag-based cooperation, individuals own one of several tags and cooperate if (and only if) the individual's own tag is the same as or similar to the peer's tag. Then, tag-based cooperation is functionally equivalent to in-group favoritism, where the tag corresponds to the group. There are some slight differences, however. First, groups implied in in-group favoritism in society often mean more than just partitioning of the population according to group identities. Individuals in such a group may be more likely to encounter in-group members owing to the common ethnicity, tribe membership, hobby, and so on, as compared to out-group members (see section on Community structure and homophily below). In contrast, tags in the context of tag-based cooperation do not a priori imply frequent intra-tag interaction. Second, in tag models, tags can be continuously distributed [25,26], whereas groups are usually discrete objects. Nevertheless, whether the tag is discrete or continuous does not usually affect the possibility of in-group favoritism [27].
Green-beard
The green-beard effect is a term coined by Dawkins [28,29] (also see [30]). In the context of genetic evolution, which Dawkins referred to as, a single gene is simultaneously responsible for tag and behavior (such as whether or not to cooperate) in this mechanism. Then, inheritance of this gene simultaneously changes the tag and behavior. If a gene encodes both the tendency of cooperation and the tag, a population of cooperators would be resistant to invasion by defectors because the tag encodes cooperativity of players. In theory, the green-beard effect is a viable mechanism for in-group favoritism.
Differently from the green-beard effect, general forms of in-group favoritism allow the tag and behavior to evolve independently by definition. If the tag and behavior do evolve independently, however, there is room for defectors possessing the tag shared by many cooperators to prosper, which undermines cooperation. Cooperation based on green beards dictates that the tag and behavior are inherited together, genetically or culturally. In fact, evidence of the green-beard effect in humans is poor [31] (but see [32]).
Armpit effect
The “armpit effect” is another term coined by Dawkins [29], who related anecdotally that a dog smells its own armpit and then others' armpits to identify which peers smell the same. A dog would exclusively cooperate with others with the same smell. To recognize tags, the armpit effect stipulates a mechanism of self-inspection, which is not necessarily explicit in the green-beard effect [21] and in the general in-group favoritism. Most of the mechanisms of in-group favoritism require that individuals can recognize and compare tags or group identity of the self and others. Self-inspection serves this purpose.
Community structure and homophily
By definition, in-group favoritism necessitates group structure of the population, may it be artificially introduced or natural. In human society, group structure is a norm rather than an exception. The fact that various social networks possess so-called community structure (i.e. community = group), whereby links connecting two individuals are more often within a community rather than across different communities [33], is consistent with this observation. Group structure may be induced by homophily, that is, love of the same “feather” in terms of, for example, sex, ethnicity, and social status [34] (homophily occurs even at the level of individual genes [35]). In a similar vein, homophily also leads to social segregation and polarization on the macroscopic level of groups [36]. Mathematical models show that an increase in the frequency of encountering similar others facilitates the evolution of homophily (see supplementary information in [37]) and also in-group favoritism (see supplementary information in [38]). However, community structure and homophily are not merely the consequence or cause of in-group favoritism. Coevolutionary dynamics of homophily and cooperation in experimental social networks show that these two traits are often tightly linked and mutually reinforced [39–42].
Assortative interaction, where individuals tend to interact with others possessing the same strategy (or phenotype in general), facilitates cooperation because unconditional cooperators then tend to interact within themselves, as do unconditional defectors [43]. In fact, many mechanisms of cooperation are machinery to induce assortative interaction. In this situation, the unconditional cooperators and unconditional defectors separately form groups, or communities. However, unconditional cooperators do not carry out in-group favoritism because they also cooperate with out-group members, who are defectors. This thought example suggests that assortative interaction in its original form is distinct from, but may act synergistically with, in-group favoritism.
Group selection
In-group favoritism is a behavioral phenomenon or a strategy, whereas group selection is a rule of natural selection. Therefore, the two concepts are not comparable. Furthermore, cooperation occurring as in-group favoritism and cooperation under group selection are different [44], as we discuss in the following.
There are two types of group selection [45,46]. In the first type, groups are assumed to explicitly compete for selection [47,48]. Then, a group containing many cooperators is favored in selection, although a selfish individual in such a group obtains a larger fitness than a cooperator in the same group. The frequency of cooperators can be maintained at a high level because groups possessing many cooperators proliferate through selection. Because intergroup competition implies hostility between groups, group competition and in-group favoritism may sound akin to each other. However, group competition is a rule of selection and not a behavioral trait of individuals or groups of individuals, unless the intensity of group competition is affected by the behavior of individuals as assumed in recent models [23,24]. Frequent cooperation under group competition does not imply that individuals cooperate more with in-group than out-group peers. In many models with group competition, individuals are not assumed to interact with out-group peers, such that in-group favoritism is an irrelevant question. Technically, both the lack of interaction with out-group peers and defection to out-group peers may yield the same zero payoff. However, we distinguish the two cases because the rule of the game is different. Finally, some arguments attempt to relate cultural group competition to in-group favoritism observed in human society [12], although the connection is yet unclear.
In the second type of group selection, interaction between individuals occurs only within groups [49]. Then, individuals migrate across groups in an assortative way; cooperators tend to find other cooperators within the new group, and the same for selfish individuals. Owing to the assortative migration, cooperation is sustained in the entire population, although selection prefers selfish individuals to cooperators in each group. This mechanism is unrelated to in-group favoritism because individuals in different groups do not interact in any way.
Experimental results
Humans
The field study by Sherif and colleagues is a classic example of in-group favoritism [50]. In their study, boys participating in a summer camp were divided into two groups in an arbitrary way and made by the camp authorities to perform several tasks in which the two groups competed. Then, the boys rapidly developed in-group favoritism in their daily life during the camp. Now there is way more empirical evidence of in-group favoritism by humans than can be surveyed here (see reviews cited in the Introduction for references). Here we briefly mention some seminal work and just a few recent experiments.
Tajfel produced in-group favoritism in laboratories in a systematic way [4,5]. He divided the participants into groups based on, for example, the liking of different paintings or the performance of non-essential counting tasks. Even in such minimal group experiments, participants showed in-group favoritism. A conceptual theory for explaining this phenomenon is the so-called social identity theory, according to which humans pursue the distinctiveness of the group to maintain the social identity of the self through in-group favoritism behavior [5,6,18]. Alternatively, Yamagishi and colleagues proposed reciprocity heuristics (so-called “group heuristics”) as underlying in-group favoritism. According to this view, in-group favoritism occurs because the participants expect that in-group members (as opposed to out-group members) will reciprocate in the future because interaction with in-group members is perceived to be more likely [8,51].
Recent experiments include the following: in-group favoritism in the context of altruistic punishment was examined with indigenous groups in highland Papua New Guinea [52]. The punisher showed in-group favoritism toward potential recipients of the endowment; they tended to punish norm violators when victim recipients belonged to the same group as the punisher. In [53], coevolution of tags and in-group favoritism was explored in laboratory experiments. In this work, in-group favoritism meant inclination to in-group interaction as opposed to across-group interaction, and coordination rather than costly helping was the task. Participants showed coevolving in-group favoritism (in their sense) and coordination. In-group favoritism also occurs in the context of politics; voters show in-group favoritism based on political affiliation [54,55]. Last, humans preferentially cooperate with those whose faces are similar to their own [56,57].
Other animals
In a broad sense, in-group favoritism is widely observed in non-humans as well, ranging from primates to microbes [21,22,58–62]. Field observational studies often focus on large group-living animals, and primary examples include monkeys [59], dolphins [60], meerkats [62], and elephants [63], to name a few. The nature of interactions is context-based, such as grooming [59], companionship [60], territory defense [61], and water searching [63]. Moreover, grouping of animals that mediates the nature of interactions can be multi-level. For example, animals jointly living in the same territory/nest/site are regarded as belonging to the same group in empirical studies [58–62], whereas, within individual groups, subgroups can further emerge and be identified with various clustering algorithms in social network analysis [60,62,63] (see Community structure and homophily section above). Intergroup interactions often involve hostile competition, such as red fire ants that kill others with different odor cues [58], and competitive mating of side-blotched lizards with different morph colors [61]. In contrast, within-group interactions are more likely to be cooperative with (sub)group members in the same territory/nest/site [61–63].
In recent years, there has been a surge of interest in the search for green-beard genes, especially through knock-out laboratory experiments using microbial populations. Unlike humans, microbes are far easier to manipulate, and there are no ethical issues. In this case, grouping as well as interaction is determined by whether microbes carry “green-beard” genes (hence referred to as kin/kind-mediated interactions [64]). Homophilic adhesive interactions (i.e. like binds like) among microbes in particular biofilms are an important way to preferentially channel benefits towards in-group members [65–67]. Under harsh conditions, microbes aggregate into a multicellular structure by secreting homophilic adhesion proteins to help them stick together. Such differential cell-cell adhesion is mediated by green-beard genes, providing the molecular and cellular basis for in-group favoritism. When starved, social amoeba can aggregate and develop into fruiting bodies to produce spores that continue further generations, while many cooperating cells in the stalk die without sporulations. When mixed with wildtype populations, knockout mutants cannot easily adhere and thus are excluded from aggregation for potential sporulations, thereby resulting in in-group favoritism by the wildtype strain (see [64] for a more detailed discussion of a variety of discrimination behaviors in microbes). The FLO1 adhesion of yeast cells allows them to form clumps and flocculate to protect themselves against harsh environments, such as acidity [68]. Because one-way binding between FLO1 cells and knockout mutants is weaker than two-way binding between FLO1 cells, the FLO1 adhesion protein functions like a “green beard” that, albeit indirectly, identifies and favors others bearing the same gene. Expressing FLO1 genes and producing adhesive protein both incur fitness costs, leading to a slower growth. Therefore, cooperation is essential in this flocculation process and is potentially prone to defection. Mechanisms such as those discussed below are needed to support in-group cooperation.
Models of in-group favoritism
In fact, many models showing in-group favoritism provide dependent mechanisms of in-group favoritism. In these models, in-group favoritism emerges or is facilitated because the model assumes another known mechanism that enables assortative interaction of in-group cooperators and hence cooperation at a population level. Assortative interaction here refers to that of individuals of the same kind (i.e. group), more broadly than the same genetic kin. A few other models provide independent mechanisms, which do not require a different cooperation-enhancing mechanism.
Ingredients of models of in-group favoritism
Most models of in-group favoritism have at least the following components.
Groups
A population of players is divided into a finite or infinite number of groups (Figure 1). A two-group model is a minimal population structure with which to discuss in-group favoritism. In accordance with empirical evidence of homophily and community structure of contact networks, interaction between players is often assumed to be more frequent within a group than across different groups. Although such assortative interaction often promotes in-group favoritism, it does not imply in-group favoritism.
Donation game
In studying in-group favoritism, it is convenient to use the donation game (also known as the gift-giving game), which is a form of the prisoner's dilemma game. In each round of the game, two players are selected from the population either from the same group or different groups. One player is designated as the donor, and the other player as the recipient. The donor refers to its strategy to decide whether to cooperate with (i.e. help) the recipient. If the donor cooperates, the donor loses cost c (>0), and the recipient gains benefit b (>c). If the donor does not cooperate (i.e. does not help, or defects), nobody's payoff changes. We repeat this procedure to determine the total payoff that each player gains. Strategies yielding large payoffs are stable against invasion by other strategies or more likely to spread in evolutionary dynamics.
Strategies
To study in-group favoritism, donors should be able to cooperate or not depending on whether the donor and recipient belong to the same group. Four representative strategies are shown in Table 1. In-group cooperators (also called tag users, discriminators, parochialists, and ethnocentrism) exclusively cooperate with in-group recipients. The donor shown in Figure 1 is a noisy in-group cooperator. Unconditional cooperators and unconditional defectors are assumed to always cooperate and defect, respectively. Because the homogeneous population of unconditional defectors is usually stable, the competition between in-group cooperators and unconditional defectors is a minimal situation to be analyzed. Lastly, out-group cooperators (also called anti-discriminators and traitors) exclusively cooperate with out-group recipients and implement out-group favoritism. However, in general, out-group cooperators are quickly wiped out by other strategies.
Table 1. Four discrete, binary strategies relevant to in-group favoritism.
| Strategy | Behavior toward in-group members | Behavior toward out-group members |
|---|---|---|
| Unconditional cooperator | Cooperate | Cooperate |
| In-group cooperator | Cooperate | Defect |
| Out-group cooperator | Defect | Cooperate |
| Unconditional defector | Defect | Defect |
We can generalize these strategies to stochastic ones by introducing the probability of cooperation with in-group members and the probability of cooperation with out-group members [38].
In fact, in-group favoritism observed in fields and laboratory experiments usually accompanies some cooperation with out-group recipients. As explained in the Introduction, in-group favoritism is operationally defined as a higher probability of cooperation with in-group rather than out-group recipients. This stochastic situation can be modeled with two additional parameters: probability to cooperate with in-group and out-group recipients, which are denoted by p and q, respectively [38]. In-group favoritism, unconditional strategies, out-group favoritism corresponds to p>q, p = q, and p<q, respectively. This parameterization is akin to that used in reactive strategy in repeated games [2,69–72]; a reactive strategy is defined by a pair of the probability of cooperation immediately after receiving cooperation in the previous round and the probability of cooperation after receiving defection.
Tolerance
In the context of tag-based reciprocity, the tags (i.e., groups) are often aligned on a metric space, such as the one-dimensional lattice, and the distance in the space represents dissimilarity between two tags. In this situation, a strategy is specified by a threshold distance, often called the tolerance [25]. A donor cooperates with a recipient if (and only if) the distance between the two players is at most the tolerance value. A negative tolerance value (which is in fact excluded in [25]) corresponds to the unconditional defector. An infinitesimally large tolerance value corresponds to the unconditional cooperator. Intermediate tolerance values correspond to in-group cooperators.
Direct reciprocity
As succinctly discussed in [44], direct reciprocity (i.e. mutual cooperation via repeated interaction between the same pair of players) [73–75] in combination with the assumption of a higher frequency of interaction with in-group rather than out-group members can lead to in-group favoritism. The latter assumption is empirically supported (see Community structure and homophily section).
Innate tendency to cooperate with the same “feather”
Riolo and colleagues were among the first to investigate the evolution of in-group favoritism using computational models [25]. Within the framework of tag-based cooperation, they assumed continuously distributed tags and tolerances. The tag and tolerance of each player experience random drifts throughout evolutionary dynamics. They numerically showed that tag-based cooperation evolved.
As pointed out in [76], in-group favoritism in this model relies on two key assumptions. First, players always cooperate with those with the same tag. In other words, the unconditional defector is disallowed. This criticism bears a resemblance to the situation of the green beards; in-group favoritism erodes if we allow unconditional defectors, who bear green beards but are selfish. Second, mutation (i.e. random drift) of the tolerance is positively biased. By definition, the tolerance cannot be less than zero, and the zero value works as a reflective boundary in evolutionary dynamics. The tolerance tends to increase near this boundary. If unconditional defectors represented by negative tolerance values are allowed, cooperation does not evolve [76]. The same results were confirmed by an analysis of a minimal model with two tags [27]. In fact, we need to explain why in-group cooperators emerge in the presence of unconditional defectors.
Spatial extensions of the minimal model, without unconditional defectors and with a positive tolerance bias, were also examined [77]. In that study, spatial patterns, such as the segregation of different tag-strategy pairs and spiral waves, were observed.
Group selection
As reviewed above, two forms of group selection facilitate cooperation. The first one, group competition, has been used to account for in-group favoritism particularly in the context of evolutionary anthropology. If a group represents a tribe or nation, hostility toward out-group members corresponds to warfare. In contrast to minimalist modeling approaches to in-group favoritism, which govern most of the other sections, the models explained in this section have a relatively large number of parameters and aim to capture realistic social situations, such as war between tribes.
In the model by Choi and Bowles, intergroup interaction in the sense of group competition (i.e. war) occurs if the fraction of fighters, corresponding to in-group cooperators, is sufficiently large in at least one of the two groups [23]. If war occurs, the group with the larger number of fighters wins. Numerical simulations of the model result in evolution of hostility toward out-group members and altruism towards in-group members (i.e. intragroup coalition), which is a form of in-group favoritism. In [24], the authors analytically derived the conditions for in-group favoritism in a related model of warfare by assuming diploid populations with two sexes and weak selection. An important departure of these models from traditional models of group competition is that the frequency of group competition evolves in these models. On the other hand, players belligerent towards out-groups and helpfully providing workforce to their own groups in these models do not represent in-group favoritism in an ordinary sense because the models do not compare the amount of cooperation towards in-group versus out-group members.
In [78], the authors explored the possibility of in-group favoritism with a model extending that in [79]. In these models, groups can grow and split. Players with large fitness values tend to produce their offspring to grow their groups. A group splits into two when the group size has exceeded a predetermined threshold. Both group competition and intergroup interaction between individual players are in operation. Numerical simulations examining fixation probability of each strategy suggest that in-group favoritism evolves under certain conditions, in particular when group competition is strong [78].
Spatial structure
Cooperation can evolve when players only interact with neighbors in a spatially embedded lattice, or so-called spatial reciprocity [1,2,80–82], also referred to as (cooperation via) viscosity of population. This spatial reciprocity is a stand-alone mechanism for cooperation. In fact, the possibility of in-group favoritism has been numerically examined with some spatial models. In the following models, players do not move in the space, and tags as well as strategies evolve.
In-group favoritism occurs in two-tag [83] or four-tag [84] models embedded in a two-dimensional lattice with empty nodes. However, the behavior of these models was not compared with those with tags and without spatial structure of the population or those with spatial structure and without tags. Therefore, the amount of synergistic effects of spatial reciprocity and tags on in-group favoritism remained unknown.
Later, four-tag models made such comparisons [85,86]. The authors compared the behavior of their model with that of the null model in which the spatiality, but no tag, exists. Cooperation was enhanced by the presence of tags as compared to its absence in the same two-dimensional lattice. In addition, tags do not facilitate cooperation when the population lacks spatial structure. Also on various networks, cooperation is more frequent when tags are present rather than absent [87].
When the evolution of a tag is loosely coupled with that of strategy, in-group favoritism can evolve on the square lattice and random graph with empty sites [88]. The loose coupling means that the tag and strategy (e.g. degree of cooperation) are often, but not always, inherited together. If the linkage between the tag and strategy is too strong, as implied by the green-beard effect, the evolutionary dynamics becomes unstable and does not sustain cooperation; defectors with a single tag dominate the population most of the time [88]. In fact, the mechanism of cooperation in these spatial models can be understood as an evolutionary hide-and-seek, which does not require spatial structure (Figure 2). We will get back to this point later.
Figure 2. Schematic explanation of the independent mechanism of tag-based cooperation with mutation of tags.
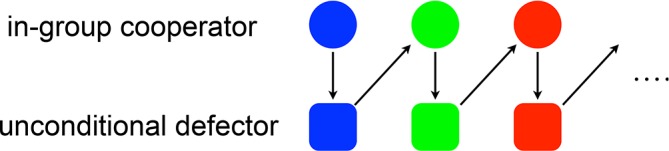
A circle and square represent the situation in which the in-group cooperator and unconditional defector are dominant in the population, respectively. Each color represents a tag.
The viability of strategic alteration of the tag has also been studied [89]. In that model, players only interact with the neighbors in the spatial lattice possessing the same tag, and are allowed to change their tags depending on the result of the game [89]. Then, cooperation increases as the number of possible tags increases. In different spatial models with binary tags, out-group favoritism evolves when b/c (i.e., benefit-to-cost ratio) is large, whereas the in-group favoritism evolves when b/c is intermediate [90]. However, this effect does not generalize to the case of more than two tags. In this model also, the effect of tags is unclear because the comparison with a spatial model without tags has not been made. Last, Traulsen and Claussen [77] investigated spatial versions of a minimalist model with two tags of the continuous-tag model [25]. As in [25], they did not assume unconditional defectors and focused on spatial pattern formation emerging via competition between in-group cooperators and unconditional cooperators.
Indirect reciprocity
In indirect reciprocity, cooperation of a donor is reimbursed by somebody different from the recipient [91]. In particular, reputation-based indirect reciprocity (also known as downstream reciprocity) is theoretically known to enable cooperation under appropriate rules according to which donor receives a reputation after an action (e.g. good or bad), which is called the social norm [92–94].
Masuda and Ohtsuki showed in-group favoritism using a two-tag model combined with a type of indirect reciprocity [95]. In the model, players are assumed not to change the tag. It is also assumed that the donor can perceive the tolerance parameter of the recipient (i.e. strategy) with a fixed probability before the interaction. If the recipient's tolerance is revealed to be small, the donor decides not to cooperate with the recipient even if the donor is an in-group cooperator. Concretely, the donor does not cooperate if the recipient's tolerance is smaller than the difference between the donor's tag and the recipient's tag. This is intuitively because the recipient would not reciprocate to the donor if such an opportunity were given to the recipient. The information about the tolerance serves as a reputation of the recipient.
The relationship between social norms and in-group favoritism was explored in a different study [96]. The model in [96] assumes a homogeneous population, and one of the three social norms can be used to assess the donor, depending on the type of interaction. In other words, the players possess a triple standard to assess the donor's actions: one used when the donor and recipient are both in-group members in the eyes of the observer (who assigns a reputation to the donor); another used when the donor is an in-group member and the recipient is an out-group member; and the other when both donor and recipient are out-group members. In-group favoritism can be stable when the following conditions are met. First, the social norm used in the first situation is one enabling cooperation in a normal homogeneous population without group structure, such as simple standing (i.e. it is regarded as good to cooperate with good recipients and do whatever towards bad recipients) and stern judging (i.e. it is regarded as good to cooperate with good recipients and defect against bad recipients) [91–94]. Second, the social norms used in the second and third situations are not the ones enabling cooperation in a homogeneous population. If the social norms used in the latter two situations are also ones enabling cooperation in a homogeneous population (e.g. simple standing), full cooperation and not in-group favoritism is stable.
The triplet social norm may be too complex for humans to implement. In a subsequent study, Nakamura and Masuda confined themselves to a small set of social norms and looked at the effect of the error in reputation assignment [97]. When the reputations of players are shared within but not across groups, in-group favoritism is stable under the simple standing or stern judging social norm. In contrast to the aforementioned study [96], in-group favoritism can occur with a single standard (i.e. same social norm used for assessing donors in any type of interaction). In this model, even the group identity of players is unused when a donor selects the action and a new reputation is assigned to a donor that has acted.
Independent mechanisms based on phenotypic matching
All mechanisms explained so far realize in-group favoritism in combination with other mechanisms that sustain cooperation on their own. In this section, we review independent mechanisms for in-group favoritism. Essentially, all models reviewed below are based on phenotypic similarity: interactions/strategies require phenotypic matching (i.e. games in phenotypic space). They are independent in the sense that no extra mechanisms, such as physical space, more fine-grained population structure, and reputation are needed for the evolution of in-group favoritism.
The original thought experiment of green beards conceived a jumbo gene that codes both phenotype expression and conditional behavioral strategy (or assumes pleiotropic effects between phenotype and strategy). Biologically, mutations would happen during reproduction to generate genetic variants. Also socially, or culturally, agents often explore different behaviors [98,99]. Here, we call both cases mutations. Central to a mutation process is a structure that controls how new variants arise. We refer to this mechanism as a mutation structure. Previous studies have adopted specific mutation structures, for example, to ensure the speed of convergence in agent-based simulations for the evolution of reciprocity [100].
We have seen that loosely connected tags and a strategy can sustain in-group favoritism in structured populations [88]. In this model, it is not only mutations but also recombinations that generate such a loosely connected green-beard gene. As opposed to natural selection, recombination strongly boosts tag diversity in a population. To study independent mechanisms for the evolution of in-group favoritism, using the long-run mutation-selection equilibrium without assuming recombination is a natural choice. Moreover, any bias in the frequency of interactions owing to population structure must be removed in order to ensure that no extra mechanism confounds the results. Therefore, one has to study a well-mixed population in which everyone is equally likely to interact with everyone else, albeit with a conditional behavioral strategy that may depend on the phenotypic similarity between players.
Motivated by the seminal numerical work on tag-based cooperation [25], Traulsen and Nowak analytically approached the problem of tag-based cooperation [101] (also see [102]). They considered a set of K arbitrary tags and assumed in-group cooperators and unconditional defectors. Because each player is characterized by the combination of a tag and a strategy, there are 2K phenotypes. Evolutionary updating is based on the so-called pairwise comparison rule. Among others, the following mutation structure is analyzed: each mutation event can lead to random phenotypic switching from one to another out of the total 2K types. A consequence is that tags switch with a much higher chance than strategies do if K is much larger than 2. In this way, cooperation is preferentially channelled toward other cooperators of the same tag, and it is hard for defectors to acquire the right tag to exploit.
Derivation of analytical results requires that the mutation rate is vanishingly small, such that the waiting time to another mutation is much longer than the fixation time of a currently existing mutant. In this limit, stochastic dynamics of invasion and fixation are approximated by a Markov chain on homogeneous population states, such that the transition rate between two states is proportional to the mutation rate multiplied by the fixation probability. Under these assumptions, a large number of tags, K, enables in-group cooperators to outcompete with unconditional defectors. When K → ∞, in-group cooperation is always predominant. In contrast, an increase in the mutation rate harms in-group favoritism.
This model leads to cyclic dominance of in-group cooperation and (unconditional) defection, particularly with changes in one tag at a time in between, thereby termed chromodynamics of cooperation if tags are envisaged with colors (Figure 2). In-group cooperators and defectors play the game of hide and seek as follows. First, if everybody owns the same tag, defectors invade in-group cooperators. Second, in-group cooperators that happen to mutate to a different tag gain a larger fitness than staying in the old tag. This is because the new tag is not yet contaminated by defectors. Third, in-group cooperators with the new tag proliferate. Fourth, defectors whose tag mutates to the in-group cooperator's one would proliferate. Fifth, in-group cooperators flee to a new tag, which may be a previously used and currently unpopular tag. These steps continue such that in-group cooperators are dynamically maintained; the fractions of different tags and strategies vary over time. Mutation is an indispensable component here. It is also suggested that similar mechanisms operate in spatial models [85,88]. Interestingly, when interaction is strongly assortative with respect to the strategy, overall cooperation decreases, as compared to the case of unconditional cooperation at the population level. This is because in-group cooperators dynamically dominate unconditional cooperators [103].
Other studies put different mutation structures of phenotype into scrutiny, requiring substantial mathematical dexterity. Antal and colleagues considered evolutionary competition dynamics between in-group cooperators and unconditional defectors in a phenotype space, assuming independent, separate mutations of phenotype and strategy [26]. The phenotype space is the one-dimensional, infinite line, and an integer defines a phenotype. The phenotype can mutate into one of the two nearest neighbor phenotypes; a mutation can be seen as a local migration between groups. When the strategy mutates, it randomly chooses one of two strategies. Evolutionary updating obeys the Wright-Fisher process. Interaction between players occurs in a well-mixed manner. In the limit of weak selection, the evolutionary dynamics can be analytically solved using coalescent theory to calculate the correlations of strategies and phenotypes among two or three randomly chosen individuals under neutrality. The critical b/c ratio above which in-group cooperators are more abundant than defectors in the mutation-selection equilibrium decreases with the phenotype mutation rate and increases with the strategy mutation rate. In the limit of fast phenotype mutation and rare strategy mutation, the critical b/c value approaches 1+ (2/√3) ≈ 2.15. The necessity of fast phenotypic mutation as compared to strategy mutation was also pointed out in previous numerical work [104,105].
The same mathematical machinery [26] can be extended to the case of multidimensional phenotype spaces in which each individual is distributed over multiple sets, forming so-called set-structured populations [106]. Further generalizing [26], this work considers conditional interactions as well as conditional strategies. Social interactions are assumed to occur according to phenotypic matching: sharing more set memberships yields more interactions. Conditional cooperation can be triggered only if two players share a minimum number of similar phenotypes, L, larger than or equal to one. This model leads to weighted, dynamic graphs of interactions, as individuals imitate, as well as mutate to, any new set memberships. The study shows that preferential help behavior, when mediated by the degree of phenotypic similarity, can prosper as cooperators possess similar sets. Departing from [26], Tarnita and colleagues assumed global mutation of phenotypes in the sense that individuals could mutate to any other set membership [106].
In stark contrast to the results obtained from the aforementioned modeling studies, in-group favoritism is empirically differential rather than all-or-nothing. In other words, individuals also help out-group members, albeit to a lesser extent. In light of this, Fu and colleagues considered a continuum of strategies that modulate behavior toward in-group and out-group recipients [38]. The authors derived the conditions under which preferential in-group cooperation and also preferential out-group cooperation emerge (K>−1 in [38]). They also identified the condition under which maximum in-group favoritism is most favored under weak selection (K>0 [38]). Moreover, computer simulations show that in-group cooperation erodes as the selection intensity increases beyond weak selection (see Figure 3a in [38]).
In all works reviewed in this section, natural selection can favor the evolution of cooperation based solely on in-group favoritism, particularly in some limiting cases (such as rare mutations and weak selection). A necessary ingredient of these models is the flexibility in group identities with which individuals are allowed to freely move across groups, through imitation of successful groups and experimentation of new groups. This “voting with one's feet” phenomenon is frequently observed in human groups, from hunter-gatherers [107,108] to modern societies.
Conclusions
In-group favoritism seems to need evolutionary underpinning [7,12,44]. However, we have seen that most models of in-group favoritism rely on other stand-alone mechanisms of cooperation. Other models of in-group favoritism (without such a stand-alone mechanism), which provide independent mechanisms of in-group favoritism, require that players can switch the group membership (i.e. tag) much easier than the strategy. Such a fast mutation of group membership may be relevant to non-human populations. However, humans show in-group favoritism even when the group membership is fixed (e.g. sex and ethnicity) or defined by the experimenters, as in minimal group experiments. Therefore, the relevance of independent mechanisms to human society may be limited.
In this review, we have focused on the good nature of in-group favoritism, which is characterized by preferential costly helping behavior to in-group members. In reality, however, in-group favoritism can manifest itself for ill, characterized by out-group hatred in which costly harming (e.g. race discrimination [5], war [23], and bacteriocins [64]) is targeted towards out-group members. Empirical and theoretical research is needed for understanding when and how nature selects positive interactions in and between groups, as opposed to negative interactions, in the real world. Research of this kind may help to reduce unnecessary and unwanted antagonism, due to intolerance toward out-groups [109].
Recent neuroimaging studies suggest that alternative approaches to understanding in-group favoritism may be profitable. Helping in-group members was accompanied by activation in the left anterior insula [110]. The anterior insula is associated with affective empathy [111–116]. The involvement of the anterior insula [110] suggests that in-group favoritism may be evoked by empathy rather than material payoff maximization. This idea is consistent with the fact that most theoretical models, all of which implicitly assume material payoff maximization, cannot explain in-group favoritism on their own. It was also previously shown that not helping out-group members (as compared to in-group members) activated the nucleus accumbens in the brain [110]. Prior studies using different tasks suggest that the nucleus accumbens is activated when participants derive pleasure from others' misfortune [116,117]. Other studies found activation in different brain regions during in-group favoritism behavior [118,119]. Interpretation of these results would seem to need further examination.
In-group favoritism may have evolved in conjunction with affective empathy in general or with other empathy-related behaviors, which have nothing to do with cooperation. It may be useful to take this into account in modeling frameworks. Alternatively, it may be fruitful to assume in-group favoritism without asking about evolutionary origins and examining its influence on collective behavior in populations of agents. It should be noted that upstream reciprocity (i.e. a type of indirect reciprocity, also called pay-it-forward reciprocity), which also lacks evolutionary explanation on its own [44], involves the anterior insula [120]. Therefore, theoretical studies of in-group favoritism and upstream reciprocity may cross-fertilize.
When it comes to genetic evolution, passing on genetic information is crucial for individuals. Therefore, kin selection may have the most important impact. When it comes to cultural evolution (knowledge, norm, language, beliefs, etc.), in-group cooperation is vital to maintain cultural inheritance and advance complexity of culture. Nevertheless, genes and cultures can be linked and coevolve over time [121–125]. Relationships between gene-culture coevolution and in-group favoritism are still unclear, despite some theoretical studies [126].
Acknowledgments
We thank Hisashi Ohtsuki, Kohei Tamura, and Arne Traulsen for the careful reading of the manuscript. Naoki Masuda acknowledges the support provided through CREST, JST. Feng Fu is supported by an ERC advanced grant (PBDR 268540).
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/7/27
References
- 1.Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314:1560–63. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowak MA. Evolutionary Dynamics. Cambridge: Belknap Press of Harvard University Press; 2006. [Google Scholar]
- 3.Sigmund K. The Calculus of Selfishness. Princeton: Princeton University Press; 2010. [DOI] [Google Scholar]
- 4.Tajfel H, Billig MG, Bundy RP, Flament C. Social categorization and intergroup behaviour. Eur J Soc Psychol. 1971;1:149–78. doi: 10.1002/ejsp.2420010202. [DOI] [Google Scholar]
- 5.Tajfel H. Social psychology of intergroup relations. Annu Rev Psychol. 1982;33:1–39. doi: 10.1146/annurev.ps.33.020182.000245. [DOI] [Google Scholar]
- 6.Brewer MB, Kramer RM. The psychology of intergroup attitudes and behavior. Annu Rev Psychol. 1985;36:219–43. doi: 10.1146/annurev.ps.36.020185.001251. [DOI] [Google Scholar]
- 7.Brewer MB. The psychology of prejudice: ingroup love and outgroup hate? J Soc Issues. 1999;55:429–44. doi: 10.1111/0022-4537.00126. [DOI] [Google Scholar]
- 8.Yamagishi T, Jin N, Kiyonari T. Bounded generalized reciprocity - ingroup boasting and ingroup favoritism. Thye SR, Lawler EJ, Macy MW, Walker HA, editors. Bingley: Emerald Group Publishing Limited; In Advances in Group Processes. Volume 16. 1999:161–97. [Google Scholar]
- 9.Brown R. Social identity theory: Past achievements, current problems and future challenges. Eur J Soc Psychol. 2000;30:745–78. doi: 10.1002/1099-0992(200011/12)30:6<745::AID-EJSP24<3.0.CO;2-O. [DOI] [Google Scholar]
- 10.Hewstone M, Rubin M, Willis H. Intergroup bias. Annu Rev Psychol. 2002;53:575–604. doi: 10.1146/annurev.psych.53.100901.135109. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta N. Implicit ingroup favoritism, outgroup favoritism, and their behavioral manifestations. Soc Justice Res. 2004;;17:143–69. doi: 10.1023/B:SORE.0000027407.70241.15. [DOI] [Google Scholar]
- 12.Mesoudi A. How cultural evolutionary theory can inform social psychology and vice versa. Psychol Rev. 2009;116:929–52. doi: 10.1037/a0017062. [DOI] [PubMed] [Google Scholar]
- 13.Aberson CL, Healy M, Romero V. Ingroup bias and self-esteem: a meta-analysis. Pers Soc Psychol Rev. 2000;4:157–73. doi: 10.1207/S15327957PSPR0402_04. [DOI] [Google Scholar]
- 14.Bettencourt BA, Charlton K, Dorr N, Hume DL. Status differences and in-group bias: a meta-analytic examination of the effects of status stability, status legitimacy, and group permeability. Psychol Bull. 2001;127:520–42. doi: 10.1037/0033-2909.127.4.520. [DOI] [PubMed] [Google Scholar]
- 15.Robbins JM, Krueger JI. Social projection to ingroups and outgroups: a review and meta-analysis. Pers Soc Psychol Rev. 2005;9:32–47. doi: 10.1207/s15327957pspr0901_3. [DOI] [PubMed] [Google Scholar]
- 16.Riek BM, Mania EW, Gaertner SL. Intergroup threat and outgroup attitudes: a meta-analytic review. Pers Soc Psychol Rev. 2006;10:336–53. doi: 10.1207/s15327957pspr1004_4. [DOI] [PubMed] [Google Scholar]
- 17.Sedikides G, Schopler J, Insko CA, editors. Intergroup Cognition and Intergroup Behavior. New York: Psychology Press; 1998. [Google Scholar]
- 18.Brown R. Group Processes (Second Edition) Malden: Blackwell Publishing; 2000. [Google Scholar]
- 19.Dovidio JF, Glick P, Rudman LA, editors. On the Nature of Prejudice. Malden: Blackwell Publishing; 2005. [DOI] [Google Scholar]
- 20.Lehmann L, Keller L. The evolution of cooperation and altruism - a general framework and a classification of models. J Evol Biol. 2006;19:1365–76. doi: 10.1111/j.1420-9101.2006.01119.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1071802
- 21.Gardner A, West SA. Greenbeards. Evolution. 2010;64:25–38. doi: 10.1111/j.1558-5646.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 22.West SA, Gardner A. Altruism, spite, and greenbeards. Science. 2010;327:1341–44. doi: 10.1126/science.1178332. [DOI] [PubMed] [Google Scholar]
- 23.Choi JK, Bowles S. The coevolution of parochial altruism and war. Science. 2007;318:636–40. doi: 10.1126/science.1144237. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann L, Feldman MW. War and the evolution of belligerence and bravery. Proc R Soc B. 2008;275:2877–85. doi: 10.1098/rspb.2008.0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riolo RL, Cohen MD, Axelrod R. Evolution of cooperation without reciprocity. Nature. 2001;414:441–3. doi: 10.1038/35106555. [DOI] [PubMed] [Google Scholar]
- 26.Antal T, Ohtsuki H, Wakeley J, Taylor PD, Nowak MA. Evolution of cooperation by phenotypic similarity. Proc Natl Acad Sci USA. 2009;106:8597–600. doi: 10.1073/pnas.0902528106. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718408306
- 27.Traulsen A, Schuster HG. Minimal model for tag-based cooperation. Phys Rev E. 2003;68:046129. doi: 10.1103/PhysRevE.68.046129. [DOI] [PubMed] [Google Scholar]
- 28.Dawkins R. The Selfish Gene. Oxford: Oxford University Press; 1976. [Google Scholar]
- 29.Dawkins R. The Extended Phenotype. Oxford: Oxford University Press; 1982. [Google Scholar]
- 30.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 31.Fehr E, Fischbacher U. Altruists with green beards. Analyse & Kritik. 2005;27:73–84. [Google Scholar]
- 32.Frank RH. Altruists with green beards: still kicking? Analyse & Kritik. 2005;27:85–96. [Google Scholar]
- 33.Fortunato S. Community detection in graphs. Phys Rep. 2010;486:75–174. doi: 10.1016/j.physrep.2009.11.002. [DOI] [Google Scholar]
- 34.McPherson M, Smith-Lovin L, Cook JM. Birds of a feather: homophily in social networks. Annu Rev Sociol. 2001;27:415–44. doi: 10.1146/annurev.soc.27.1.415. [DOI] [Google Scholar]
- 35.Christakis NA, Fowler JH. Friendship and natural selection. Proc Natl Acad Sci USA. 2014;111:10796–801. doi: 10.1073/pnas.1400825111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schelling TC. Dynamic models of segregation. J Math Sociol. 1971;1:143–86. doi: 10.1080/0022250X.1971.9989794. [DOI] [Google Scholar]
- 37.Fu F, Nowak MA, Christakis NA, Fowler JH. The evolution of homophily. Sci Rep. 2012;2:845. doi: 10.1080/0022250X.1971.9989794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu F, Tarnita CE, Christakis NA, Wang L, Rand DG, Nowak MA. Evolution of in-group favoritism. Sci Rep. 2012;2:460. doi: 10.1038/srep00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fehl K, van der Post DJ, Semmann D. Co-evolution of behaviour and social network structure promotes human cooperation. Ecol Lett. 2011;14:546–51. doi: 10.1111/j.1461-0248.2011.01615.x. [DOI] [PubMed] [Google Scholar]
- 40.Rand DG, Arbesman S, Christakis NA. Dynamic social networks promote cooperation in experiments with humans. Proc Natl Acad Sci USA. 2011;108:19193–98. doi: 10.1073/pnas.1108243108. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/723901494
- 41.Wang J, Suri S, Watts DJ. Cooperation and assortativity with dynamic partner updating. Proc Natl Acad Sci USA. 2012;109:14363–8. doi: 10.1073/pnas.1120867109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirado H, Fu F, Fowler JH. Quality versus quantity of social ties in experimental cooperative networks. Nat Comm. 2013;4:2814. doi: 10.1038/ncomms3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eshel I, Cavalli-Sforza LL. Assortment of encounters and evolution of cooperativeness. Proc Natl Acad Sci USA. 1982;79:1331–5. doi: 10.1073/pnas.79.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rand DG, Nowak MA. Human cooperation. Trends Cog Sci. 2013;17:413–25. doi: 10.1016/j.tics.2013.06.003. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718039505
- 45.West SA, Griffin AS, Gardner A. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J Evol Biol. 2007;20:415–32. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1071807
- 46.West SA, Griffin AS, Gardner A. Social semantics: how useful has group selection been? J Evol Biol. 2008;21:374–85. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 47.Wynne-Edwards VC. Animal Dispersion in Relation to Social Behavior. Edinburgh: Oliver & Boyd; 1962. [Google Scholar]
- 48.Mayr E. The objects of selection. Proc Natl Acad Sci USA. 1997;94:2091–4. doi: 10.1073/pnas.94.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson DS. A theory of group selection. Proc Natl Acad Sci USA. 1975;72:143–6. doi: 10.1073/pnas.72.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherif M, Harvey OJ, White BJ, Hood WR, Sherif CW. Intergroup Conflict and Cooperation: the Robbers Cave Experiment. Norman: University of Oklahoma Book Exchange; 1961. [Google Scholar]
- 51.Yamagishi T, Jin N, Miller AS. In-group bias and culture of collectivism. Asian J Soc Psychol. 1998;1:315–28. doi: 10.1111/1467-839X.00020. [DOI] [Google Scholar]
- 52.Bernhard H, Fischbacher U, Fehr E. Parochial altruism in humans. Nature. 2006;442:912–5. doi: 10.1038/nature04981. [DOI] [PubMed] [Google Scholar]
- 53.Efferson C, Lalive R, Fehr E. The coevolution of cultural groups and ingroup favoritism. Science. 2008;321:1844–9. doi: 10.1126/science.1155805. [DOI] [PubMed] [Google Scholar]
- 54.Fowler JH, Kam CD. Beyond the self: social identity, altruism, and political participation. J Politics. 2007;69:813–27. doi: 10.1111/j.1468-2508.2007.00577.x. [DOI] [Google Scholar]
- 55.Rand DG, Pfeiffer T, Dreber A, Sheketoff RW, Wernerfelt NC, Benkler Y. Dynamic remodeling of in-group bias during the 2008 presidential election. Proc Natl Acad Sci USA. 2009;106:6187–91. doi: 10.1073/pnas.0811552106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krupp DB, Debruine LM, Barclay P. A cue of kinship promotes cooperation for the public good. Evol Human Behav. 2008;29:49–55. doi: 10.1016/j.evolhumbehav.2007.08.002. [DOI] [Google Scholar]
- 57.Sigmund K. Sympathy and similarity: the evolutionary dynamics of cooperation. Proc Natl Acad Sci USA. 2009;106:8405–6. doi: 10.1073/pnas.0903947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller L, Ross KG. Selfish genes: a green beard in the red fire ant. Nature. 1998;394:573–5. doi: 10.1038/29064. [DOI] [Google Scholar]
- 59.Kutsukake N, Suetsugu N, Hasegawa T. Pattern, distribution, and function of greeting behavior among black-and-white colobus. Int J Primatol. 2006;27:1271–91. doi: 10.1007/s10764-006-9072-x. [DOI] [Google Scholar]
- 60.Lusseau D, Wilson B, Hammond PS, Grellier K, Durban JW, Parsons KM, Barton TR, Thompson PM. Quantifying the influence of sociality on population structure in bottlenose dolphins. J Anim Ecol. 2006;75:14–24. doi: 10.1111/j.1365-2656.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 61.Sinervo B, Chaine A, Clobert J, Calsbeek R, Hazard L, Lancaster L, McAdam AG, Alonzo S, Corrigan G, Hochberg ME. Self-recognition, color signals, and cycles of greenbeard mutualism and altruism. Proc Natl Acad Sci USA. 2006;103:7372–7. doi: 10.1073/pnas.0510260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH. The social network structure of a wild meerkat population: 3. Position of individuals within networks. Behav Ecol Sociobiol. 2011;65:1857–71. doi: 10.1007/s00265-011-1194-2. [DOI] [Google Scholar]
- 63.de Silva S, Ranjeewa ADG, Kryazhimskiy S. The dynamics of social networks among female Asian elephants. BMC Ecol. 2011;11:17. doi: 10.1186/1472-6785-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strassmann JE, Gilbert OM, Queller DC. Kin discrimination and cooperation in microbes. Annu Rev Microbiol. 2011;65:349–67. doi: 10.1146/annurev.micro.112408.134109. [DOI] [PubMed] [Google Scholar]
- 65.Rainey PB, Rainey K. Evolution of cooperation and conflict in experimental bacterial populations. Nature. 2003;425:72–4. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1015249
- 66.Hallatschek O, Hersen P, Ramanathan S, Nelson DR. Genetic drift at expanding frontiers promotes gene segregation. Proc Natl Acad Sci USA. 2007;104:19926–30. doi: 10.1073/pnas.0710150104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Claessen D, Rozen DE, Kuipers OP, Søgaard-Andersen L, van Wezel GP. Bacterial solutions to multicellularity: a tale of biofilms, filaments and fruiting bodies. Nature Rev Micro. 2014;12:115–24. doi: 10.1038/nrmicro3178. [DOI] [PubMed] [Google Scholar]
- 68.Smukalla S, Caldara M, Pochet N, Beauvais A, Guadagnini S, Yan C, Vinces MD, Jansen A, Prevost MC, Latgé J, Fink GR, Foster KR, Verstrepen KJ. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell. 2008;135:726–37. doi: 10.1016/j.cell.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1135875
- 69.Nowak M, Sigmund K. Game-dynamical aspects of the prisoner's dilemma. Appl Math Comp. 1989;30:191–213. doi: 10.1016/0096-3003(89)90052-0. [DOI] [Google Scholar]
- 70.Nowak M, Sigmund K. The evolution of stochastic strategies in the Prisoner's Dilemma. Acta Appl Math. 1990;20:247–65. doi: 10.1007/BF00049570. [DOI] [Google Scholar]
- 71.Nowak MA, Sigmund K. Tit for tat in heterogeneous populations. Nature. 1992;355:250–53. doi: 10.1038/355250a0. [DOI] [Google Scholar]
- 72.Kraines D, Kraines V. Learning to cooperate with Pavlov. An adaptive strategy for the iterated Prisoner's Dilemma with noise. Theory Decis. 1993;35:107–50. doi: 10.1007/BF01074955. [DOI] [Google Scholar]
- 73.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. doi: 10.1086/406755. [DOI] [Google Scholar]
- 74.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211:1390–6. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 75.Axelrod R. Evolution of Cooperation. New York: Basic Books; 1984. [Google Scholar]
- 76.Roberts G, Sherratt TN. Does similarity breed cooperation? Nature. 2002;418:499–500. doi: 10.1038/418499b. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718574177
- 77.Traulsen A, Claussen JC. Similarity-based cooperation and spatial segregation. Phys Rev E. 2004;70:046128. doi: 10.1103/PhysRevE.70.046128. [DOI] [PubMed] [Google Scholar]
- 78.García J, van den Bergh JCJM. Evolution of parochial altruism by multilevel selection. Evol Human Behav. 2011;32:277–87. doi: 10.1016/j.evolhumbehav.2010.07.007. [DOI] [Google Scholar]
- 79.Traulsen A, Nowak MA. Evolution of cooperation by multilevel selection. Proc Natl Acad Sci USA. 2006;103:10952–5. doi: 10.1073/pnas.0602530103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nowak MA, May RM. Evolutionary games and spatial chaos. Nature. 1992;359:826–9. doi: 10.1038/359826a0. [DOI] [Google Scholar]
- 81.Nowak MA, May RM. The spatial dilemmas of evolution. Int J Bifu Chaos. 1993;3:35–78. doi: 10.1142/S0218127493000040. [DOI] [Google Scholar]
- 82.Nowak MA, Bonhoeffer S, May RM. More spatial games. Int J Bifu Chaos. 1994;4:33–56. doi: 10.1142/S0218127494000046. [DOI] [Google Scholar]
- 83.Hochberg ME, Sinervo B, Brown SP. Socially mediated speciation. Evolution. 2003;57:154–8. doi: 10.1111/j.0014-3820.2003.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 84.Axelrod R, Hammond RA, Grafen A. Altruism via kin-selection strategies that rely on arbitrary tags with which they coevolve. Evolution. 2004;58:1833–8. doi: 10.1111/j.0014-3820.2004.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 85.Hammond RA, Axelrod R. The evolution of ethnocentrism. J Conflict Resol. 2006;50:926–36. doi: 10.1177/0022002706293470. [DOI] [Google Scholar]
- 86.Hammond RA, Axelrod R. Evolution of contingent altruism when cooperation is expensive. Theor Popul Biol. 2006;69:333–8. doi: 10.1016/j.tpb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Kim JW. A tag-based evolutionary prisoner's dilemma game on networks with different topologies. J Artif Soc Soc Simul. 2010;13:2. [Google Scholar]
- 88.Jansen VAA, van Baalen M. Altruism through beard chromodynamics. Nature. 2006;440:663–6. doi: 10.1038/nature04387. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718567352
- 89.Wu T, Fu F, Zhang Y, Wang L. Adaptive tag switching reinforces the coevolution of contingent cooperation and tag diversity. J Theor Biol. 2013;330:45–55. doi: 10.1016/j.jtbi.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 90.Laird RA. Green-beard effect predicts the evolution of traitorousness in the two-tag Prisoner's dilemma. J Theor Biol. 2011;288:84–91. doi: 10.1016/j.jtbi.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 91.Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437:1291–8. doi: 10.1038/nature04131. [DOI] [PubMed] [Google Scholar]
- 92.Leimar O, Hammerstein P. Evolution of cooperation through indirect reciprocity. Proc R Soc Lond B. 2001;268:745–753. doi: 10.1098/rspb.2000.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brandt H, Sigmund K. The logic of reprobation: assessment and action rules for indirect reciprocation. J Theor Biol. 2004;231:475–86. doi: 10.1016/j.jtbi.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 94.Ohtsuki H, Iwasa Y. How should we define goodness? - reputation dynamics in indirect reciprocity. J Theor Biol. 2004;231:107–20. doi: 10.1016/j.jtbi.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 95.Masuda N, Ohtsuki H. Tag-based indirect reciprocity by incomplete social information. Proc R Soc B. 2007;274:689–95. doi: 10.1098/rspb.2006.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Masuda N. Ingroup favoritism and intergroup cooperation under indirect reciprocity based on group reputation. J Theor Biol. 2012;311:8–18. doi: 10.1016/j.jtbi.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Nakamura M, Masuda N. Groupwise information sharing promotes ingroup favoritism in indirect reciprocity. BMC Evol Biol. 2012;12:213. doi: 10.1186/1471-2148-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Traulsen A, Hauert C, De Silva H, Nowak MA, Sigmund K. Exploration dynamics in evolutionary games. Proc Natl Acad Sci USA. 2009;106:709–12. doi: 10.1073/pnas.0808450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Traulsen A, Semmann D, Sommerfeld RD, Krambeck HJ, Milinski M. Human strategy updating in evolutionary games. Proc Natl Acad Sci USA. 2010;107:2962–6. doi: 10.1073/pnas.0912515107. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/723912199
- 100.Nowak M, Sigmund K. A strategy of win-stay, lose-shift that outperforms tit-for-tat in the Prisoner's Dilemma game. Nature. 1993;364:56–8. doi: 10.1038/364056a0. [DOI] [PubMed] [Google Scholar]
- 101.Traulsen A, Nowak MA. Chromodynamics of cooperation in finite populations. PLOS ONE. 2007;2:e270. doi: 10.1371/journal.pone.0000270. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/724194873
- 102.Traulsen A. Mechanisms for similarity based cooperation. Eur Phys J B. 2008;63:363–71. doi: 10.1140/epjb/e2008-00031-3. [DOI] [Google Scholar]
- 103.García J, van Veelen M, Traulsen A. Evil green beards: tag recognition can also be used to withhold cooperation in structured populations. J Theor Biol. 2014;360:181–6. doi: 10.1016/j.jtbi.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 104.Hales D. Understanding tag systems by comparing tag models. Edmonds B, Troitzsch KG, Iglesias CH, editors. Hershey: IGI Global. In Social Simulation: Technologies, Advances and New Discoveries. 2008:68–80. doi: 10.4018/978-1-59904-522-1.ch006. [DOI] [Google Scholar]
- 105.Hales D, Edmonds B. Applying a socially inspired technique (tags) to improve cooperation in P2P networks. IEEE Trans Syst Man Cybern A. 2005;35:385–95. doi: 10.1109/TSMCA.2005.846399. [DOI] [Google Scholar]
- 106.Tarnita CE, Antal T, Ohtsuki H, Nowak MA. Evolutionary dynamics in set structured populations. Proc Natl Acad Sci USA. 2009;106:8601–4. doi: 10.1073/pnas.0903019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hill KR, Walker RS, Božičević M, Eder J, Headland T, Hewlett B, Hurtado AM, Marlowe F, Wiessner P, Wood B. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331:1286–89. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/10705957
- 108.Apicella CL, Marlowe FW, Fowler JH, Christakis NA. Social networks and cooperation in hunter-gatherers. Nature. 2012;481:497–501. doi: 10.1038/nature10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sigmund K, Nowak MA. Tides of tolerance. Nature. 2001;414:403–5. doi: 10.1038/35106672. [DOI] [PubMed] [Google Scholar]
- 110.Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron. 2010;68:149–60. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722584306
- 111.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 112.Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–8. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 113.Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 114.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–70. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 115.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 116.Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- 117.Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Emphatic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–69. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/8207
- 118.Rilling JK, Dagenais JE, Goldsmith DR, Glenn AL, Pagnoni G. Social cognitive neural networks during in-group and out-group interactions. NeuroImage. 2008;41:1447–61. doi: 10.1016/j.neuroimage.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 119.Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. NeuroImage. 2010;51:1468–75. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 120.Watanabe T, Takezawa M, Nakawake Y, Kunimatsu A, Yamasue H, Nakamura M, Miyashita Y, Masuda N. Two distinct neural mechanisms underlying indirect reciprocity. Proc Natl Acad Sci USA. 2014;111:3990–5. doi: 10.1073/pnas.1318570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feldman MW, Laland KN. Gene-culture coevolutionary theory. Trends Ecol Evol. 1996;11:453–7. doi: 10.1016/0169-5347(96)10052-5. [DOI] [PubMed] [Google Scholar]
- 122.Gintis H. The hitchhiker's guide to altruism: gene-culture coevolution, and the internalization of norms. J Theor Biol. 2003;220:407–18. doi: 10.1006/jtbi.2003.3104. [DOI] [PubMed] [Google Scholar]
- 123.Richerson PJ, Boyd R, Henrich J. Gene-culture coevolution in the age of genomics. Proc Natl Acad Sci USA. 2010;107:8985–92. doi: 10.1073/pnas.0914631107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boyd R, Richerson PJ. Culture and the Evolutionary Process. Chicago: The University of Chicago Press; 1985. [Google Scholar]
- 125.Durham WH. Coevolution: Genes, Culture, and Human Diversity. Stanford: Stanford University Press; 1991. [Google Scholar]
- 126.Ihara Y. Evolution of culture-dependent discriminate sociality: a gene-culture coevolutionary model. Phil Trans R Soc B. 2011;366:889–900. doi: 10.1098/rstb.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]


