Abstract
Objective:
To determine the relationship between exposure to repeated head impacts through tackle football prior to age 12, during a key period of brain development, and later-life executive function, memory, and estimated verbal IQ.
Methods:
Forty-two former National Football League (NFL) players ages 40–69 from the Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests (DETECT) study were matched by age and divided into 2 groups based on their age of first exposure (AFE) to tackle football: AFE <12 and AFE ≥12. Participants completed the Wisconsin Card Sort Test (WCST), Neuropsychological Assessment Battery List Learning test (NAB-LL), and Wide Range Achievement Test, 4th edition (WRAT-4) Reading subtest as part of a larger neuropsychological testing battery.
Results:
Former NFL players in the AFE <12 group performed significantly worse than the AFE ≥12 group on all measures of the WCST, NAB-LL, and WRAT-4 Reading tests after controlling for total number of years of football played and age at the time of evaluation, indicating executive dysfunction, memory impairment, and lower estimated verbal IQ.
Conclusions:
There is an association between participation in tackle football prior to age 12 and greater later-life cognitive impairment measured using objective neuropsychological tests. These findings suggest that incurring repeated head impacts during a critical neurodevelopmental period may increase the risk of later-life cognitive impairment. If replicated with larger samples and longitudinal designs, these findings may have implications for safety recommendations for youth sports.
It was previously thought that greater plasticity in the developing brain would support better recovery following injury.1 Recent evidence indicates that children and adolescents are more vulnerable than adults to poor outcomes and prolonged recovery from concussions.2–5 Furthermore, concussions in youth may negatively affect social development and educational success.4–6 Recent research suggests that subconcussive head impacts experienced in sports also have acute7–9 and long-term10–14 neuroanatomical and functional consequences. Youth football players ages 9–12 can incur an average of 240, and up to 585, head impacts per season at magnitudes that parallel those experienced by high school and collegiate football players,15–17 several of which exceed 80g. With millions of youth athletes participating in contact sports annually, including 4.8 million football players,15,18 the long-term consequences of brain trauma in youth sports are a growing public health concern.
The timing of key neurodevelopmental processes could contribute to windows of vulnerability to brain trauma. A critical stage of brain development occurs between ages 10 and 12.19–24 However, the long-term consequences of repeated head impacts (RHI) incurred during this important neurodevelopmental period are unknown. To investigate the relationship between age of first exposure (AFE) to RHI through tackle football and later-life cognition, we evaluated 2 groups of former National Football League (NFL) players with the hypothesis that those who began playing football before age 12 would perform significantly worse on measures of executive function, memory, and premorbid estimated verbal IQ (eVIQ) than those who started playing at age 12 or older.
METHODS
This research was part of Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests (DETECT), an ongoing study aiming to develop methods for diagnosing the neurodegenerative disease chronic traumatic encephalopathy (CTE) during life. Participants undergo numerous tests, including neuroimaging, CSF protein analysis, genetic testing, neurologic and psychiatric evaluations, neuropsychological testing, and a history interview. Only neuropsychological tests and exposure-related variables and demographic information from the history interview were used in this analysis. DETECT recruitment efforts began in November 2011 and included e-mails through distribution lists of the NFL Players Association and NFL Alumni Association, presentations at NFL alumni meetings, Boston University CTE Center Web site postings, and word of mouth.
Participants.
DETECT participants include former NFL players and a control group of former elite noncontact sport athletes. Controls were not included in this analysis. Inclusion criteria for the former NFL players are as follows: male, ages 40–69, played at least 2 years in the NFL and 12 years of organized football, and self-reported complaints of cognitive, behavioral, and mood symptoms for at least the last 6 months. Exclusion criteria included general MRI and lumbar puncture contraindications and history of any other diagnosed CNS disease.
Head impact exposure variables.
AFE to tackle football was treated as a dichotomous variable and used to divide subjects into 2 cohorts: before age 12 (AFE <12) and age 12 or older (AFE ≥12). Age 12 was chosen as the cutoff based on neurodevelopmental literature19–24 and previous work from our center.25 The duration of football play (i.e., total number of years) differed between AFE groups and was therefore used as a covariate and treated as a continuous variable.
Outcome measures.
A focused set of neuropsychological outcome measures was selected from the DETECT test battery for this preliminary study in order to reduce the likelihood of type I error. The measures were selected based on a priori hypotheses regarding primary functional areas expected to be impaired in CTE14,26 and neurodevelopmental literature.19–24 Each test is described briefly here; detailed administration and interpretation guidelines are described by Strauss et al.27
Wisconsin Card Sort Test.
The Wisconsin Card Sort Test (WCST) is a widely used measure of multiple aspects of executive function, including shifting cognitive set, response inhibition, perseveration, and strategic planning.27 A 128-card computerized form was used in this study.28 WCST scores analyzed include percent errors (%E), percent perseverative responses (%PR), percent perseverative errors (%PE), percent nonperseverative errors (%NPE), and percent conceptual level responses (%CLR).
Neuropsychological Assessment Battery List Learning test.
The Neuropsychological Assessment Battery List Learning test (NAB-LL) is a measure of verbal episodic memory that is sensitive to impairment due to both neurodegenerative disease29 and traumatic brain injury.30 To evaluate different aspects of learning and memory, 3 NAB-LL variables were used: Immediate Recall (IR), Short Delay Recall, and Long Delay Recall.
Wide Range Achievement Test, 4th edition, Reading subtest.
The Wide Range Achievement Test, 4th edition (WRAT-4)31 Reading subtest is a word pronunciation test commonly used to measure premorbid eVIQ.27,31
Statistical analysis.
Raw scores were converted to T scores for all WCST and NAB-LL measures based on each test's demographically corrected normative data. WRAT-4 Reading raw scores were converted to age-corrected standard scores. Paired-sample t tests were conducted for unadjusted between-group comparisons. A mixed-effects linear model was used to determine the adjusted effect of AFE to tackle football on all outcome measures. This model adjusted for duration of play and education as well as for correlations within the age-matched pairs, between outcomes from the same subject, and between outcomes of the same test for the WCST and NAB-LL in order to account for possible inflation of type I error. To ensure internal validity and further control for type I error at α = 0.05, bootstrap analysis was conducted on 1,000 replicates. All analyses were conducted using SAS 9.3.
Standard protocol approvals, registrations, and patient consents.
All study procedures were approved by the Boston University Medical Center Institutional Review Board. Subjects provided written informed consent prior to participation.
RESULTS
Demographic information and athletic history are described in table 1. Seventy-four former NFL players from DETECT were eligible for this analysis. Age differed significantly between groups when all subjects were divided by AFE to tackle football (AFE <12 mean = 50.4 years, SD = 6.5; AFE ≥12 mean = 57.5 years, SD = 7.7; p < 0.001). To account for this, subjects were matched a priori by age such that one subject from the AFE <12 group was paired with a subject of the same age from the AFE ≥12 group. Of the 74 eligible participants, 42 subjects (age range 41–65 years) could be randomly matched by age (within 2 years), with 21 subjects in each AFE group. The AFE to tackle football ranged from age 7 to age 17. Duration of football play differed significantly between groups.
Table 1.
Demographics

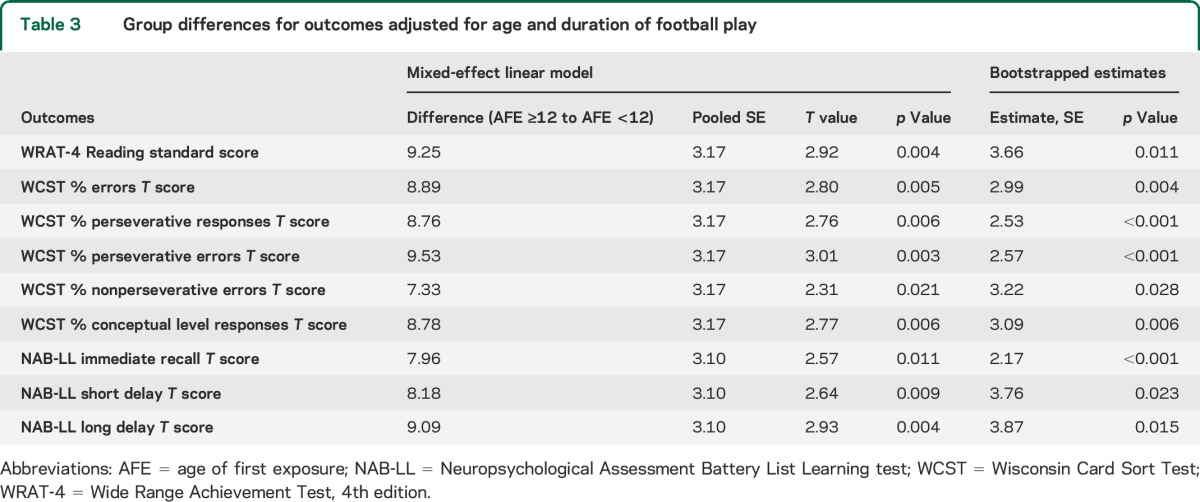
Mean outcomes scores from paired-sample t tests are shown in table 2. Due to uncompleted tests, 19 pairs of subjects were examined for the WCST; 21 pairs were examined for all other measures. Mean scores for the WCST %E, %PR, %PE, and %CLR; NAB-LL IR; and WRAT-4 Reading differed significantly between groups. The AFE <12 group had significantly lower scores than the AFE ≥12 group on all outcomes, indicating poorer performance. Results from the mixed-effects linear model and bootstrap analyses are presented in table 3. After controlling for education, duration of play, and multidimensional correlations between tests and within subjects, all measures of the WCST, NAB-LL, and WRAT-4 Reading tests differed significantly between groups, with the AFE <12 group performing significantly worse than the AFE ≥12 group. These differences remained significant following bootstrap analysis.
Table 2.
Unadjusted group differences for outcomes
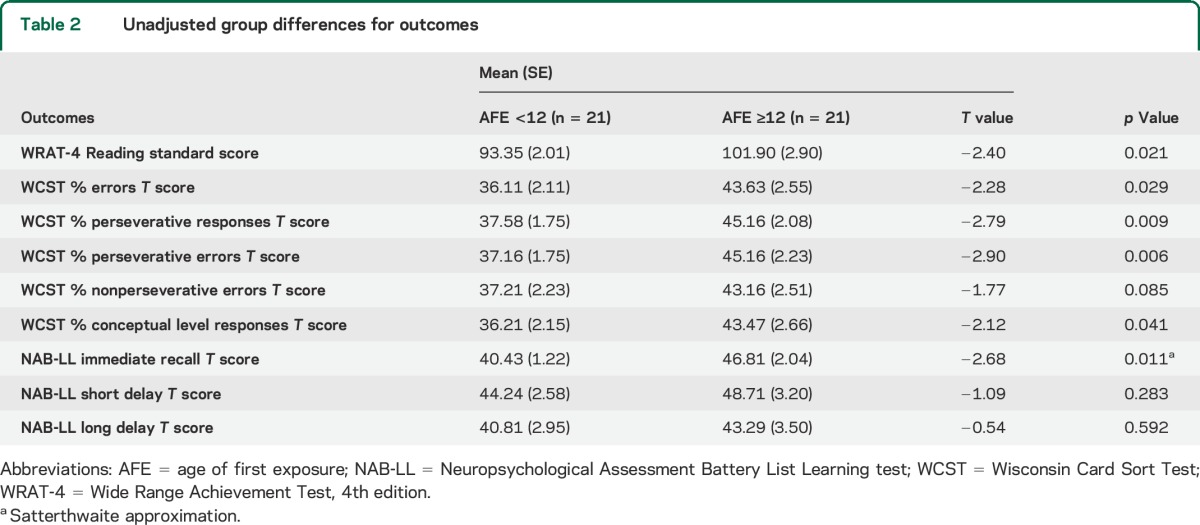
Table 3.
Group differences for outcomes adjusted for age and duration of football play
DISCUSSION
The objective of this study was to evaluate the relationship between AFE to RHI through tackle football and later-life cognitive function. We found that former NFL players in the AFE <12 group demonstrated significantly greater impairment on objective measures of executive functioning, immediate and delayed recall, and eVIQ than those in the AFE ≥12 group. These results are consistent with preliminary work by our group indicating that former football players of all levels with an AFE <12 self-reported significantly worse executive function, depression, and apathy than those with an AFE ≥12.25 The results of this study suggest that sustaining RHI during critical periods of brain maturation could alter neurodevelopmental trajectories, leading to later-life cognitive impairments.
The relationship between AFE to tackle football and later-life executive dysfunction, memory impairment, and lower eVIQ remained significant, and for some variables became significant, after adjusting for duration of football play and education. Although the difference did not reach significance, it is noteworthy that the AFE <12 group played an average of 2 fewer years in the NFL than the AFE ≥12 group. Longer duration of NFL play could have been expected to contribute to poorer cognitive performance in the AFE ≥12 group. However, despite playing fewer years in the NFL, the AFE <12 group performed worse on all measures.
The disruption of key neurodevelopmental processes by RHI may be the underlying cause of the results of this study. A period of peak myelination rates and increased cerebral blood flow, which has been shown to predict rapid neurodevelopmental periods, occurs between ages 10 and 12.5,19 In males, peak cortical thickness in the frontal and parietal cortices20 and peak amygdalar and hippocampal volume are reached during this same preadolescent period.22 At the onset of puberty, occurring around age 12 in males, volumes in these regions begin to decrease due to synaptic pruning, allowing for more efficient information processing.32 RHI incurred around the time of peak volume and early synaptic pruning in the hippocampus could alter the course of hippocampal development, contributing to the later-life memory impairments observed in this study.
The findings of this study are consistent with research demonstrating that children and adolescents are more susceptible to prolonged recovery and poor outcomes from concussions. Reduced intelligence has been reported following concussions in children.4–6 While the rate of intellectual development may return to normal following pediatric brain injury, the loss of normal development time during recovery may cause the injured child to fall behind and never return to the levels of his or her uninjured peers.5,6 Children may appear to fully recover from concussions due to functional compensatory mechanisms despite a lack of neuronal recovery.5 Prefrontal cortical regions, including the dorsolateral prefrontal cortex (DLPFC), continue to develop into the early 20s and are critical for executive functioning and intelligence.24,33 Studies have reported changes in DLPFC activation following both acute concussive injury34 and prolonged exposure to RHI.9 Functional impairments resulting from RHI occurring in childhood may not become apparent until early adulthood when environmental demands increase but cognitive skills associated with later-maturing regions fail to properly develop. These mechanisms could explain the executive dysfunction displayed on the WCST and lower WRAT-4 Reading scores in the AFE <12 group.
Brain trauma as an environmental factor may play a larger role in neurodevelopmental processes in children than in adolescents and adults. The influence of environmental vs genetic factors on brain structure and function evolves over childhood and adolescence.35,36 Lenroot et al.36 used the same age cutoff as this study to examine the heritability of cortical thickness. They found that cortical thickness of later-developing brain regions, including the DLPFC, was heritable in the older group, but environmental factors had a greater influence than genetics in these regions in the group under age 12. Cortical growth trajectories in children and adolescents have been associated with intelligence,24 and the influence of environmental factors on intelligence has also been shown to give way to genetic influence at age 12.35 RHI may influence neurodevelopment of some brain regions more in those younger than age 12 due to the strong environmental influence prior to that age.
Youth football players may incur hundreds of subconcussive head impacts each season.15,16 Recent neuroimaging research has identified altered white matter structural integrity and functional changes postseason compared to preseason baseline measures in college ice hockey8 and college and high school football players.7,9 One study found that these changes persisted through 6 months of noncontact rest.7 RHI has also been associated with long-term consequences, including CTE.14 Although executive dysfunction and memory impairment are common CTE symptoms,14,26 our results do not suggest that the participants in our study have or will develop CTE. The neurobiological consequences of RHI on neurodevelopmental trajectories may differ from the pathogenesis and progression of CTE, leading to functionally similar but neuropathologically distinct outcomes. Further research is needed to determine whether incurring RHI during periods of neurodevelopmental vulnerability may contribute to the neuropathogenetic cascade leading to the tauopathy of CTE.
The WRAT-4 Reading subtest is frequently used to measure premorbid eVIQ in studies of neurodegenerative disease.37 However, scores on this and similar word pronunciation tests have been associated with delayed memory performance and dementia scores in other neurodegenerative diseases,37,38 leading to an underestimation of premorbid intelligence. Furthermore, research suggests that declines in intelligence may persist following concussion.4–6 However, the impact of RHI on intelligence has yet to be elucidated. Several other factors can influence performance on the WRAT-4 Reading subtest, including quality of education, race, cultural experience, and socioeconomic status.39 Although race was not significantly different between groups, there were 6 more African American participants in the AFE ≥12 group, suggesting that lower WRAT-4 Reading scores in the AFE <12 group were not driven by race-related differences. However, socioeconomic status and acculturation information was unavailable for this cohort and should be examined in future research. Due to the variety of factors that could influence WRAT-4 Reading scores in this population, this measure was not included as a covariate when analyzing other outcomes. Incurring RHI through football before age 12 could negatively affect intellectual development, resulting in differences in knowledge acquisition abilities and lower later-life IQ. However, it is unclear whether the results of this study are due to exposure to RHI during critical neurodevelopmental stages, later-life acquired memory impairment from possible neurodegenerative disease, premorbid intelligence differences unrelated to RHI exposure, or other demographic factors. Moreover, it is possible that children with lower verbal abilities are drawn to playing football at an earlier age. Future longitudinal studies beginning prior to the start of football participation in youth and using more comprehensive measures of intelligence are needed in order to determine the relationship between RHI in youth and intelligence.
Although this study found an association between participation in tackle football before age 12 and later-life cognitive deficits, this does not suggest that incurring RHI at age 12 or older is safe or free from long-term consequences. Although the AFE <12 group performed significantly worse, the AFE ≥12 group still scored below average on several WCST and NAB-LL measures. There are likely other factors, such as aspects of exposure (e.g., type, frequency, and severity), genetics, or other health-related issues, that could influence the risk of later-life consequences.
There are several important limitations to address in this study. While the study of former NFL players allows for investigation of a group with high exposure to RHI, the results may not be generalizable to other groups. The nature and number of head impacts incurred in other youth sports, including hockey and soccer, may differ from those incurred in youth football and may affect neurodevelopmental processes differently. Furthermore, timing of brain development stages and milestones differs between males and females.20,22 Future studies should investigate the later-life effects of RHI from other youth contact sports and in female athletes. Total exposure to RHI cannot be definitively determined retrospectively within this cohort; however, we used years of football play as a proxy for total exposure. The use of helmet accelerometer technology will make it possible to obtain a better estimate of total exposure in future studies. In addition, fewer opportunities to participate in youth football before age 12 were available to the older participants in this study, causing the AFE ≥12 group to be significantly older than the AFE <12 group in the DETECT cohort. Simply adjusting for age would not have accounted for era-related differences in the style of football the participants played. Therefore, we used age-matched pairs in this study, which greatly reduced the sample size. The cross-sectional study design does not allow for the determination of causality between early-life exposure to RHI and later-life impairments, and the age range of participants limits the generalizability to older individuals. Future research would benefit from longitudinal designs with larger sample sizes beginning at younger ages.
It is possible that participants in this study were motivated to perform poorly on neuropsychological testing due to outside influences, such as litigation against the NFL. If this were the case, we would not expect to find a difference between groups based on AFE to football as we did in this study. It is also noteworthy that we found objective differences between AFE groups despite the fact that all participants reported behavioral and cognitive difficulties in order to be included in this study. This potential limitation may be addressed by eliminating inclusion criteria requiring self-report of symptoms in future research.
Increased awareness about the long-term effects of brain trauma has led to rule changes in sports at all levels. While these changes may be effective in minimizing RHI in youth sports,15 empirical data are necessary to guide these rule changes with regard to AFE and other modifiable risk factors. More evidence is needed to assist stakeholders, including legislators, league officials, health care providers, coaches, parents, and athletes, in decision-making when considering the potential detrimental effects of exposure to RHI relative to the countless benefits of participation in youth sports.
Supplementary Material
ACKNOWLEDGMENT
The authors gratefully acknowledge David Riley and Clifford Robbins for their assistance with data acquisition, as well as Jane Pleskunas for her assistance with data analysis. They also extend their appreciation to the study participants who make this work possible.
GLOSSARY
- %CLR
conceptual level responses
- %E
percent errors
- %PE
percent perseverative errors
- %PR
percent perseverative responses
- AFE
age of first exposure
- CTE
chronic traumatic encephalopathy
- DETECT
Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests
- DLPFC
dorsolateral prefrontal cortex
- eVIQ
estimated verbal IQ
- IR
Immediate Recall
- NAB-LL
Neuropsychological Assessment Battery List Learning test
- NFL
National Football League
- RHI
repeated head impacts
- WCST
Wisconsin Card Sort Test
- WRAT-4
Wide Range Achievement Test, 4th edition
Footnotes
Editorial, page 1068
AUTHOR CONTRIBUTIONS
Ms. Stamm is the primary author. She was responsible for drafting the manuscript and interpretation of the data and participated in data acquisition and analysis. Ms. Bourlas participated in data acquisition and drafting and revising the manuscript. Ms. Baugh participated in revising the manuscript, acquisition of data, and study design. Mr. Fritts participated in drafting the manuscript and acquisition of data. Mr. Daneshvar participated in revising the manuscript and study design. Mr. Martin participated in data management and analysis for this study. Dr. McClean participated in revising the manuscript and interpreting the data. Dr. Tripodis conducted the statistical analysis and participated in interpretation of the data. Dr. Stern is the principal and corresponding author. He was responsible for study concept and design, revising the manuscript, and analysis and interpretation of data. He also played a role in obtaining funding.
STUDY FUNDING
Supported by NIH (R01 NS 078337; F31 NS 081957; P30 AG13846; UL1-TR000157), and participant travel funded by gifts from JetBlue Airlines, the National Football League (NFL), and the NFL Players Association.
DISCLOSURE
J. Stamm is funded by NIH Grant F31 NS 081957. A. Bourlas, C. Baugh, N. Fritts, D. Daneshvar, B. Martin, M. McClean, and Y. Tripodis report no disclosures relevant to the manuscript. R. Stern is funded by NIH grants R01 NS078337, R01 CA129769, P30 AG13846, U01 AG10483, and U01 AG015477; and has received research support from Sports Legacy Institute, the Alzheimer's Association, the National Operating Committee on Standards for Athletic Equipment, Avid Radiopharmaceuticals, Eli Lilly, Eisai Pharmaceuticals, Janssen Alzheimer's Immunotherapy, Pfizer, and Medivation. He is a paid consultant to Athena Diagnostics and serves as an expert advisor to attorneys for cases pertaining to the long-term consequences of repetitive brain trauma. He receives royalties from Psychological Assessment Resources for the publication of neuropsychological tests. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Schneider GE. Is it really better to have your brain lesion early? A revision of the “Kennard principle”. Neuropsychologia 1979;17:557–583. [DOI] [PubMed] [Google Scholar]
- 2.Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res 2005;157:11–22. [DOI] [PubMed] [Google Scholar]
- 3.Zuckerman SL, Lee YM, Odom MJ, Solomon GS, Forbes JA, Sills AK. Recovery from sports-related concussion: days to return to neurocognitive baseline in adolescents versus young adults. Surg Neurol Int 2012;3:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moser RS, Schatz P, Jordan BD. Prolonged effects of concussion in high school athletes. Neurosurgery 2005;57:300–306. [DOI] [PubMed] [Google Scholar]
- 5.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 2011;134:2197–2221. [DOI] [PubMed] [Google Scholar]
- 6.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld JV. Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics 2009;124:e1064–e1071. [DOI] [PubMed] [Google Scholar]
- 7.Bazarian JJ, Zhu T, Zhong J, et al. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One 2014;9:e94734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koerte IK, Kaufmann D, Hartl E, et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus 2012;33:E3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talavage TM, Nauman EA, Breedlove EL, et al. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma 2014;31:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005;57:719–726. [DOI] [PubMed] [Google Scholar]
- 11.Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc 2007;39:903–909. [DOI] [PubMed] [Google Scholar]
- 12.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seichepine DR, Stamm JM, Daneshvar DH, et al. Profile of self-reported problems with executive functioning in college and professional football players. J Neurotrauma 2013;30:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobb BR, Urban JE, Davenport EM, et al. Head impact exposure in youth football: elementary school ages 9-12 years and the effect of practice structure. Ann Biomed Eng 2013;41:2463–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel RW, Rowson S, Duma SM. Head impact exposure in youth football. Ann Biomed Eng 2012;40:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnebel B, Gwin JT, Anderson S, Gatlin R. In vivo study of head impacts in football: a comparison of National Collegiate Athletic Association Division I versus high school impacts. Neurosurgery 2007;60:490–495. [DOI] [PubMed] [Google Scholar]
- 18.National Council of Youth Sports. Report on trends and participation in organized youth sports, 2008 edition. Available at: www.ncys.org/pdfs/2008/2008-ncys-market-research-report.pdf. Accessed January 17, 2015. [Google Scholar]
- 19.Epstein HT. Stages of increased cerebral blood flow accompany stages of rapid brain growth. Brain Dev 1999;21:535–539. [DOI] [PubMed] [Google Scholar]
- 20.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–863. [DOI] [PubMed] [Google Scholar]
- 21.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008;40:1044–1055. [DOI] [PubMed] [Google Scholar]
- 22.Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One 2012;7:e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol 1987;22:487–497. [DOI] [PubMed] [Google Scholar]
- 24.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature 2006;440:676–679. [DOI] [PubMed] [Google Scholar]
- 25.Bourlas A, Stamm J, Baugh C, et al. Relationship between age of first exposure to tackle football and later-life mood, behavior, and cognition. Brain Inj 2014;28:755–756. Abstract. [Google Scholar]
- 26.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical Subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther 2014;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strauss E, Sherman EMS, Spreen O, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed New York: Oxford University Press; 2006. [Google Scholar]
- 28.Wisconsin Card Sorting Test Computer Version 4-Research Edition (WCST: CV4) [computer program]. Version 4. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 29.Gavett BE, Poon SJ, Ozonoff A, et al. Diagnostic utility of the NAB List Learning test in Alzheimer's disease and amnestic mild cognitive impairment. J Int Neuropsychol Soc 2009;15:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zgaljardic DJ, Temple RO. Neuropsychological Assessment Battery (NAB): performance in a sample of patients with moderate-to-severe traumatic brain injury. Appl Neuropsychol 2010;17:283–288. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson GS, Robertson G. WRAT-4: Wide Range Achievement Test Professional Manual, 4th ed Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 32.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 2006;47:296–312. [DOI] [PubMed] [Google Scholar]
- 33.Kaller CP, Heinze K, Mader I, et al. Linking planning performance and gray matter density in mid-dorsolateral prefrontal cortex: moderating effects of age and sex. Neuroimage 2012;63:1454–1463. [DOI] [PubMed] [Google Scholar]
- 34.Slobounov SM, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp Brain Res 2010;202:341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brouwer RM, van Soelen IL, Swagerman SC, et al. Genetic associations between intelligence and cortical thickness emerge at the start of puberty. Hum Brain Mapp 2014;35:3760–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenroot RK, Schmitt JE, Ordaz SJ, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp 2009;30:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Rourke JJ, Adams WH, Duff K, et al. Estimating premorbid functioning in Huntington's disease: the relationship between disease progression and the wide range achievement test reading subtest. Arch Clin Neuropsychol 2011;26:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarlane J, Welch J, Rodgers J. Severity of Alzheimer's disease and effect on premorbid measures of intelligence. Br J Clin Psychol 2006;45:453–463. [DOI] [PubMed] [Google Scholar]
- 39.Manly JJ, Byrd DA, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American elders. Appl Neuropsychol 2004;11:37–46. [DOI] [PubMed] [Google Scholar]
- 40.Robbins CA, Daneshvar DH, Picano JD, et al. Self-reported concussion history: impact of providing a definition of concussion. Open Access J Sports Med 2014;5:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


