Abstract
Objective:
To examine the association between head injuries throughout life and the risk for Parkinson disease (PD) in an interview-based case-control study.
Methods:
We identified 1,705 patients diagnosed with PD at 10 neurologic centers in Denmark in 1996–2009 and verified their diagnoses in medical records. Patients were matched to 1,785 controls randomly selected from the Danish Central Population Register on sex and year of birth. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using unconditional logistic regression.
Results:
We observed no association between any head injury before first cardinal symptom and PD (OR 1.02; 95% CI 0.88, 1.19). Examination of number of head injuries (1: OR 1.02; 95% CI 0.87, 1.20; ≥2: OR 1.03; 95% CI 0.72, 1.47) or hospitalization for a head injury (OR 0.89; 95% CI 0.70, 1.12) did not show an association with PD. For 954 study subjects with at least one head injury, there was no evidence of an association between loss of consciousness (OR 0.89; 95% CI 0.67, 1.17), duration of loss of consciousness (≤1 minute: OR 0.93; 95% CI 0.58, 1.49; 1–5 minutes: OR 0.74; 95% CI 0.51, 1.08; ≥5 minutes: OR 0.81; 95% CI 0.53, 1.24), or amnesia (OR 1.31; 95% CI 0.88, 1.95) and risk for PD. Application of a lag time of 10 years between head injury and first cardinal symptom resulted in similar risk estimates.
Conclusions:
The results do not support the hypothesis that head injury increases the risk for PD.
Parkinson disease (PD) is a common movement disorder characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta coupled with abnormal aggregates of proteins known as Lewy bodies.1 Despite intensive research into etiologic factors, little is known about the factors that cause the neurodegeneration. The hypothesis that head injury increases the risk for PD has been examined in numerous studies during past decades2–10; however, the findings have been highly inconsistent, with reported relative risks ranging from 0.6 to 11.7.
In a previous register-based case-control study, we examined the association between hospital contact for head injuries in middle or late adulthood and a diagnosis of PD. The reported positive association was due almost entirely to injuries that occurred during the months preceding the first hospital contact for PD.11 Because of the register-based design of the study, detailed diagnostic information was lacking to distinguish PD from other types of parkinsonism. Furthermore, although previous studies have found positive associations between both milder and repeated head injuries and PD,12,13 these 2 aspects could not be investigated in our large register-based study. Thus, the aim of the present study was to examine the association between head injuries throughout life and the risk for PD in a large interview-based case-control study among 1,705 patients with PD.
METHODS
Patients.
In the Danish National Hospital Register, we identified 3,508 patients aged 35 years or over who were registered with a hospital contact for a primary diagnosis of PD (ICD-8 code 342 and ICD-10 code G20) in 1996–2009 at one of 10 major neurologic treatment centers in Denmark (figure). All the patients identified before January 1, 2007, were also included in the large register-based study by Rugbjerg et al.11 Those patients who were alive and available for contact, spoke Danish and English, and were well enough to participate in an interview between January 2008 and December 2010 were eligible for the study (n = 2,762). We excluded 179 patients for whom review of their medical record prior to contact did not confirm PD. Of the remaining 2,583 patients we contacted, 2,086 (81%) agreed to be interviewed, and we obtained medical records for 2,066 (99%) of the interviewed patients. The medical records were reviewed rigorously by trained reviewers supervised by a specialist in movement disorders in order to distinguish cases of PD from other forms of parkinsonism that feature the main characteristics of PD. In this review, we applied the standard diagnostic criteria of the UK Brain Bank14 and the Gelb criteria.15 In general, we considered that patients had PD if at least 2 of 4 cardinal symptoms (resting tremor, bradykinesia, rigidity, asymmetrical onset) were present; the patient responded to antiparkinsonian medication; the patient had no atypical features (including a diagnosis of dementia before cardinal symptoms, early falls, severe symptomatic dysautonomia, very rapid progression of the disease, sudden onset of symptoms, supranuclear gaze palsy, hallucinations unrelated to medication, freezing phenomena, and Babinski sign); and there was no sign of a differential diagnosis, e.g., cerebrovascular disease. After review of the medical records, 1,828 (89%) of the 2,066 patients interviewed were considered to have PD.
Figure. Flow chart of patient recruitment.

PD = Parkinson disease.
Population controls.
For each of the 2,583 patients initially contacted for interview, 5 potential controls matched to cases on sex and year of birth were density sampled from the Danish Central Population Register. All eligible controls had to fulfil the same inclusion criteria as the patients and be without a prior hospital diagnosis of PD at the index date (i.e., the date of diagnosis of their respective case). The controls were contacted in random order until one consented to participate. Of 3,626 eligible controls contacted, 1,909 (53%) consented to participate and completed an interview. Among the interviewed controls, 3 were registered with a first hospital contact for PD in the Hospital Register after they were selected as controls.
Exclusion of patients and controls.
We have shown previously that patients with PD start treatment with antiparkinson drugs on average 3 years before their first hospital contact for PD, indicating that the patients have had symptoms of PD before they were in contact with a hospital due to their disease.11 Thus, in order to further reduce the risk for including patients with PD symptoms due to other neurologic conditions than PD, we excluded 14 interviewed patients and 22 controls who had had a hospital contact for dementia (ICD-8 codes 290.09-290.19 or 293.09; ICD-10 codes F00-03, F05.1, or G30) or cerebrovascular disease (ICD-8 codes 430-438; ICD-10 codes I60-69, G45, or G46) any time between the start of the Hospital Register in 1977 to 3 years before the index date. We also excluded one case with unknown onset of first symptoms in addition to 108 cases and 102 controls for whom there was no self-reported information on the never/ever question on head injury, resulting in 1,705 cases and 1,785 controls for the analyses.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the Danish Data Protection Agency (no. 2011-41-7025) and by the Los Angeles institutional review board for human subjects, University of California. All participants provided written informed consent.
Information on head injuries, lifestyle, and sociodemographic factors.
We obtained information on head injuries, other medical history, lifestyle factors, and family history of PD in a structured telephone interview. Because of speaking difficulties or generally poor health, 568 study subjects responded to the questionnaire by mail only. All subjects were asked about all types of head injury before their diagnosis of PD, including head injuries that had caused loss of consciousness, subsequent amnesia, or memory problems, and hospitalization for their head injuries. The questionnaire also elicited information on lifetime tobacco use, caffeine intake, and alcohol consumption as well as educational background and family history of PD. A positive family history of PD was defined as having at least one first-degree relative with PD. We used the participants' home municipality at the date of first hospital contact for PD to assess the degree of urbanization. The date of the first cardinal symptom noted on the medical records was used as the referent date for calculating exposure; controls were assigned the date of their respective case.
Statistical analysis.
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) to estimate an association between head injury and PD by unconditional logistic regression. The exclusion of patients without PD led to an excess number of controls, because most of the patients had their medical record reviewed after the interview. Thus, we chose unconditional regression as our primary model to increase the number of subjects in analyses.
Main analyses were performed for head injury (ever, never), number of head injuries (0, 1, ≥2), and hospitalization for a head injury (no, yes) before the first cardinal symptom. Separate analyses were also conducted for age at head injury (<13, 13–19, 20–49, ≥50 years). For patients who reported a head injury, we also analyzed loss of consciousness (never, ever), duration of consciousness (0, ≤1–5, ≥5 minutes), and amnesia (no, yes). The multivariate analyses were adjusted for sex, year of birth, age at first cardinal symptom, alcohol use/units per week (continuous), pack-years of smoking (continuous), highest attained education (basic, 7–12 years; vocational, 10–12 years; higher, ≥13 years), family history of PD (none, suspected, confirmed), and degree of urbanization (capital area, provincial town, rural area, peripheral region). Only those participants with elucidated information on head injuries were included in the analyses.
In secondary analyses, we truncated head injuries 10 years before the first cardinal symptom to ensure that the head injuries had not been caused by latent PD. We also stratified the main and secondary analyses on sex, early (<60 years) vs late (≥60 years) onset of PD, family history of PD (no, yes), and type of data collection (questionnaire, telephone interview). Stratified analyses were also performed by number of years between the index date and the date of interview (<5, ≥5) in order to determine any effect of different survival of patients. All analyses were performed with R statistical software.16
RESULTS
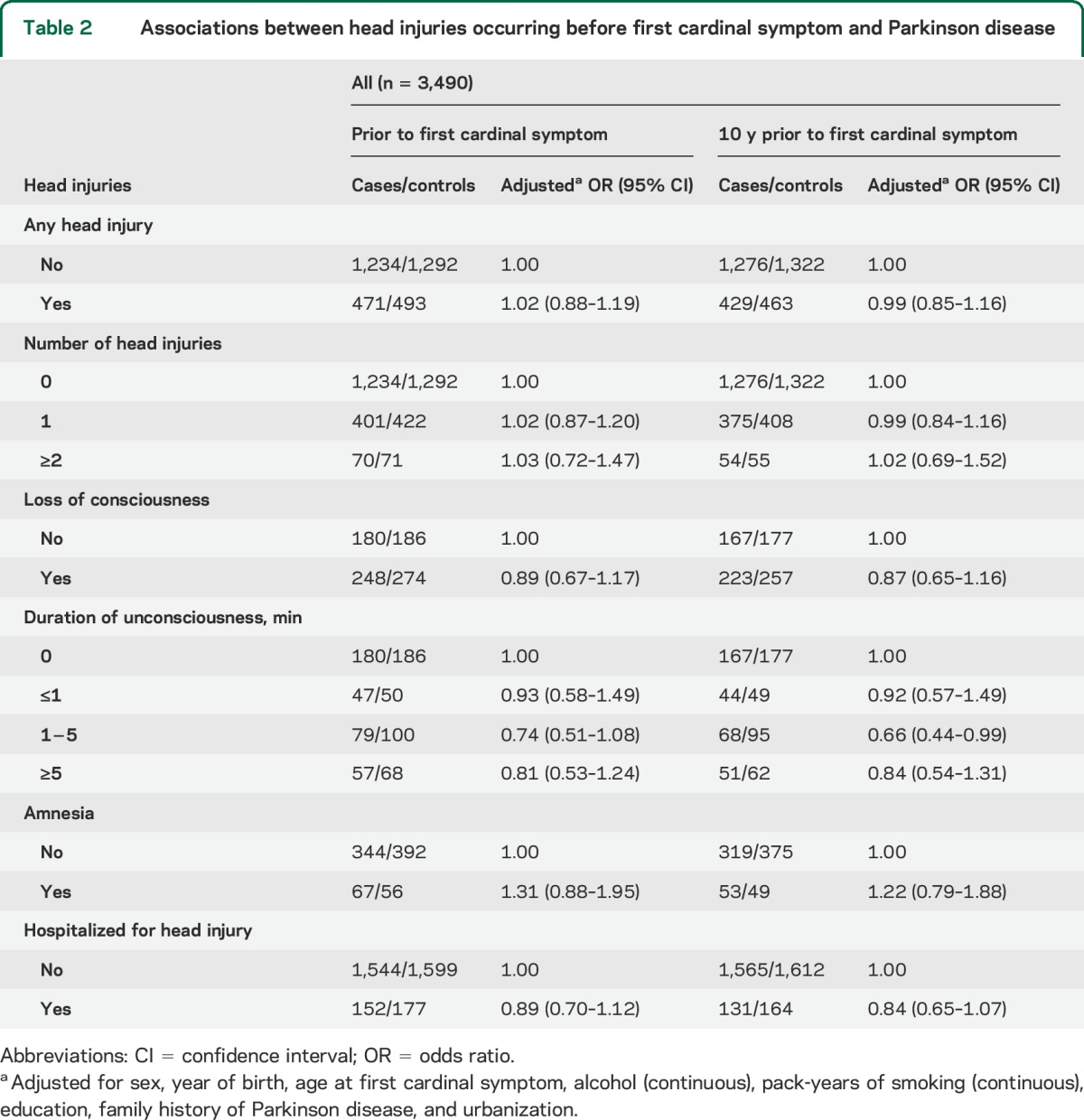
The descriptive characteristics of the 1,705 cases and 1,785 controls are shown in table 1. The reported frequency of at least one head injury before first cardinal symptom was similar for cases (27.3%) and controls (27.4%) (table 2). We did not find an association with PD for patients who reported one head injury or 2 or more head injuries or had ever been hospitalized for a head injury as compared with those who had never experienced a head injury. For 964 patients who reported at least one head injury, we found no association between loss of consciousness, duration of loss of consciousness, or amnesia and the risk for PD. The risk estimates were similar for men and women (data not shown). Exclusion of all head injuries that had occurred 10 years before the first cardinal symptom did not change any of the risk estimates (table 2). We found no effect of age at head injury on risk for PD, except for adolescents (22 cases; 9 controls) who reported 2 or more head injuries (OR 2.45; 95% CI 1.13, 5.72) (table e-1 on the Neurology® Web site at Neurology.org). When the analyses were stratified on family history of PD (no, yes), there was no indication of an increased risk associated with any head injury (no: OR 1.07; 95% CI 0.91, 1.26; yes: OR 0.67; 95% CI 0.39, 1.16). Further, no association between any head injury and risk for PD was observed when stratified on age at first cardinal symptom (<60 years: OR 1.12; 95% CI 0.89, 1.41; ≥60: OR 0.95; 95% CI 0.77, 1.17) or type of data collection (questionnaire: OR 1.01; 95% CI 0.64, 1.58; telephone interview: OR 1.04; 95% CI 0.88, 1.23). We also stratified the analyses on time between the index date and the date of interview (<5, ≥5) and found similar risk estimates in both groups (data not shown). Finally, we did not observe any differences in risk estimates between the results from the conditional and unconditional analyses (data not shown).
Table 1.
Descriptive characteristics of 1,705 patients with Parkinson disease and 1,785 population controls

Table 2.
Associations between head injuries occurring before first cardinal symptom and Parkinson disease
DISCUSSION
In this large case-control study of 1,705 patients with PD, we found no association between head injuries occurring before the first cardinal symptom and risk for PD. We evaluated the influence of severity and repeated head injuries occurring at all ages on the risk for PD but found no evidence of an association between head injuries and PD, except in adolescence. However, as we found no other increase in risk for PD among those reporting a head injury, there was no dose-response relation, and the results are based on a small number of patients with head injuries (22 cases; 9 controls), this result might represent a spurious finding.
Numerous studies of head injury and PD have been conducted, but the findings have been highly inconsistent. The largest studies were based on data from registries.8,11,17 In a Danish cohort study, severe head injury was not found to be a risk factor for PD, whereas a Danish and a Swedish case-control study reported overall positive associations between a hospital contact for head injury and PD (Rugbjerg et al.11: OR 1.5; 95% CI 1.4, 1.7; Fang et al.17: OR 1.17; 95% CI 1.04, 1.31). As all 3 studies lacked information on first cardinal symptom, date of first hospital contact for PD was used as the date of diagnosis. This resulted in an average age of patients at their first hospital contact for PD of more than 70 years (Rugbjerg et al.11: 73.0 years; Fang et al.17: 71.7 years). In our study, the average age of patients with PD at first cardinal symptom was 61.4 years, suggesting that the patients included in the register studies may have had symptoms of PD years before their first hospital contact was registered. A prodromal phase of 20 years has been suggested for PD; therefore, when clinical symptoms lead to a diagnosis of PD, the disease is already neuropathologically severely advanced and possibly irreversible because of the neuronal dysfunction and cell loss.18,19 Hence, it is important to exclude exposures shortly before onset of PD when studying etiologic risk factors for PD. This was confirmed by the results from the register-based case-control studies; i.e., when only patients with at least 1 year of lag time between head injury and date of first hospital contact for PD were included, the association weakened, and after 10 years of lagging no overall association was found in either study.11,17 The results of these 2 large register-based studies can therefore be explained largely by reverse causality, i.e., hospital contacts for head injuries are a consequence of the movement disorder rather than its cause.
Two recently published meta-analyses of head injury in relation to PD reported a moderate increased risk for PD associated with a history of a head injury that resulted in concussion (pooled OR 1.57; 95% CI 1.35, 1.83)4 or head injury with or without loss of consciousness (pooled OR 1.58; 95% CI 1.30, 1.91).7 Most of the studies included in the meta-analyses, however, did not report risk estimates that accounted for a lag time. The largest studies with most influence in these meta-analytical results were the 2 register-based case-control studies,11,17 but the authors included only the risk estimates with no or minimal lag time in their analyses. In addition, they did not present analyses where these large studies were excluded, thus it is not possible to evaluate the influence the other studies may have had on the reported pooled risk estimate.
Several biologically plausible mechanisms have been posited by which a head injury could lead to neuronal degeneration. A head injury triggers a physiologic cascade that involves some of the processes implicated in the development of PD, such as neuroinflammation and activation of microglia,20 accumulation of α-synuclein,21 disruption of mitochondrial function, and increased free radical production.22 In our study, however, we do not find any association between head injury and PD.
In a recent study, an association between head injury and PD was only found among carriers of the α-synuclein (SNCA) Rep1 promoter risk allele,23 which has previously been linked to PD.24 We currently lack information on this susceptibility gene for PD and thus cannot rule out the possibility that head injury triggers PD in people with an increased genetic susceptibility. We stratified our analysis by family history of PD, but we did not find an increased risk in patients with a positive family history of PD. Furthermore, as we did not examine head injury in combination with another environmental risk factor, such as exposure to pesticides, we cannot exclude the possibility that these combinations increase the risk for PD, as suggested previously.25
The strengths of the study include its large sample size, the population-based design and a moderate rate for controls (53%), and the extensive information collected on all head injuries, including severity, throughout life. Further, all medical records were rigorously abstracted to include only patients with PD. A re-evaluation of a random sample of 50 medical records of patients initially considered to have PD showed that 48 had PD (96%), indicating limited misclassification of diagnosis. The rigorous review made it possible to only include exposures prior to the first cardinal symptoms rather than using the date of diagnosis stated by the patients or first hospital contact for PD. Case–control studies are prone to recall bias because patients often search for explanations for their disease and are more likely to report exposure. The frequency of head injury in our study was, however, similar in patients and controls; if the results were affected by differential misclassification due to recall bias, the patients would be expected to over-report the number of head injuries. As we found no association between head injury and risk for PD, this seems unlikely. The study population was, however, elderly, and use of self-reported data collected late in life to elicit information on lifetime habits is a limitation of this study. All study participants probably reported some data inaccurately, resulting in some degree of nondifferential misclassification of exposure.
The participation rate was high among patients (81%), but moderate among controls (53%). Further examination of all eligible participants showed that twice as many nonparticipants than participants had died as of March 2013 (38% vs 18% patients and 13% vs 6% controls). We cannot rule out if the selection of patients were biased due to a potential association between head injury and, e.g., a more progressive PD. In addition, some of the patients had their first hospital contact for PD back in 1996, thus the study includes both prevalent and incident patients. When we stratified the analyses on time between first cardinal symptom and interview (<5, ≥5), however, the risk estimates were similar for both groups of patients, indicating no or minor influence of survival bias.
The results of this large study, based on 1,705 patients with a verified diagnosis of PD, do not support the hypothesis that head injury increases the risk for PD. Future studies may want to focus on head injuries in combination with genetics or environmental risk factors when examining the relation between head injury and risk for PD and allow for appropriate lag time to exclude head injuries close to symptom date.
Supplementary Material
GLOSSARY
- CI
confidence interval
- OR
odds ratio
- PD
Parkinson disease
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Line Kenborg: contributed to the study design, design of analyses, and interpretation of data and drafted the manuscript. Kathrine Rugbjerg: contributed to the study design, design of analyses, and interpretation of data and revised the manuscript for intellectual content. Pei-Chen Lee: contributed to the study design, design of analyses, and interpretation of data and revised the manuscript for intellectual content. Line Ravnskjær: contributed to the design of analyses, conducted the statistical analyses, and revised the manuscript for intellectual content. Jane Christensen: contributed to the design of analyses, conducted the statistical analyses, and revised the manuscript for intellectual content. Beate Ritz: contributed to the overall conception and design and developed the protocol, the design of the analyses, and interpretation of data and revised the manuscript for intellectual content. Christina F. Lassen: contributed to the study design, design of analyses, and interpretation of data and revised the manuscript for intellectual content.
STUDY FUNDING
Supported by grant R01ES013717 from the NIH.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology 2009;72:S1–S136. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha NE, Stokes L, Schoenberg BS, et al. A case-control study of twin pairs discordant for Parkinson's disease: a search for environmental risk factors. Neurology 1986;36:284–288. [DOI] [PubMed] [Google Scholar]
- 3.Dong JQ, Zhang ZX, Zhang KL. Parkinson's disease and smoking: an integral part of PD's etiological study. Biomed Environ Sci 2003;16:173–179. [PubMed] [Google Scholar]
- 4.Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013;28:1222–1229. [DOI] [PubMed] [Google Scholar]
- 5.Lai BC, Marion SA, Teschke K, Tsui JK. Occupational and environmental risk factors for Parkinson's disease. Parkinsonism Relat Disord 2002;8:297–309. [DOI] [PubMed] [Google Scholar]
- 6.Morano A, Jimenez-Jimenez FJ, Molina JA, Antolin MA. Risk-factors for Parkinson's disease: case-control study in the province of Caceres, Spain. Acta Neurol Scand 1994;89:164–170. [DOI] [PubMed] [Google Scholar]
- 7.Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 2012;72:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spangenberg S, Hannerz H, Tuchsen F, Mikkelsen KL. A nationwide population study of severe head injury and Parkinson's disease. Parkinsonism Relat Disord 2009;15:12–14. [DOI] [PubMed] [Google Scholar]
- 9.Tan EK, Tan C, Fook-Chong SM, et al. Dose-dependent protective effect of coffee, tea, and smoking in Parkinson's disease: a study in ethnic Chinese. J Neurol Sci 2003;216:163–167. [DOI] [PubMed] [Google Scholar]
- 10.Wright JM, Keller-Byrne J. Environmental determinants of Parkinson's disease. Arch Environ Occup Health 2005;60:32–38. [DOI] [PubMed] [Google Scholar]
- 11.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. BMJ 2008;337:a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick FD, De PG, Ahmadi A, et al. Environmental risk factors for Parkinson's disease and parkinsonism: the Geoparkinson study. Occup Environ Med 2007;64:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson's disease risk in twins. Ann Neurol 2006;60:65–72. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 16.R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2001. [Google Scholar]
- 17.Fang F, Chen H, Feldman AL, Kamel F, Ye W, Wirdefeldt K. Head injury and Parkinson's disease: a population-based study. Mov Disord 2012;27:1632–1635. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Del TK. Invited article: nervous system pathology in sporadic Parkinson disease. Neurology 2008;70:1916–1925.18474848 [Google Scholar]
- 19.Hawkes CH, Del TK, Braak H. A timeline for Parkinson's disease. Parkinsonism Relat Disord 2010;16:79–84. [DOI] [PubMed] [Google Scholar]
- 20.Smith C, Gentleman SM, Leclercq PD, et al. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol 2013;39:654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol 2007;208:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma 2000;17:843–855. [DOI] [PubMed] [Google Scholar]
- 23.Goldman SM, Kamel F, Ross GW, et al. Head injury, alpha-synuclein Rep1, and Parkinson's disease. Ann Neurol 2012;71:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maraganore DM, de AM, Elbaz A, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA 2006;296:661–670. [DOI] [PubMed] [Google Scholar]
- 25.Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology 2012;79:2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


