Abstract
Epithelial cancers including breast and prostate commonly progress to form incurable bone metastases. For this to occur, cancer cells must adapt their phenotype and behaviour to enable detachment from the primary tumour, invasion into the vasculature, and homing to and subsequent colonisation of bone. It is widely accepted that the metastatic process is driven by the transformation of cancer cells from a sessile epithelial to a motile mesenchymal phenotype through epithelial–mesenchymal transition (EMT). Dissemination of these motile cells into the circulation provides the conduit for cells to metastasise to distant organs. However, accumulating evidence suggests that EMT is not sufficient for metastasis to occur and that specific tissue-homing factors are required for tumour cells to lodge and grow in bone. Once tumour cells are disseminated in the bone environment, they can revert into an epithelial phenotype through the reverse process of mesenchymal–epithelial transition (MET) and form secondary tumours. In this review, we describe the molecular alterations undertaken by breast cancer cells at each stage of the metastatic cascade and discuss how these changes facilitate bone metastasis.
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related death in females worldwide.1 Despite this, the majority of primary tumours that remain confined to the breast are amenable to currently available treatments, and 5-year survival rates for patients with non-metastatic disease is ∼93%. However, once the tumour has metastasised to a distant site, 5-year survival decreases to ∼22% (National Cancer Institute SEER database). For breast cancer, the most common site of metastasis is bone, and patients with this condition have a median survival of around 2–3 years following initial diagnosis of bone involvement. Identification of new therapeutic approaches is therefore needed to improve outcome for patients with tumour spread to the skeleton. A better understanding of the molecular determinants that drive the different stages of breast cancer metastasis to bone is essential for the future development of successful therapeutic strategies.
Metastatic conversion of breast cancer cells is driven by genetic, epigenetic and phenotypic adaptations that change tumour cells from an epithelial to a mesenchymal phenotype (EMT). This process is initiated by the overexpression of mesenchymal proteins such as fibronectin and metalloproteinases2,3 in addition to the loss of cell adhesion molecules including E-cadherin and B-cadherin. Loss of E-cadherin is thought to be fundamental in this process, resulting in reduced adhesion of epithelial cells to desmosomes, increased cellular motility and dissemination of tumour cells into the circulation.4 Once in the circulation tumour cells must home to a secondary environment where they will be capable of forming metastases only if the environment is appropriate.5 On homing to bone it is thought that tumour cells occupy specific niches that are identical to, or overlapping with, the haematopoietic stem cell (HSC) niche.6,7 This niche is made up of two primary cell types: stromal cells and transient cells. Stromal cells include adipocytes, fibroblasts and osteoblasts, and these originate from mesenchymal cells in the marrow. These cells contribute to the proliferation and differentiation of cancer cells via the secretion of molecules such as vascular cell adhesion molecule 1, syndecan-1 and matrix metalloproteinase 2 (MMP2).8 Transient cells include T cells, erythrocytes and platelets, all of which have been shown to stimulate tumour growth and metastasis.9 Furthermore, the continuous process of bone remodelling, involving osteoclast-mediated bone resorption resulting in the release of a multitude of growth factors, cytokines and cell adhesion molecules from the bone matrix, makes the bone an attractive site for metastatic tumour cells.10,11
The interactions between tumour cells and their microenvironment are important regulators of cancer metastasis, and many excellent reviews have been published on this subject.10,12,13 However, increasing evidence suggests that metastasis occurs as a result of a stepwise accumulation of genetic mutations, with different molecular alterations being required for different stages in the metastatic process. In the current review, we focus on the molecular alterations that drive the different stages of metastasis: tumour cell invasion and dissemination into the circulation, tumour cell homing to bone and tumour cell colonisation and growth in the metastatic site (bone).
Tumour cell invasion and dissemination into the circulation
Amassing evidence shows that cells escaping primary tumours and becoming disseminated into the circulation have a mesenchymal phenotype, and it is widely accepted that these cells originate from a subset of primary tumour cells that have undergone EMT. The exact molecular mechanisms that dictate EMT in tumour cells remain enigmatic; however, studies of EMT that occur during developmental processes such as gastrulation and neural crest delamination provide clues on how EMT may occur in metastasis.14 Currently, EMT is divided into three separate subtypes, which are associated with distinct biological functions:
Implantation of embryogenesis and organ development.
Tissue regeneration and organ fibrosis and wound healing process.
Cancer progression and metastasis.
The induction of type 3 EMT at the onset of metastasis is facilitated by genetic alterations acquired by cancer cells, and these generate cells with invasive properties that enable them to move into the blood stream (Figure 1). In this review, we will discuss the key transcriptional and cellular molecules that coordinate to drive EMT (as shown in Figure 2). We will describe experimental evidence for new emerging concepts that challenge some current hypothesis to establish an up-to-date functional role for EMT mediators in the facilitation of tumour cell escape from the primary tumour and dissemination into the circulation.
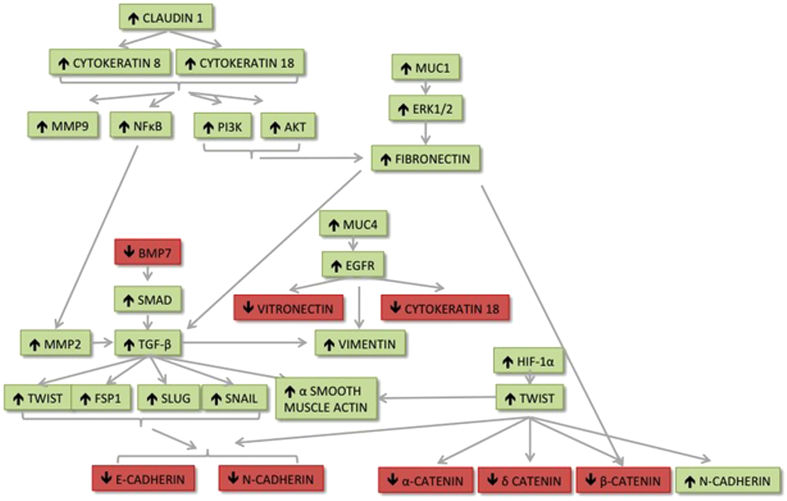
Figure 1.
Breast cancer metastasis to bone is initiated by tumour cell dissemination into the circulation. Diagrammatic representation showing the molecular alterations that drive phenotypic alterations in cancer cells leading to tumour cell dissemination into the blood and lymphatic vessels. α-SMA, α-smooth muscle actin.
Figure 2.
Molecular pathways involved in EMT, enabling cells to escape from the primary site and metastasise to bone. Molecules shown in red are downregulated and molecules shown in green are upregulated during EMT.
The transforming growth factor-β superfamily
Members of the transforming growth factor-β (TGF-β) superfamily, including bone morphogenic proteins (BMPs) and TGF-β, influence the EMT via regulation of transcription factors SIP1, Slug and Snail, which in turn suppress the adhesion molecule E-cadherin causing loss of cell–cell adhesion.15
Experimental evidence from murine mammary cancer 4T1 cells highlights the critical early role that TGF-β has in inducing EMT. Cells treated with TGF-β acquired a spindle-like morphology and expression of the mesenchymal markers N-cadherin and vimentin. These changes were not seen when the cells were treated simultaneously with TGF-β and the TGF-β antagonist Schisandrin B (SchB). In addition, treatment of mice bearing 4T1 cells in a mammary fat pad with SchB resulted in significant suppression of metastasis to bone and lung but did not affect tumour growth at the primary site. Furthermore, TGF-β appears to be specific to early events associated with tumour cell dissemination into the circulation, as SchB did not inhibit lung metastasis when 4T1 cells were injected into the tail vein.16
TGF-β activity is controlled by the expression of BMP7, and upregulation of BMP7 in vitro counteracts physiological EMT via inhibition of Smad-mediated TGF-β signalling and decreased expression of vimentin. In agreement with this, BMP7 expression has been shown to be inversely proportional to the tumourigenicity and invasive behaviour of MDA-MB-231 breast cancer cells.17,18 However, expression of BMP7 in primary tumour biopsies appears to contradict this finding. In a clinical setting, the presence of high levels of BMP7 expression in primary tumours has been strongly associated with accelerated bone metastasis, especially from ductal carcinomas.19 Therefore, the role of BMP7 in EMT remains inconclusive, but it is possible that BMP7 is more closely related to bone homing and colonisation than to EMT in a clinical setting.
TWIST
The role of TWIST as a master regulator of type 1 EMT is well documented.20,21,22 In addition, there is a considerable amount of evidence suggesting that TWIST also has a key role in type 3 EMT and metastasis (reviewed in Khan et al.23 and Mallini et al.24), and increased expression of this protein is associated with increased severity of bone metastases.25 TWIST is expressed in a variety of invasive and metastatic breast cancer cells including mouse 4T1 cells and human SUM1315, MDA-MB-231 and MDA-MB-435 cells. In contrast, this gene is not expressed on non-metastatic MDA-MB-436, MCF7 and BT20. Small interfering RNA (siRNA) knockdown of TWIST in 4T1 cells significantly reduces lung metastasis in vivo and the number of tumour cells shed into the circulation, without altering primary tumour growth.26 The role of TWIST in stimulating EMT is further evident from experiments carried out in human mammary epithelial cells in which retroviral transfection with TWIST resulted in loss of cell–cell contact with elevated expression of vimentin, α-smooth muscle actin and N-cadherin, as well as loss of E-cadherin, α-catenin, β-catenin and δ-catenin from the cell membrane.26 Laboratory studies demonstrating that TWIST activity induces metastasis have been extensively validated with clinical samples. Microarray analysis of different breast cancer subtypes revealed that TWIST is expressed by 70% of invasive lobular carcinomas and that tumour samples expressing TWIST also demonstrated other features of having undergone EMT, including almost universal loss of E-cadherin (97%).26
The mechanism by which TWIST stimulates breast cancer cell migration and invasion is regulated by epigenetic pathways. Evidence from mouse and human breast cancer cells has demonstrated that TWIST induces expression of the microRNA, miR-10b. This in turn inhibits translation of the mRNA encoding homeobox D10 resulting in increased expression of the prometastatic gene RHOC.27 In support of these findings, overexpression of TWIST1 increases miR-10b expression and the metastatic potential of bone-seeking, MDA-MB-231/B02 breast cancer cells in vitro. Conversely, the repression of miR-10b substantially decreased the ability of TWIST1 expressing MDA-MB-231/B02 breast cancer cells to home to and colonise the bone environment in vivo.25 Taken together with the clinical correlation showing that high miR-10b expression in primary breast carcinomas correlates with clinical progression,27 these findings demonstrate that epigenetic as well as genetic alterations influence EMT and progression to bone metastasis.
HIF-1α
It has been hypothesised that tumour cells undergo EMT in response to unfavourable conditions such as hypoxia to allow them to move to an environment that will better support their growth.28 Evidence from in vitro studies using MCF7 cells shows that hypoxia-inducible factor-1α (HIF-1α) drives EMT and onset of metastasis via direct regulation of TWIST expression.29,30 Under hypoxic conditions, HIF-1α binds directly to the hypoxia response element of the TWIST promoter, leading to increased expression of TWIST. This in turn results in upregulation of vascular endothelial growth factor and the formation of new blood vessels, a subsequent increase in E-cadherin followed by loss of vimentin and N-cadherin. This process of hypoxia-induced EMT can be completely reversed by inhibiting HIF-1α with siRNA, implying that HIF-1α is a key regulator of EMT.29 It therefore appears that hypoxia drives the onset of metastasis by inducing HIF-1α and TWIST signalling, arming the cells with a repertoire of molecules enabling escape into the circulation.
E-cadherin
Expression of E-cadherin has become a hallmark of many EMT assays, and it is generally accepted that loss of this molecule is critical for the initial escape and migration of individual cells from the primary tumour site.31 E-cadherin is a single span transmembrane glycoprotein that interacts with ϒ-catenin in adjacent cells to form intercellular adhesion junctions.32 Loss of E-cadherin disrupts cell–cell contact, leading to loss of the apical–basal barrier and cellular polarity, allowing growth factors that would normally have been segregated from the basolateral surface to interact with receptors in an autocrine manner and further drive the EMT phenotype.33 The critical role of E-cadherin in EMT and metastasis is well documented.2,3 Reduced expression of this molecule in non-metastatic human mammary cell lines (HMLE) with short hairpin RNA (shRNA) results in loss of cell-to-cell contact and an increase in markers of mesenchymal cells. Furthermore, orthotopic injection of E-cadherin knockdown HMLE cells into the mammary fat pad of nude mice resulted in significantly more micro- and macrometastases in the lungs compared with mice injected with control, transformed HMLE-like cells.34
Loss of E-cadherin has generally been considered pivotal for the onset of metastasis; however, emerging evidence suggests that cell-to-cell contact is the critical key to metastasis and that disrupting this process can induce metastasis without altering E-cadherin. Plakoglobin is the gene that encodes the Υ-catenin protein, which links E-cadherin to the actin cytoskeleton.35 Knockdown of plakoglobin by miRNA in MCF7 and T47D breast cancer cells leads to loss of cell–cell contact and increased tumour cell invasion. Furthermore, implanting plakoglobin knockdown cells into mammary fat pads of nude mice resulted in increased shedding of tumour cells into the blood stream compared with animals injected with control cells.36 Importantly, reduced plakoglobin expression resulted in an 80% reduction in Υ-catenin but did not alter E-cadherin expression or location to the cell membrane. Interestingly, however, decreased plakoglobin was found to reduce NM23-H1 (encoding nucleoside diphosphate kinase A, metastasis suppressor), which disrupts cell–cell adhesion via α- and β-catenin. Plakoglobin has been shown to promote α- and β-catenin nuclear translocation,36 implying that increased metastatic capability of cells can be driven independently of E-cadherin expression by loss of plakoglobin and NM23-H1.
Mucins
Aberrant expression of the transmembrane glycoproteins MUC1 and MUC4 has been associated with invasive epithelial carcinomas, including carcinoma of the breast. Recent evidence is emerging for a role of these proteins in EMT and metastasis. Experiments using an in vitro model of mouse mammary tumour cells (DA3) have demonstrated that overexpression of truncated human MUC1 leads to EMT, associated with ERK1/2-dependent activation of fibronectin and an increase in cell invasion.37 Furthermore, in prostate cancer cells, the cytoplasmic tail of MUC1 has been shown to interact with the DNA-binding domain of the androgen receptor, resulting in activation of EMT and increased invasiveness of cancer cells.38 Direct evidence for an association between MUC1 and initiation of EMT in cancer has been shown in a mouse model of pancreatic ductal adenocarcinoma. Overexpression of MUC1 led to elevated levels of vimentin, Slug and Snail compared with levels in mutant MUC1 (MUC1 CT) mice. In addition, MUC1 in MUC CT mice did not immunoprecipitate with or cause nuclear translocation of β-catenin, thereby blocking the transcription of genes associated with EMT.39
Studies utilising models of triple-negative breast cancer have shown that MUC4 mucin enhances the invasive and migratory potential of cancer cells through upregulation of EGFR family proteins. A separate study demonstrated that knockdown of MUC4 in breast cancer cells leads to molecular and biochemical alterations that are necessary for EMT, including reduced expression of mesenchymal markers such as vimentin and vitronectin and increased expression of the epithelial marker cytokeratin 18.40 These data suggest that MUC4 has an important role in EMT, transforming breast cancer cells into a more migratory and aggressive phenotype.
Cytokeratins 8, 18 and 19
Cytokeratins are the protein components of intermediate filaments that make up the supporting scaffolding within cells. The organisation and expression of these proteins change as epithelial cells gradually adopt an epithelial phenotype.41 The change in expression profile of cytokeratins during EMT provides insight into how changes in the structural integrity of cancer cells can drive invasion during cancer progression. In human breast cancer cells, expression levels of cytokeratins K8 and CK18 and 19 correlate with invasive potential, with high levels of K8 and K18 expressed in less invasive MCF7 compared with more invasive MDA-MB-231 and MDA-MB-436 cells and CK19 being undetectable in invasive cells. Conversely, a negative correlation was seen between CK8, CK18 and CK19 and filament proteins with increased vimentin and fibronectin observed in MDA-MB-231 and MDA-MB-436 compared with MCF7.42
Direct evidence supporting an active role of cytokeratins in metastasis has come from a recent study that used shRNA to knock down CK8 and CK18 in epithelial cancer cells, hepatocellular carcinoma (EppG2) and cervical carcinoma (HeLa). Reduced expression of CK8 and CK18 in these cells led to hyperactivity of PI3K, NF-κB and Akt, as well as increased expression of MMP2 and MMP9. Although it is recognised that CK8 and CK18 are involved in intracellular signalling and that these molecules are specific to epithelial cells, loss of these markers does not modify other markers of EMT. Instead, CK8 and CK18 are modulated by claudin-1, which influences the phenotype of epithelial cells independently of other EMT markers.43 Therefore, it is likely that, in addition to loss of K8 and K18 being a hallmark of EMT, modulation of these proteins at the transcriptional level influences cancer cell phenotype by orchestrating structural alterations that enable cells to escape from the primary tumour.
Fibronectin
The extracellular matrix protein fibronectin has a critical role in cellular adhesion, migration and transformation by activating the PI3/Akt pathway when bound to αvβ1 integrin.44 Normal adult breast tissue is largely devoid of fibronectin; however, during the process of tumourigenesis, levels of this protein increase in the stroma with highest levels of expression detected at the invasive front of tumours during EMT.44 In vitro evidence from human mammary breast cancer cells, MCF-10A, has highlighted a role for fibronectin in promoting the onset of EMT. Exposure of MCF-10A cells to exogenous fibronectin stimulated cell migration and induced an EMT response including upregulation of the EMT markers fibronectin Snail, N-cadherin, vimentin, MMP2, α-smooth muscle actin and phospho-Smad2. In addition, exogenous fibronectin is able to induce EMT under serum-free conditions; this process could be reversed following addition of a TGF-β-neutralising antibody.45 These data suggest that fibronectin can induce EMT in breast cancers by enhancing the activity of endogenous TGF-β.
Vimentin
The functional role of vimentin in EMT is yet to be fully elucidated; however, high levels of expression of this molecule are associated with an increased metastatic phenotype. Overexpression of vimentin in MCF7 breast cancer cells results in increased motility, whereas knocking down vimentin expression with siRNA in MDA-MB-231 cells downregulates genes associated with an invasive phenotype, Axl, ITGB4 and PLAU, and upregulates normal mammary epithelial genes, REAB25 and EHF.46 Under non-cancerous conditions, vimentin is involved in processes that require cell migration, such as wound healing, where it has a pivotal role in determining cell polarity, regulation of cell-to-cell contact and transport of signalling proteins.47 It therefore seems likely that altered expression of this molecule in cancerous tissue may also affect cell–cell adhesion and cell polarity, enabling cells to detach from the primary tumour and invade the surrounding stroma.
The role of cancer stem cells in EMT and progression to bone metastasis
Historically, there have been two major hypotheses relating to the induction and progression of breast cancer: The first of these, termed ‘clonal evolution theory', states that all cells within a tumour are equally tumourigenic with the same ability to invade and metastasise. The second concept describes the idea that solid tumours, including breast, originate and are sustained by a small proportion of self-renewing stem-like cells termed ‘cancer stem cells'. Current evidence strongly supports the cancer stem cell hypothesis, and experiments carried out using the cell surface markers CD44/CD24 to identify CD44low/CD24high breast cancer stem cells have shown that this population is capable of generating heterogenic tumours in vivo.48 Furthermore, when breast cancer progresses to form bone metastases, it appears that not all of the tumour cells that reach the bone environment are capable of colonising this site. Only a subset of cells that colonise bone develop into overt metastases,49,50 providing additional support for the idea that tumourigenesis is driven by a small population of cancer stem cells. It has been suggested that the process of differentiation and dedifferentiation between cancer stem cells and transient amplifying cells in breast cancer is plastic and dedifferentiation of transient amplifying tumour cells can generate increased numbers of cancer stem cells (reviewed in Puisieux et al.51). In support of this, recent studies have identified a link between induction of EMT and the gain of cancer stem cell characteristics.52,53 Both human and mouse breast tumours undergoing EMT transformation have high levels of cancer stem cells,54 and overexpression of TWIST1, ZEB1 or SNAI1 in transformed or immortalised mammary epithelial cells results in increased CD44high/CD24low/− cells and heightened ability to form mamospheres, indicating EMT induced acquisition of a cancer stem cell phenotype.52,53,55
Although many studies demonstrate a direct relationship between EMT and stemness (reviewed in Puisieux et al.51 and Medema56), it is evident that for cells to colonise a distant site, such as bone, they must undergo the reverse process of epithelial-to-mesenchymal transition (MET) while still retaining a population of cancer stem cells capable of inducing the metastatic tumour. The plasticity required for these rapid phenotypic changes suggests that they are regulated by epigenetic rather than genetic mechanisms. Recent experimental evidence has demonstrated that, during EMT, PRRX1 cooperates with TWIST1 to induce all EMT factors relevant to migration and invasion of cancer cells from the primary tumour into the blood, and at the same time PRRX1/TWIST suppresses stemness properties in the EMT state and needs to be downregulated for stemness properties to be reactivated to allow metastatic colonisation of tumour cells.57,58 Therefore, although EMT increases the number of CD44high/CD24low/− cells, these cannot be classified as cancer stem cells, but they can gain stemness properties as they lose TWIST activity and undergo MET during the process of bone colonisation (see below).
Tumour cell homing to bone
Following degradation of the extracellular matrix, tumour cells cross the basement membrane and enter the circulation. Once in the blood and/or lymphatic systems, tumour cells must home and disseminate to a secondary site where the microenvironment is favourable for them to proliferate and establish metastases. In theory, breast cancer cells can metastasise to any organ of the body; however, it has been demonstrated that breast cancer cells prefer to home to certain organs such as bone, lungs, liver and brain.5,6 Although the exact mechanism of the metastasis of breast cancer cells to these specific sites is still not completely understood, it is generally accepted that as cancer stem cells are the only cells capable of seeding a new tumour, they are also responsible for initiation of metastasis. In 1889, Stephen Paget59 proposed that both cancer cells (seed) and the secondary sites (soil) facilitate the process by releasing factors, signals and molecules that make the microenvironment appropriate and increase the probability of cancer cells to grow there (‘seed and soil hypothesis');59 this hypothesis still appears to hold today, and molecules involved in the process of bone homing are shown in Figure 3.
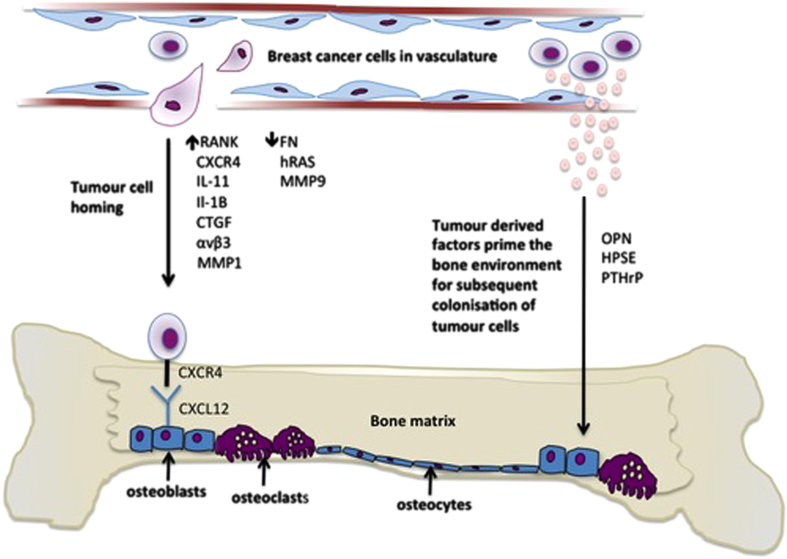
Figure 3.
Breast cancer cells home to the bone microenvironment. Bone is made up of different cell types that express a variety of molecules including the chemokine CXCL12 that attract breast cancer cells to this microenvironment. Breast cancer cells also express molecules including the chemokine receptor CXCR4 that enable them to adhere to the osteoblast-rich HSC cell niche and disseminate in the bone. Once in the bone microenvironment, tumour-derived growth factors prime the bone for subsequent colonisation of tumour cells. OPN, osteopontin; HPSE, heparanase.
Chemokines
One group of molecules that have been shown to have a crucial role in organ-specific metastasis of breast cancer cells are the chemokines and their receptors (G-protein-coupled receptors). The role of chemokines in organ-specific homing was first demonstrated in the homing of lymphocytes and haematopoietic cells to different organs.60 Studies have shown that breast cancer cells express these molecules leading to the hypothesis that they function as homing factors for breast cancer metastasis.6,61 More specifically, the chemokine receptors CCR7 and CXCR4 were found to be upregulated in metastatic breast cancer cell lines as well as in primary breast tumours that metastasise.6 Moreover, higher levels of their respective ligands CCL21/6Ckine and CXCL12/SDF-1a were found in sites that breast cancer cells preferentially migrate to, such as bone, lung, liver and lymph nodes, compared with lower levels in the small intestine and kidney, which are sites that breast cancer cells rarely metastasise to.6,62 CXCR4 has been shown to specifically promote bone metastasis by stimulating tumour cell recruitment and homing to bone in response to its interaction with the CXCL12/SDF-1a ligand.51 Furthermore, inhibition of CXCL12/CXCR4 interaction using neutralising antibodies or RNA resulted in reduced breast tumour growth, as well reduced cell migration, invasion and metastasis.6,63,64 The role of CXCR4 in breast cancer cell homing to bone has further been demonstrated by genetic analysis of bone-homing MDA-MB-231, non-bone-homing MDA-MB-231 breast cancer cells and normal human mammary epithelium (MCF10A). In these experiments, bone-homing cells expressed significantly more CXCR4 compared with their parental clone and normal mammary epithelium, and this overexpression was associated with a higher number of bone metastases in vivo.2 However, it should be noted that expression of CXCR4 is not a universal feature in breast cancer bone homing. We and others have generated bone-homing clones of MDA-MB-231 cells (BO2 and MDA-IV cells) using the same method as described by Kang et al.2,65,66 Interestingly, CXCR4 was not detected in either of these bone-homing cell lines by microarray or PCR analysis.
Intergrins
Intergrins are cell surface glycoproteins that facilitate cell–cell and cell–extracellular matrix adhesion and cell migration (reviewed in Hiraga et al.67). Expression of these molecules on both tumour cells in bone and the supporting host stromal cells (osteoclasts, new blood vessels, inflammatory cells, platelets and bone marrow stromal cells) has a key role in enhancing bone metastasis. There are 8 β and 18 α integrin subunits that can make up 24 different combinations in different cell types, each characterised by distinct ligand-binding specificities, signalling abilities and regulatory mechanisms (reviewed in Schneider et al.68). Metastatic tumour cells show differential integrin heterodimerisation and activation compared with non-metastatic tumour cells that enable cells to home and colonise specific metastatic sites.69 In bone metastasis, the β1 and β3 family members appear to be of major importance.
In breast cancer, αvβ3 is increased in the expression in bone metastatic cells compared with non-metastatic cells.70 Binding of αvβ3 integrin to bone extracellular matrix proteins including osteopontin, vitronectin and bone sialo protein promotes adhesion of breast cancer cells to bone.71 Therefore, it is likely that breast cancer cells expressing integrins that can interact with such proteins are more likely to home to bone. In 2002, Pecheur and co-workers72 showed that expression of αvβ3 integrin by breast cancer cells increased the adhesion of cancer cells to bone and the incidence of bone metastasis. Moreover, inhibition of αvβ3 integrin using a small-molecule antagonist suppresses breast cancer bone metastasis, indicating an explicit role for αvβ3 integrin in bone-specific homing.73 The β1 family member, α5β1, specifically binds to fibronectin on human bone marrow stroma, and expression of this molecule on breast cancer cells facilitates interaction with the bone stroma. The interaction between tumour cell α5β1 and host stromal cell fibronectin appears to contribute to the survival of growth-arrested breast cancer cells, a potential mechanism through which tumour cells can become sequestered and dormant within the bone marrow cavity and may later begin to proliferate and establish skeletal metastases.74
Additional molecules associated with bone homing
In addition to chemokines and intergrins, a multitude of other molecules have been shown to have important roles in chemotaxis of breast cancer cells to bone (see Figure 3): increased expression of MMP-1, interleukin 11 (IL-11) and connective tissue factor has been identified in bone-homing clones of MDA-MB-231 breast cancer cells compared with parental cells in vivo.2 Using a similar approach in which bone-homing clones of MDA-MB-231 cells were compared with parental cells, we and others have found that bone homing is associated with decreased cell–cell adhesion and migration, coupled with significantly reduced levels of the cell adhesion molecule fibronectin and calcium signal binding protein S100A4.65,66 Interestingly, we also found a strong link between IL-1B expression and bone homing in both MDA-MB-231 cells and primary tumours from breast cancer patients, indicating that this molecule may promote an invasive and motile phenotype in breast cancer cells.66
In fitting agreement with the hypothesis that breast cancer cells acquire genotypic similarities to cancer stem cells during EMT and progression to bone metastasis, Hiraga et al.67 showed that CD44 overexpression in breast, myeloma and prostate cancer cells promotes bone metastasis by enhancing tumourigenicity, cell migration, invasion and production of extracellular matrix haluronan. Whether high expression of CD44 is because of these cell lines having high numbers of cancer stem cells or whether this is a result of EMT-induced expression of CD44 remains to be established. However, binding of CD44 to haluronan is a possible mechanism by which cancer cells colonise bone once they have entered this environment.
Tumour cell colonisation and growth to bone
Tumour cells are disseminated into the bone environment through the blood stream, and as a result the most common homing site is the highly vascularised metaphysis in the long bones. Once in the bone microenvironment, it is hypothesised that breast cancer cells compete for the HSC niche, and once within this niche, they can be stimulated to proliferate.66,75 In addition to molecules with well-defined roles in bone colonisation, discussed below, we have previously demonstrated that this process is also associated with increased levels of MMP9, HRAS and fibronectin.66 MMP9 has strong associations with increased tumour cell invasion in a number of cancer types,63 and increased MMP9 expression has been shown to increase the capacity of tumour cells to extravasate as well as to cause activation of bone-resorbing osteoclasts.76,77 In addition to the proinvasive properties of MMP9, HRAS overexpression has been shown to increase the invasion of MCF10A breast cells and to transform these from an endothelial to an epithelial cell type (MET).78 Fibronectin on the other hand has well-characterised properties as a cell adhesion molecule and has been shown to be upregulated in bone metastatic deposits compared with primary breast tumours.79 It is therefore likely that increased expression of fibronectin enables tumour cells to adhere to the HSC niche once they have successfully entered the bone microenvironment.60,68
MET and bone colonisation
To enable colonisation of bone, tumour cells undergo a reverse EMT transition known as MET, reactivating their epithelial cell properties and increasing their adhesion and interactions with the bone marrow microenvironment80,81 (Figure 4). The re-expression of E-cadherin is considered the fundamental hallmark of MET, as it allows tumour cells to interact with the bone marrow and adhere to the HSC niche.33,80 Once in the bone, tumour cells first form micrometastases that can either proliferate and form overt metastatic lesions or remain dormant for long periods until they reactivate and establish tumours.82 The mechanisms by which some tumour cells remain dormant whereas others are stimulated to proliferate remain to be established. However, amassing evidence suggests that factors that increase bone turnover, including menopause/ovariectomy, may stimulate the cells within the mesenchymal stem cell niche and drive proliferation of dormant tumour cells.83 Increased proliferation of tumour cells in the bone marrow causes the production of molecules and growth factors by both tumour cells and bone resorption that promote tumour growth, osteoclast production, osteoblast inactivation and bone destruction (osteolysis), a process known as a ‘vicious cycle', as shown in Figure 3.82 More specifically, proliferating cancer cells have been shown to release factors such as parathyroid hormone-related protein (PTHrP), interleukins (IL-8, -11), MMP-1, cyclooxygenase-2 (COX-2), transcription factor GLI2 and HIF-1α that promote both their growth in the bone marrow and osteolysis.
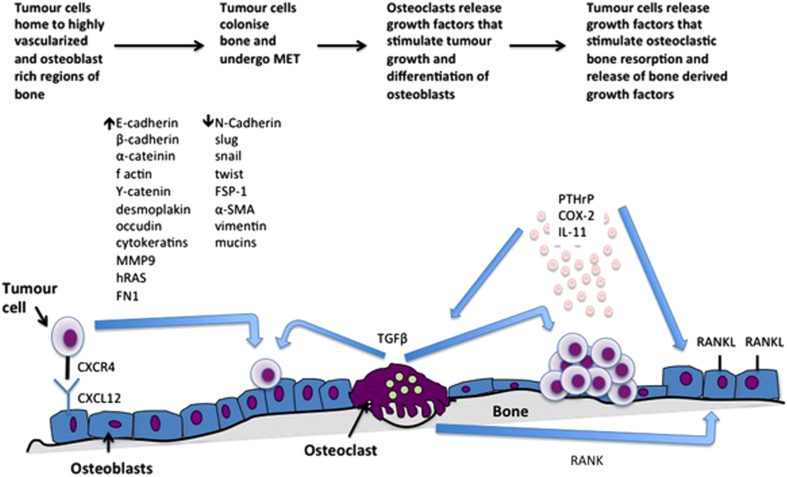
Figure 4.
Breast cancer cells disseminated in bone colonise this environment. Disseminated breast cancer cells in the bone microenvironment undergo MET, enabling colonisation and growth at this metastatic site. Tumour cells growing in bone secrete growth factors that stimulate osteoclastic bone resorption. This in turn results in a vicious cycle whereby osteoclasts release bone-derived growth factors that further stimulate tumour growth.
PTHrP
PTHrP is a major regulator of osteolytic lesion formation and a key molecule responsible for the humoral hypercalcaemia of malignancy.84,85 Moreover, PTHrP has been shown to be expressed in 92% of primary breast cancers that metastasised to bone compared with tumours that metastasised to non-bone sites (17%) and primary tumours that did not metastasise (60%).86 It is hypothesised that release of PTHrP by tumour cells primes the bone environment for growth of metastatic tumour cells by increasing bone turnover. Release of TGF-β from the bone marrow increases PTHrP secretion from breast cancer cells via activation of Smad and p38 mitogen-activated protein kinase pathways.87 This stimulates the production of receptor activator of nuclear factor-κB ligand (RANKL), which subsequently binds to RANK and induces osteoclast differentiation and activation.88 Under normal physiological conditions, the binding of RANK to RANKL is homeostatically controlled by binding of RANK to its decoy receptor, soluble ligand, osteoprotegerin (OPG).89 However, production of PTHrP by tumour cells has been shown to inhibit OPG activity, thus promoting bone metastasis. Breast cancer cells also express COX-2, which has an important role in the development of osteolytic bone metastasis by driving the overexpression of prostaglandin E2.90 Moreover, the production of osteolytic factors including IL-8 and IL-11 by breast cancer cells appears to have an important role in osteoclast formation. Bendre et al.91 showed a direct effect of IL-8 in osteoclast differentiation and activation leading to the knock-on effect of bone destruction. In addition, it was shown that IL-11 mediates bone resorption by increasing the osteoblast production of RANKL.2 Evidence from IL-11-overexpressing cell lines indicates that this molecule also has direct effects on promoting bone metastasis in vivo.92 Similarly to IL-11, MMP-1 promotes osteolytic bone metastasis by increasing the osteoblast production of RANKL and also by suppressing the expression of osteoprotegerin through activation of the EGFR-dependent signalling pathway.93 Metastatic breast cancer cells often express HIF-1α, which promotes osteoclast formation and inhibits osteoblast differentiation (activation),94 as well as transcription factor GLI2, which promotes the production of PTHrP, facilitating osteolysis.95
TGF-β
As a result of bone destruction stimulated by the osteoclast-promoting factors released from breast cancer cells, growth factors such as TGF-β stored in a latent form in bone marrow are released.96 The activation of TGF-β signalling has been observed in several studies and correlates with breast cancer bone metastasis formation.97,98 It therefore appears that breaking the vicious cycle between tumour growth and bone destruction may be necessary for successful treatment of breast cancer bone metastasis. In an attempt to address this, a small molecule targeted against the TGF-β receptor 1 has been developed. YR-290 blocks TGF-β signalling by inhibiting nuclear translocation of Smad2 and reducing phosphorylation of Smad2/3. Treatment of HaCaT cells with YR-290 inhibits TGF-β-induced EMT, resulting in dose-dependent increases in the expression of vimentin and fibronectin and an increase in E-cadherin. Furthermore, in a 4T1 mouse model, YR-290 blocked spontaneous metastasis to the lung by 74.71% and 97.38% in groups treated with 1 and 5 mg kg−1 per day, respectively. Moreover, it has been demonstrated that blocking of TGF-β signalling in MDA-MB-231 breast cancer cells inhibited the development of osteolytic bone metastases in vivo.96 Although these data are from early-stage experiments, they provide insight into the potential therapeutic benefits of targeting EMT/MET to produce novel treatments for preventing the development and progression of breast cancer bone metastasis.
Conclusions/future perspectives
The molecular alterations associated with driving breast cancer metastasis to bone are still not fully understood; however, it is clear that this involves a vast number of molecules leading to large-scale functional and architectural change of the cancer cell. Metastasis appears to be primarily driven by alterations in adhesion complexes and the cytoskeleton, enabling the cell to first escape from the primary tumour and then lodge in its metastatic site. The exact chronology of individual changes remains unclear, and a number of molecular pathways converge to generate an altered phenotype. The switch between one phenotype and another is rapid and the exact order in which these happen may prove difficult to identify. Furthermore, it is still unclear which, if any, of these molecules has the overriding influence on EMT/MET. Being a trigger of EMT is a claim common to TGF-β, TWIST, HIF-1α and E-cadherin, with alterations in the remaining molecules having an effect on EMT to differing extents. Furthermore, emerging evidence suggests that the phenotypic plasticity required for cells to undergo EMT/MET is driven as much, if not more, by epigenetic changes. This research is still in its infancy, and it is expected that current research efforts in this area will increase our understanding around the epigenetic regulation of metastatic processes. Redundancy that is typical in biological systems may prove to be problematic when choosing a suitable EMT/MET-related target for blocking the spread of cancer. However, as seeding of breast cancer cells into the bone is an early event, it is likely that tumour cells may already be present in the bones of patients before they are diagnosed with breast cancer.50,99 Although molecules associated with early onset of metastasis may not prove useful as therapeutic targets, these may represent potential biomarkers for predicting disease recurrence, enabling early treatment intervention/strategy change. Identification of novel molecules associated with tumour growth in bone may generate improved therapeutic strategies for this currently incurable condition.
Footnotes
The authors declare no conflict of interest.
References
- Cancer Research UK. Europe and the world (females). Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/incidence/#world, accessed on November 2014.
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003; 3: 537–549. [DOI] [PubMed] [Google Scholar]
- Karakosta A, Golias CH, Charalabopoulos A, Peschos D, Batistatou A, Charalabopoulos K. Genetic models of human cancer as a multistep process. Paradigm models of colorectal cancer, breast cancer, and chronic myelogenous and acute lymphoblastic leukaemia. J Exp Clin Cancer Res 2005; 24: 505–514. [PubMed] [Google Scholar]
- Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell–cell adhesion. Curr Opin Cell Biol 1998; 10: 572–577. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2: 563–572. [DOI] [PubMed] [Google Scholar]
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–56. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002; 62: 1832–1837. [PubMed] [Google Scholar]
- Park SY, Kim HJ, Kim KR, Lee SK, Lee CK, Park KK et al. Betulinic acid, a bioactive pentacyclic triterpenoid, inhibits skeletal-related events induced by breast cancer bone metastases and treatment. Toxicol Appl Pharmacol 2014; 275: 152–162. [DOI] [PubMed] [Google Scholar]
- Riether C, Schürch CM, Ochsenbein AF. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ 2014; 22: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling JA, Guelcher SA. Bone structural components regulating sites of tumor metastasis. Curr Osteoporos Rep 2011; 9: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2011; 11: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olechnowicz SW, Edwards CM. Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res 2014; 74: 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannuru KC, Singh RK. Tumor–stromal interactions in bone metastasis. Curr Osteoporos Rep 2010; 8: 105–113. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest 2009; 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Ju HZ, Liu SF, Lee TC, Shih YW, Chuang LY et al. BMP-2 suppresses renal interstitial fibrosis by regulating epithelial–mesenchymal transition. J Cell Biochem 2011; 112: 2558–2565. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang B, Liu K, Ding Z, Hu X. Schisandrin B attenuates cancer invasion and metastasis via inhibiting epithelial-mesenchymal transition. PLoS ONE 2012; 7: e40480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R et al. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res 2007; 67: 8742–8751. [DOI] [PubMed] [Google Scholar]
- Buijs JT, van der Horst G, van den Hoogen C, Cheung H, de Rooij B, Kroon J et al. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene 2012; 31: 2164–2174. [DOI] [PubMed] [Google Scholar]
- Buijs JT, Petersen M, van der Horst G, van der Pluijm G. Bone morphogenetic proteins and its receptors; therapeutic targets in cancer progression and bone metastasis? Curr Pharm Des 2010; 16: 1291–1300. [DOI] [PubMed] [Google Scholar]
- Saunders LR, McClay DR. Sub-circuits of a gene regulatory network control a developmental epithelial-mesenchymal transition. Development 2014; 141: 1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDusen NJ, Firulli AB. Twist factor regulation of non-cardiomyocyte cell lineages in the developing heart. Differentiation 2012; 84: 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Hemmings BA. Phosphorylation of basic helix-loop-helix transcription factor Twist in development and disease. Biochem Soc Trans 2012; 40: 90–93. [DOI] [PubMed] [Google Scholar]
- Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumour Biol 2013; 34: 2497–2506. [DOI] [PubMed] [Google Scholar]
- Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev 2014; 40: 341–348. [DOI] [PubMed] [Google Scholar]
- Croset M, Goehrig D, Frackowiak A, Bonnelye E, Ansieau S, Puisieux A et al. TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J Bone Miner Res 2014; 29: 1886–1899. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–939. [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007; 449: 682–689. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J et al. Hypoxia induces epithelial–mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC Cancer 2013; 13: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 2008; 10: 295–305. [DOI] [PubMed] [Google Scholar]
- Yang MH, Wu KJ. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle 2008; 7: 2090–2096. [DOI] [PubMed] [Google Scholar]
- van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone 2011; 48: 37–43. [DOI] [PubMed] [Google Scholar]
- Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem 2002; 277: 39209–39216. [DOI] [PubMed] [Google Scholar]
- Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metast 2008; 25: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68: 3645–3654. [DOI] [PubMed] [Google Scholar]
- Yin T, Getsios S, Caldelari R, Godsel LM, Kowalczyk AP, Müller EJ et al. Mechanisms of plakoglobin-dependent adhesion: desmosome-specific functions in assembly and regulation by epidermal growth factor receptor. J Biol Chem 2005; 280: 40355–40363. [DOI] [PubMed] [Google Scholar]
- Holen I, Whitworth J, Nutter F, Evans A, Brown HK, Lefley DV et al. Loss of plakoglobin promotes decreased cell-cell contact, increased invasion, and breast cancer cell dissemination in vivo. Breast Cancer Res 2012; 14: R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattrup CL, Gendler SJ. MUC1 alters oncogenic events and transcription in human breast cancer cells. Breast Cancer Res 2006; 8: R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi H, Ahmad R, Jin C, Joshi MD, Guha M, Alam M et al. MUC1-C oncoprotein confers androgen-independent growth of human prostate cancer cells. Prostate 2012; 72: 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy LD, Sahraei M, Subramani DB, Besmer D, Nath S, Tinder TL et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011; 30: 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Lakshmanan I, Ponnusamy MP, Chakraborty S, Jain M, Pai P et al. MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS One 2013; 8: e54455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosse SA, Hannemann J, Spötter J, Bauche A, Andreas A, Müller V et al. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clin Cancer Res 2012; 18: 993–1003. [DOI] [PubMed] [Google Scholar]
- Mackinder MA, Evans CA, Chowdry J, Staton CA, Corfe BM. Alteration in composition of keratin intermediate filaments in a model of breast cancer progression and the potential to reverse hallmarks of metastasis. Cancer Biomark 2012; 12: 49–64. [DOI] [PubMed] [Google Scholar]
- Fortier AM, Asselin E, Cadrin M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J Biol Chem 2013; 288: 11555–11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YK, Kim A, Kim MK, Choi JE, Kang SH, Lee SJ. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum Pathol 2013; 44: 2028–2037. [DOI] [PubMed] [Google Scholar]
- Park J, Schwarzbauer JE. Mammary epithelial cell interactions with fibronectin stimulate epithelial–mesenchymal transition. Oncogene 2014; 33: 1649–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011; 30: 1436–1448. [DOI] [PubMed] [Google Scholar]
- Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol 2013; 25: 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle 2006; 16: 1744–1750. [DOI] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA. Models. Mechanisms and evidence for cancer dormancy. Nat Rev Cancer 2007; 7: 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014; 6: 488–494. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS ONE 2008; 3: e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA 2010; 107: 15449–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesuna F, Lisok A, Kimble B, Raman V. Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia 2009; 11: 1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol 2013; 15: 338–344. [DOI] [PubMed] [Google Scholar]
- Ocaña OH, Córcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S et al. Metastatic colonization requires the repression of the epithelial–mesenchymal transition inducer Prrx1. Cancer Cell 2012; 22: 709–724. [DOI] [PubMed] [Google Scholar]
- Vaga S, Morales AV, Ocaña OH, Valdés F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 2004; 18: 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metast Rev 1989; 8: 98–101. [PubMed] [Google Scholar]
- Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol 2000; 12: 336–341. [DOI] [PubMed] [Google Scholar]
- Singh RK, Varney ML. IL-8 expression in malignant melanoma: implications in growth and metastasis. Histol Histopathol 2000; 15: 843–849. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Anderson RL. Genes involved in breast cancer metastasis to bone. Cell Mol Life Sci 2002; 59: 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 2004; 64: 8604–8612. [DOI] [PubMed] [Google Scholar]
- Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res 2005; 65: 967–971. [PMC free article] [PubMed] [Google Scholar]
- Bellahcène A, Bachelier R, Detry C, Lidereau R, Clézardin P, Castronovo V. Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells. Breast Cancer Res Treat 2007; 101: 135–148. [DOI] [PubMed] [Google Scholar]
- Nutter F, Holen I, Brown HK, Cross SS, Evans CA, Walker M et al. Different molecular profiles are associated with breast cancer cell homing compared with colonisation of bone: evidence using a novel bone-seeking cell line. Endocr Relat Cancer 2014; 21: 327–341. [DOI] [PubMed] [Google Scholar]
- Hiraga T, Ito S, Nakamura H. Cancer stem -like marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility and hyaluronan production. Cancer Res 2013; 73: 4112–4122. [DOI] [PubMed] [Google Scholar]
- Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone 2011; 48: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110: 673–687. [DOI] [PubMed] [Google Scholar]
- Yoneda T. Cellular and molecular basis of preferential metastasis of breast cancer to bone. J Orthop Sci 2000; 5: 75–81. [DOI] [PubMed] [Google Scholar]
- Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res 2005; 25: 79–83. [PubMed] [Google Scholar]
- van der Pluijm G, Vloedgraven H, Papapoulos S, Löwick C, Grzesik W, Kerr J et al. Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest 1997; 77: 665–675. [PubMed] [Google Scholar]
- Pécheur I, Peyruchaud O, Serre CM, Guglielmi J, Voland C, Bourre F et al. Integrin alpha(v)beta3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J 2002; 16: 1266–1268. [DOI] [PubMed] [Google Scholar]
- Harms JF, Welch DR, Samant RS, Shevde LA, Miele ME, Babu GR et al. A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MB-435 skeletal metastasis. Clin Exp Metast 2004; 21: 119–128. [DOI] [PubMed] [Google Scholar]
- Mastro AM, Gay CV, Welch DR. The skeleton as a unique environment for breast cancer cells. Clin Exp Metast 2003; 20: 275–284. [DOI] [PubMed] [Google Scholar]
- Woodward JK, Holen I, Coleman RE, Buttle DJ. The roles of proteolytic enzymes in the development of tumour-induced bone disease in breast and prostate cancer. Bone 2007; 41: 912–927. [DOI] [PubMed] [Google Scholar]
- Friedrich RE, Eisenmann J, Röser K, Scheuer HA, Löning T. Expression of proteases in giant cell lesions of the jaws, tendon sheath and salivary glands. Anticancer Res 2010; 30: 1645–1652. [PubMed] [Google Scholar]
- Kim MS, Lee EJ, Kim HR, Moon A. P38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res 2003; 63: 5454–5461. [PubMed] [Google Scholar]
- Dumont B, Castronovo V, Peulen O, Blétard N, Clézardin P, Delvenne P et al. Differential proteomic analysis of a human breast tumor and its matched bone metastasis identifies cell membrane and extracellular proteins associated with bone metastasis. J Proteome Res 2012; 11: 2247–2260. [DOI] [PubMed] [Google Scholar]
- Yao D, Dai C, Peng S. Mechanism of the mesenchymal–epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res 2011; 9: 1608–1620. [DOI] [PubMed] [Google Scholar]
- Gunasinghe NP, Wells A, Thompson EW, Hugo HJ. Mesenchymal–epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metast Rev 2012; 31: 469–478. [DOI] [PubMed] [Google Scholar]
- Waning DL, Guise TA. Molecular mechanisms of bone metastasis and associated muscle weakness. Clin Cancer Res 2014; 20: 3071–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res 2014; 20: 2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 1996; 98: 1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J Am Soc Nephrol 2008; 19: 672–675. [DOI] [PubMed] [Google Scholar]
- Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA et al. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res 1991; 51: 3059–3061. [PubMed] [Google Scholar]
- Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA et al. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 2002; 277: 24571–24578. [DOI] [PubMed] [Google Scholar]
- Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther 2007; 6: 2609–2617. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–319. [DOI] [PubMed] [Google Scholar]
- Singh B, Berry JA, Shoher A, Ayers GD, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene 2007; 26: 3789–3796. [DOI] [PubMed] [Google Scholar]
- Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003; 33: 28–37. [DOI] [PubMed] [Google Scholar]
- Morgan H, Tumber A, Hill PA. Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. Int J Cancer 2004; 109: 653–660. [DOI] [PubMed] [Google Scholar]
- Lin JL, Wang MJ, Lee D, Liang CC, Lin S. Hypoxia-inducible factor-1alpha regulates matrix metalloproteinase-1 activity in human bone marrow-derived mesenchymal stem cells. FEBS Lett 2008; 582: 2615–2619. [DOI] [PubMed] [Google Scholar]
- Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res 2007; 67: 4157–4163. [DOI] [PubMed] [Google Scholar]
- Sterling JA, Oyajobi BO, Grubbs B, Padalecki SS, Munoz SA, Gupta A et al. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer Res 2006; 66: 7548–7553. [DOI] [PubMed] [Google Scholar]
- Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 1999; 103: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 2005; 102: 13909–13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Chen Y, Yu L, Zheng C, Qi Y, Li Z et al. Inhibition of breast cancer metastases by a novel inhibitor of TGFβ receptor 1. J Natl Cancer Inst 2013; 105: 47–58. [DOI] [PubMed] [Google Scholar]
- Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle 2006; 5: 1744–1750. [DOI] [PubMed] [Google Scholar]