Abstract
Microdamage resulting from fatigue or ‘wear and tear' loading contributes to bone fragility; however, the full extent of its influence is not completely understood. Linear microcracks (∼50–100 μm) and diffuse damage (clusters of sublamellar-sized cracks) are the two major bone microdamage types, each with different mechanical and biological consequences. Healthy bone, due to its numerous microstructural interfaces and its ability to affect matrix level repair, deals effectively with microdamage. From a material standpoint, healthy bone behaves much like engineering composites like carbon-fiber reinforced plastics. Both materials allow matrix damage to form during fatigue loading and use microstructural interfaces to dissipate energy and limit microcrack propagation to slow fracture. The terms fracture toughness and 'toughening mechanism', respectively, describe mechanical behavior and microstructural features that prevent crack growth and make it harder to fracture a material. Critically, toughness is independent of strength. In bone, primary toughening features include mineral and collagen interfaces, lamellae and tissue heterogeneity among osteons. The damage tolerance of bone and other composites can be overcome with sustained loading and/or matrix changes such that the microstructure no longer limits microcrack propagation. With reduced remodeling due to aging, disease or remodeling suppression, microdamage accumulation can occur along with loss of tissue heterogeneity. Both contribute additively to reduced fracture toughness. Thus, the answer to the key question for bone fragility of how much microdamage is too much is extremely complex. It ultimately depends on the interplay between matrix damage content, internal repair and effectiveness of matrix-toughening mechanisms.
Introduction
Fragility fractures are a major public health problem in the United States and around the world.
According to the 2002 National Osteoporosis Foundation report 44 million individuals in the United States, over the age of 50 years, are at risk of facture, and this number is predicted to reach 61 million by 2020.1 The cost to society of osteoporosis is comprised of both direct care costs such as acute management and rehabilitation following osteoporosis-related fractures, as well as indirect costs related to poor health.2 Traditionally, bone mineral density was thought to be the primary predictor of fracture risk, but more recently it has become accepted that bone mineral density is not the only consideration in terms of fracture risk.3,4,5 It was first postulated some five decades ago that microdamage accumulation increases bone's fragility.6 Subsequently, it was shown that a relationship did indeed exist between increased fatigue-induced microdamage levels and reduced mechanical properties of bone tissue.7,8,9,10 Bone can be described as a quasi-brittle microcracking composite material that derives at least some of its fracture properties from the formation of discrete microcracks, which absorb energy and prevent catastrophic fracture.10,11,12,13 Specifically, microcracks in bone tend to form relatively frequently; however, subsequent growth or propagation of those cracks is made difficult by various ‘toughening mechanisms'. The term ‘toughening mechanism' is a general description of features within a material that help prevent crack growth by absorbing energy that might otherwise be used for crack propagation. For example, consider a simple plywood structure made up of a number of individual ‘sheets' bonded together. A starter crack between two of the sheets could easily travel along the ‘grain' of such a material if sufficient force was used to pull the adjacent sheets apart. Now imagine it was possible to include tough fibers, with some amount of elasticity that ran perpendicularly across the boundary face of each sheet. These fibers would absorb energy through elastic deformation if any two sheets were pulled apart making it harder to separate the sheets or in other words propagate the crack. In this way, the presence of these fibers could thus be considered a ‘toughening mechanism'. In reality, recent studies have shown that a ‘plywood' lamellar model for bone is too simplistic. There can be arrays (ordered and disordered mineralized fibers) within lamellae and/or twisted plywoods.14 This is just one particular example of this phenomenon; features of a material at the nano/micro/macro level that have this effect can work in the same way.
What is bone microdamage?
There are two common types of microdamage that can result from physiological habitual loading of bone tissue: ‘linear microcracks' and ‘diffuse damage'. Although linear microdamage is the more well known type, diffuse damage, although slightly less well understood, is certainly equally important. These damage morphologies do have certain similarities; however, there are also distinct differences between the two from both a mechanical and a biological perspective.
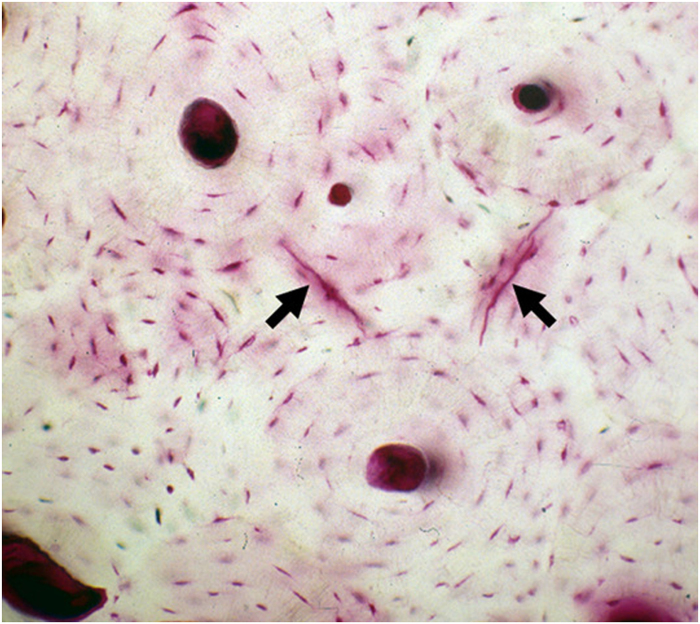
Linear microcracks are sharply defined cracks around 50–100 μm in length, when seen in bone cross-sections. They form under habitual repetitive loading experienced during walking/running (Figure 1). Fatigue is a failure process that was originally characterized in engineering materials, when relatively small loads, well below the failure strength, are applied repetitively and eventually small cracks form and grow. Eventually, failure of a material can occur from the accumulation of fatigue damage. In bone, these microcracks normally go unnoticed clinically in a normal healthy individual as they are repaired; that repair mechanism will be discussed later. However, under certain conditions if damage accumulation exceeds intrinsic repair capacity, cracks can grow incrementally during fatigue and eventually cause failure. This progress of fatigue fracture has been demonstrated in racehorses15 and presumably occurs in human bone as well. The formation/accumulation of microscopic cracks is directly correlated with deterioration of mechanical properties such as stiffness, strength and toughness. The majority of linear microcracks occur in interstitial bone. Interstitial bone is comparatively older than the surrounding tissues and thus has accumulated the greatest amount of loading cycles; this tissue is also likely to have higher levels of non-enzymatic collagen cross-linking,16 and mineralization (and the resulting decrease in water content)17 potentially allows cracking to occur more easily. Furthermore, indirect mechanisms such as reduced mineralizations in surrounding osteons may also have a role in crack initiation by altering the local stress distribution. Microstructural studies reveal that microcracks are significantly longer in the longitudinal axis of the bone than when viewed in the transverse orientation, as is more typical. This is intuitively correct, as in most cases cracks will tend to follow the preferential microstructural grain of the material. Numerous studies, using human vertebrae, tibiae and femora, have shown that the amount of linear microcracks increases substantially with age in both trabecular and cortical bone.8,18,19 Schaffler et al.8 reported an exponential increase in linear microcracks in human femoral compact bone as a function of increasing age. In a study by Courtney et al.20 aged and young bones were subjected to fatigue until yield and similarly found more linear microcracks in elderly bones. Taken together, these data suggest that linear microdamage is an important consideration in the context of bone quality and fracture risk. However, it should be noted that a definitive clinical assessment between damage content and fracture risk has not been established, which is largely due to the fact that current clinical imaging tools do not allow measurement of bone microdamage burden in patients.
Figure 1.
Photomicrograph of cross-section of basic fuchsin-stained human compact bone from a 65-year-old donor. Arrows point linear microcracks that had occurred under physiological loading conditions.
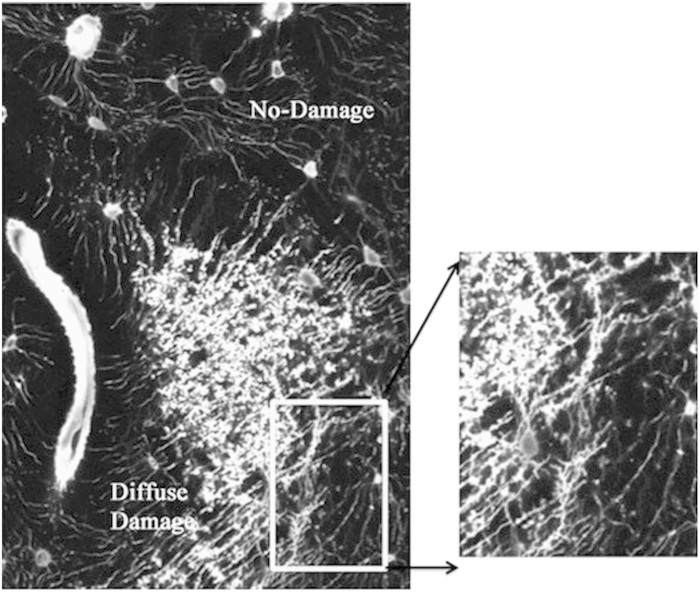
Diffuse damage has a very different set of defining characteristics. It consists of clusters of small sublamellar size cracks (Figure 2). This damage morphology was first identified in fatigue loaded cortical bone samples based on the pooling of basic fuchsin stain, which is the standard method used to visualize microdamage in bone. Subsequent high-resolution studies reveal these were crazing cracks that separate mineral aggregates from each other and from the surrounding organic matrix.9,18,21,22 This diffuse damage was subsequently found in human in cortical and trabecular bone biopsies.18 Interestingly, that study reported that no age-dependent accumulation of diffuse damage among the groups; however, it was noted that diffuse damage was higher in men compared with women, although the age-adjusted fracture rates were 1.6-fold higher in women. It appears that of all the damage types, diffuse damage occurs ‘easiest and earliest' compared with others. Diffuse damage occurs very rapidly after the onset of even modest cyclic loads. Constant loads maintained over long time periods can also induce diffuse damage, through the process that engineers refer to as creep. Furthermore, diffuse damage is formed more readily in tensile regions of bone, whereas typical linear microcracks are found in shear or compression loaded bone areas.23,24 It is also worth noting that diffuse damage does not seem to be the precursor of linear microcracks. Boyce et al. 23 and Diab et al.25 have both demonstrated that diffuse damage and linear microcracks occur at completely separate regions of fatigued bone specimens. Nevertheless, the two types of damage seem to have the same probability distribution,26 with the percentage of donors having a certain amount of damage decreasing exponentially with increasing damage. This type of distribution may be characteristic of remodeling-mediated damage repair mechanisms and suggests that diffuse damage, like linear microcracks, is associated with some form of repair mechanism. Indeed, emerging data suggest that there may also be important biological responses to diffuse damage, which are completely distinct from the response to linear microdamage.27
Figure 2.
Fluorescence photomicrograph showing diffuse damage stained with basic fuchsin, and diffuse damage was created in vivo in a rat model.27 Enlarged image on right shows that diffuse damage comprised many ultrastructural small cracks.
Microdamage, accumulation and bone toughness
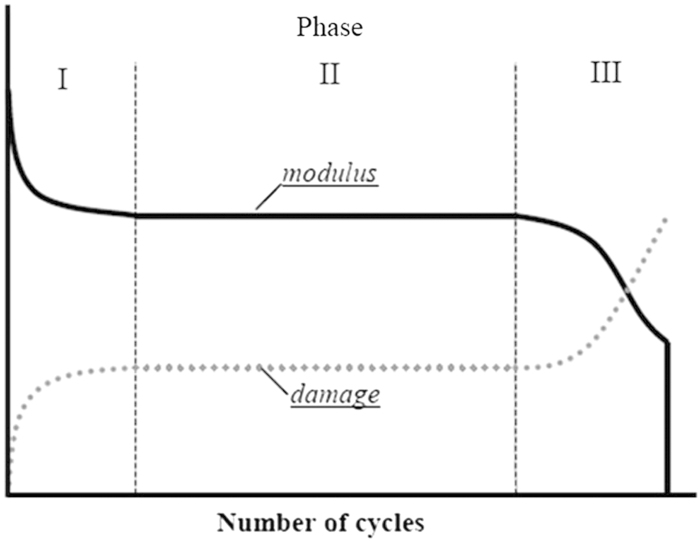
The accumulation of microdamage in fatigue loading is a non-linear process. Figure 3 shows that under cyclical loading conditions microdamage accumulation and modulus changes occur in three distinct phases in bone. In the first phase (I) damage initiates and modulus drops relatively quickly. The second phase (II) is dominated not by further crack initiation but rather by interactions between existing damage and the local microstructure as the toughening mechanisms such as lamellae, osteons and other porosities come into play. Crack growth during the second phase absorbs energy but does not cause much change in the stiffness or strength. In fact most of the useful life span of engineered and biological composite materials (including bone) is in phase II of the fatigue loading history––after the initial damage has occurred. Once again, the design criteria do not prioritize the prevention of crack initiation but rather it makes them ‘damage tolerant,' that is, composite materials allow cracking up to a point and then limit its propagation via microstructural interfaces. Finally in phase III, the amount of damage eventually overwhelms the action of the internal matrix interfaces (that is, toughening mechanisms), the microcracks start to coalescence and the material properties start to decay very rapidly leading to outright failure.
Figure 3.
Representative curves showing that cyclical loading causes modulus loss and microdamage accumulation in three distinct phases.
This model of damage accumulation and mechanical deterioration can be applied directly to bone, where microcracks are easily initiated but difficult to propagate in normal healthy tissue. In fact, we develop microcracks in our bones regularly simply by our daily activities. Schaffler et al.28 first demonstrated that loading at low strain levels readily induces damage in compact bone. More recently, an important study by Burr et al.29 showed that the dog bones develop microdamage with normal routine daily activities.
It has been shown experimentally that microdamage accumulation in fatigue has a paradoxical beneficial role in terms of energy dissipation in addition to its obvious role in ‘weakening' bone. This demonstrates that microdamage is a ‘two-edged sword'. Specifically, when damage occurs it dissipates energy at natural interfaces and prevents the acute traumatic fracture of bone from small cracks. Diffuse damage, because of the large number of interfaces created in the material, appears to be the more effective damage in terms of energy absorption. In cases of aging, disease or certain drug treatments, the impact of these natural interfaces can be reduced or removed, which can decrease the mechanical effectiveness of the material; specific examples of this will be discussed below.
Similar to engineered composites, there are various toughening mechanisms that serve to prevent crack growth. Mineral crystals, collagen fibers, lamellae, lacunae, cement lines and osteons all represent structural interfaces where damage can form and also where energy can be absorbed and microcrack growth can be attenuated or stopped. Furthermore, these structural interfaces appear to be ‘bonded' by non-collagenous proteins that absorb energy during microcracking.30,31,32 Recent data indicate that ostepontin in complex with osteocalcin is critical in this regard.33,34 This complex serves as a ‘glue' layer between the collagen and mineral phases of the tissue; these glue bonds break and reform readily and quickly after the loading and unloading of the bone––that is, they function as sacrificial bonds. An excellent paper by Fratzl and Weinkamer35 reviewed the hierarchical materials in nature extensively.
It is well established in material science that microdamage content (morphology and quantity) compromises the residual (remaining) mechanical properties of a material. Diminished residual properties (properties remaining after damage) in bone after fatigue were first demonstrated by Carter and Hayes and found that the formation of bone microdamage is accompanied by moderate reductions in stiffness and strength.36 In contrast, fatigue damage accumulation has a disproportionately large effect on the fracture toughness of the material, which is reduced in much greater relative amounts compared with stiffness and strength.8,9,13 Studies from our laboratory, and more recently from Lambers et al.,37 provide important insights into the impact of bone microdamage on fracture toughness. In experiments from our laboratory human compact bone samples were fatigued at physiological strains to increasing amounts of damage, as evidenced by stiffness loss degradation (15% and 30%, respectively).38 Linear-type microcracks were observed rarely in specimens at the lower fatigue level (15% modulus loss but were observed routinely at higher levels of fatigue (30% modulus loss). In studies of whole bone fatigue in canine long bones, Burr et al.4 also reported that linear microcracks were not observed until after 15% stiffness loss during whole bone fatigue. In contrast, in fatigue-loaded human bone specimens, patches of diffuse damage of the bone matrix were observed at all fatigue levels.
In this same study, the residual (remaining) properties of human compact bone after fatigue were measured, using samples from the matched contralateral femurs to those used in fatigue experiments described in the preceding paragraph. After completion of fatigue loading, specimens were subjected to a single monotonic test to failure to determine the residual properties of strength, work-to-fracture and postyield displacement (the latter two measures reflect the toughness of the material). Among specimens loaded to the lower level of fatigue (15% modulus loss, which caused principally diffuse damage), the residual bone strength, work-to-fracture and postyield displacement were reduced in approximate proportion to the amount of modulus degradation. In contrast, bone specimens fatigued to the higher fatigue levels (30% modulus loss) showed proportional losses of bone strength (∼30% reduction) but losses in work to fracture and postyield displacements on the order of ∼70% compared with control, non-fatigued bone. Recently, similar findings were reported by Lambers et al.37 in fatigue loaded cancellous bone from human lumbar vertebrae. They predicted that 1.5% damage volume/bone volume creates ∼30% reduction in tissue stiffness and 92% decrease in the fatigue resistance. Together these data demonstrate that accumulation of fatigue damage in bone causes a moderate decrease in bone strength but a disproportionate loss of toughness and the ability of bone to withstand a catastrophic fracture. In a simple analogy, microdamage can cause the bone to act like the material has been internally perforated (like old-fashioned postage stamps), where the presence of such defects markedly lowers the energy needed to fracture the bone.
Microdamage repair mechanisms
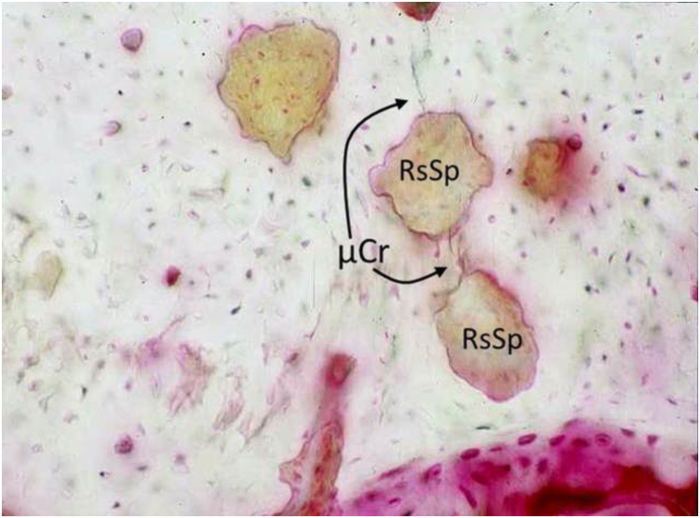
Unlike typical engineering materials, healthy bone tissue has the unique capability of self-repair. When Harold Frost first reported the existence of linear microcracks in human bone he proposed osteonal remodeling as the repair mechanism to remove and replace areas of damaged tissue.6 Since then, the intracortical remodeling response to fatigue-induced microcracks has been the focus of much research. It is now clear that fatigue-induced linear microcracks in bone tissue lead to a reparative remodeling response that is targeted at the damage site and this orchestrates removal and replacement of the damaged tissue (Figure 4). This has been shown in large and small animal models.7,39,40 Burr and coworkers observed that intracortical remodeling events were significantly associated with the presence of microcracks in a canine model.41 Subsequent studies from the same group confirmed that remodeling occurs in specific association with fatigue microdamage.7 Bentolila et al.40 using a rat ulnar fatigue model showed that the number of microcracks was reduced by approximately 40% within 10 days of loading. It is important to understand the operational efficiency of the remodeling repair response in bone, so that deviations from the normal can be assessed. Using a series of mathematical models, Burr and Martin determined the relationship between the factors involved in microdamage-induced targeted remodeling––that is, crack distribution, stress, loading frequency and the remodeling rate.42,43 The models showed strong agreement between theory and the experimental observations and also that removal of cracks by remodeling before excessive extension of the crack is the key in preventing catastrophic failure. Most importantly, they found that normal remodeling is indeed a finely balanced and efficient system. Mashiba et al.44 and Allen et al.45 reported that 40–50% suppression of remodeling from bisphosphonate treatment resulted in three-fold increase in damage burden. These authors have also shown a doubling of microdamage content in bone with a more modest (∼20%) remodeling suppression by raloxifene, suggesting that the degree of remodeling suppression of microdamage repair is the primary concern, not the specific pharmacological agent.46 Although these seminal experimental confirmations of these predictions of microdamage accumulation with remodeling suppression were conducted in healthy young adult beagle dogs receiving bisphosphonates or raloxifene, recent data from human iliac crest biopsies of treatment-naive and bisphosphonate-treated patients show similar increases in microdamage after long-term bisphosphonate therapy.47
Figure 4.
Experimentally induced microcracks (μCr, arrows) in cortical bone shown in association with newly activated intracortical resorption spaces (RsSp) at 10 days after fatigue loading of rat ulna in vivo (Photomicrograph field width 560 μm.) (Figure adapted from Bentolila et al.40 by permission.)
Recent studies have revealed much about the mechanisms by which bone remodeling is targeted to microdamage. We demonstrated that fatigue-induced microcracks cause osteocyte apoptosis in the area at microdamage sites,48,49 and this apoptosis causes the subsequent osteoclastic response.50 Kennedy et al. revealed that this osteocyte apoptosis triggers RANKL (receptor activator of nuclear factor-κB ligand) from surviving osteocytes immediately surrounding dying osteocytes.49,51,52 Furthermore, Rumpler et al.53 showed that matrix damage in devitalized bone in vitro did not stimulate or target osteoclastic activity, thus cytokines potentially released from the matrix by microdamage are not osteoclast targeting signals. Thus, activation and targeting of bone remodeling by microdamage in vivo requires a combination of localized osteocyte apoptosis and osteoclastogenic signaling from the surviving ‘bystander' osteocytes.
Few studies in the literature have specifically examined the fate of diffuse damage in living bone. Whether there are any long-term effects on the mechanical and/or biology function of the tissue is also unknown––indeed, it has been unclear whether there is any reaction at all within the bone to these sublamellar small cracks. In 2010, Herman et al.54 showed that diffuse damage in the rat loading modeling did not adversely affect the local osteocyte population, that is, did not cause apoptosis and also did not evoke intracortical remodeling. This raises the intriguing question of what happens to this type of damage during life. Using a similar model, diffuse damage was selectively introduced into the rat ulna and its natural history was assessed.27 The authors found that both mechanically and structurally this damage diminished over time providing the first direct experimental evidence for a self-healing (non-remodeling dependent) of sub-lamellar level cracking in bone. The underlying mechanism of this self-healing remains obscure at this time, but it seems reasonable to presume it involves the extensive osteocyte network that is intimately associated with regions of diffuse damage. Thus, it appears that the body has unique capabilities to deal with this most frequently created type of microdamage that does not involve traditional bone remodeling.
Is microdamage good or bad?—not the right question
The intertwined mechanical and biological effects of microdamage on bone tissue are complex. In one sense, its presence potentially weakens the bone and reduces fracture resistance. Contrastingly, microdamage can be an effective way of dissipating energy in composite materials. This is not always the case some materials with more homogeneous microstructure are designed to prevent crack initiation, rather than managing propagation, as without heterogeneity and internal interfaces a single crack could propagate to failure. However, as discussed earlier in this report, composite materials, including bone, are designed to tolerate small fatigue cracks to be damage tolerant. Consequently, rather than asking whether microdamage is ‘good or bad', perhaps the more relevant question for microdamage and bone fragility is how much damage can bone accommodate?
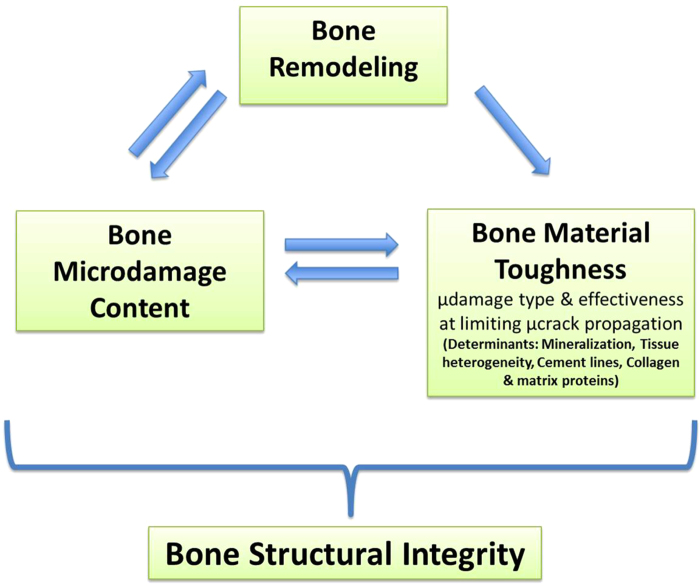
The answer to that question depends on the level of damage accumulation, the condition of the bone material and the status of remodeling in the system. In young healthy bone, the remodeling is operating well and tissue heterogeneity (mineralization differences, cement lines, differentiated lamellae) is at optimal levels. Thus such healthy bone can (i) sustain microdamage, (ii) limit microdamage propagation within its microstructure and (iii) evoke appropriate remodeling response to remove the damage (Figure 5). In contrast, older bone where remodeling has been suppressed can allow microdamage to accumulate and the effectiveness of tissue heterogeneity to limit microcrack propagation is compromised, this makes the material both more damage prone and less damage tolerant such that it will be easier to propagate a failure crack. In terms of reduced heterogeneity, studies by Boivin et al.55 and Roschger et al.56 showed that there were higher levels of mean tissue mineralization and as well as mineral homogeneity in both cortical and trabecular bone of the iliac crest from remodeling suppressed (alendronate-treated) patients after 2–3 years. Furthermore, Boskey and coworkers reported a significant loss in bone heterogeneity when remodeling is suppressed by bisphosphonate treatment and this loss in heterogeneity is highly correlated with reductions in toughness.57 In terms of linking this concept with a clinical phenomenon, Donnelly et al.58 used microspectroscopic approaches to demonstrate that reduced tissue heterogeneity was a fundamental characteristic of bone samples from patients with atypical femoral fractures.
Figure 5.
Schematic showing the mutually dependent relationship between bone remodeling, microdamage context and intrinsic bone material properties (especially fracture toughness). Each of which must be considered in the context of global fracture risk.
Microdamage and compromised remodeling
It is clear that in situations where the remodeling response is functional, and adequate tissue heterogeneity is present, bone tissue can deal with microdamage without displaying any clinically detectable symptoms. However, when one or more of these variables change, the mechanical integrity of the tissue can quickly be compromised. This is particularly true when damage accumulation is allowed to occur and fracture toughness is reduced. The bisphosphonate-treated dog studies by Burr and co-workers mentioned above provide great insight into this situation. One year of bisphosphonate-related remodeling suppression, without any increased activity or loading, had a significant effect on the amount of microdamage and by extension the mechanical properties of the tissue. Although some of the dosages used in those studies were relatively high, a plateau effect had previously been demonstrated in terms of microdamage accumulations so higher dosages do not necessarily translate into more microdamage.
Early theoretical predictions of how microdamage was related to remodeling were shown to be in good agreement with the evidence presented from the bisphosphonate-treated animal models. As bisphosphonates are in common use, it would be expected that some similar clinical manifestation would eventually arise. Atypical femoral fractures are pathological fracture of the subtrochanteric region, with a transverse or short oblique fracture pattern and lack of comminution.38 Atypical femoral fractures have been predominantly reported in patients taking bisphosphonates, although the relative contribution of remodeling suppression versus intrinsic material differences remains obscure. Furthermore, these fractures appear to fit the description of a brittle bone failure and seem to fit with the predictions that have been made in terms of targeted remodeling of bone microdamage––that is, absence of remodeling will cause microdamage to build up and material properties to decline. Accordingly, any pathology or treatment, which inhibits remodeling, regardless of mechanism, will likely have some impact on the material and mechanical properties of the tissue.59,60
In conclusion, it is clear that microscopic damage at various length scales in bone tissue is a crucial consideration in relation to its mechanical properties and to its biological homeostasis. The accumulation of cracks in normal healthy bone can be readily dealt with from a mechanical perspective by the microstructural toughening mechanisms. In addition, linear microdamage is dealt with biologically by the osteoclastic remodeling response, whereas the direct repair mechanism of diffuse damage remains unknown at this time. Under conditions of altered remodeling, resulting from aging, disease or drug treatment, fragility and fracture risk are increased markedly. Extrapolation from the well-established consequences of microdamage in composite materials and bone in laboratory studies suggest that microdamage accumulation should contribute significantly to impaired tissue fracture resistance.
Acknowledgments
Supported by grants AR041210, AR057139 and AR060445 from the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the NIH.
References
- Osteoporosis in the workplace: the social, economic and human costs of osteoporosis on employees, employers and governments. Prepared by the World Health Organization Collaborating Center, Liege, Belgium on behalf of the IOF Committee of Scientific Advisors. Available at http://www.iofbonehealth.org, 2002.
- Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep 2010; 12: 186–191. [DOI] [PubMed] [Google Scholar]
- Vashishth D. Rising crack-growth-resistance behavior in cortical bone: implications for toughness measurements. J Biomech 2004; 37: 943–946. [DOI] [PubMed] [Google Scholar]
- Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res 1997; 12: 6–15. [DOI] [PubMed] [Google Scholar]
- Hui SL, Slemenda CW, Johnston CC Jr. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988; 81: 1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost HM. Presence of microscopic cracks in vivo in bone. Bull Henry Ford Hospital 1960; 8: 27–35. [Google Scholar]
- Mori S, Burr DB. Increased intracortical remodeling following fatigue damage. Bone 1993; 14: 103–109. [DOI] [PubMed] [Google Scholar]
- Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone 1995; 17: 521–525. [DOI] [PubMed] [Google Scholar]
- Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS et al. Does microdamage accumulation affect the mechanical properties of bone? J Biomech 1998; 31: 337–345. [DOI] [PubMed] [Google Scholar]
- Vashishth D, Behiri JC, Bonfield W. Crack growth resistance in cortical bone: concept of microcrack toughening. J Biomech 1997; 30: 763–769. [DOI] [PubMed] [Google Scholar]
- Zioupos P, Currey JD. The extent of microcracking and the morphology of microcracks in damaged bone. J Mater Sci 1994; 29: 978–986. [Google Scholar]
- Jepsen KJ, Krzpow DJ, Dutta Roy T, Pizzuto T (eds). Damage accumulation during tensile yielding of human cortical bone. Transactions of the Orthopaedic Research Society 1999, Pascal Communications, Inc: Anaheim, CA, USA. [Google Scholar]
- Schaffler MB. Role of bone turnover in microdamage. Osteoporos Int 2003; 14: S73–S77 discussion S7–S80 . [DOI] [PubMed] [Google Scholar]
- Reznikov N, Shahar R, Weiner S. Three-dimensional structure of human lamellar bone: the presence of two different materials and new insights into the hierarchical organization. Bone 2014; 59: 93–104. [DOI] [PubMed] [Google Scholar]
- Martin RB, Stover SM, Gibson VA, Gibeling JC, Griffin LV. In vitro fatigue behavior of the equine third metacarpus: remodeling and microcrack damage analysis. J Orthop Res 1996; 14: 794–801. [DOI] [PubMed] [Google Scholar]
- Sroga GE, Karim L, Colon W, Vashishth D. Biochemical characterization of major bone-matrix proteins using nanoscale-size bone samples and proteomics methodology. Mol Cell Proteomics 2011; 10: M110 006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Burr DB, Sharkey NA. Skeletal Tissue Mechanics Springer-Verlag: New York, NY, USA, 1998, . [Google Scholar]
- Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, Schaffler MB et al. In vivo diffuse damage in human vertebral trabecular bone. Bone 2000; 26: 147–152. [DOI] [PubMed] [Google Scholar]
- Kruzic JJ, Ritchie RO. Comments on "Measurement of the microstructural fracture toughness of cortical bone using indentation fracture". J Biomech 2008; 41: 1379–1380. [DOI] [PubMed] [Google Scholar]
- Courtney AC, Hayes WC, Gibson LJ. Age-related differences in post-yield damage in human cortical bone. Experiment and model. J Biomech 1996; 29: 1463–1471. [DOI] [PubMed] [Google Scholar]
- Yeni YN, Hou FJ, Ciarelli T, Vashishth D, Fyhrie DP. Trabecular shear stresses predict in vivo linear microcrack density but not diffuse damage in human vertebral cancellous bone. Ann Biomed Eng 2003; 31: 726–732. [DOI] [PubMed] [Google Scholar]
- Fazzalari NL, Forwood MR, Manthey BA, Smith K, Kolesik P. Three-dimensional confocal images of microdamage in cancellous bone. Bone 1998; 23: 373–378. [DOI] [PubMed] [Google Scholar]
- Boyce TM, Fyhrie DP, Glotkowski MC, Radin EL, Schaffler MB. Damage type and strain mode associations in human compact bone bending fatigue. J Orthop Res 1998; 16: 322–329. [DOI] [PubMed] [Google Scholar]
- Reilly GC, Currey JD. The development of microcracking and failure in bone depends on the loading mode to which it is adapted. J Exp Biol 1999; 202: 543–552. [DOI] [PubMed] [Google Scholar]
- Diab T, Condon KW, Burr DB, Vashishth D. Age-related change in the damage morphology of human cortical bone and its role in bone fragility. Bone 2006; 38: 427–431. [DOI] [PubMed] [Google Scholar]
- Wenzel TE, Schaffler MB, Fyhrie DP. In vivo trabecular microcracks in human vertebral bone. Bone 1996; 19: 89–95. [DOI] [PubMed] [Google Scholar]
- Seref-Ferlengez Z, Basta-Pljakic J, Kennedy OD, Philemon CJ, Schaffler MB. Structural and mechanical repair of diffuse damage in cortical bone in vivo. J Bone Miner Res 2014; 29: 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler MB, Radin EL, Burr DB. Mechanical and morphological effects of strain rate on fatigue of compact bone. Bone 1989; 10: 207–214. [DOI] [PubMed] [Google Scholar]
- Burr DB, Diab T, Koivunemi A, Koivunemi M, Allen MR. Effects of 1 to 3 years' treatment with alendronate on mechanical properties of the femoral shaft in a canine model: implications for subtrochanteric femoral fracture risk. J Orthop Res 2009; 27: 1288–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma PK, Fantner GE, Kindt JH, Thurner PJ, Schitter G, Turner PJ et al. Sacrificial bonds in the interfibrillar matrix of bone. J Musculoskelet Neuronal Interact 2005; 5: 313–315. [PubMed] [Google Scholar]
- Yeni YN, Kim DG, Dong XN, Turner AS, Les CM, Fyhrie DP. Do sacrificial bonds affect the viscoelastic and fracture properties of bone? Clin Orthop Relat Res 2006; 443: 101–108. [DOI] [PubMed] [Google Scholar]
- Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater 2005; 4: 612–616. [DOI] [PubMed] [Google Scholar]
- Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM et al. Dilatational band formation in bone. Proc Natl Acad Sci USA 2012; 109: 19178–19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantner GE, Adams J, Turner P, Thurner PJ, Fisher LW, Hansma PK. Nanoscale ion mediated networks in bone: osteopontin can repeatedly dissipate large amounts of energy. Nano Lett 2007; 7: 2491–2498. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Weinkamer R. Nature's hierarchical materials. Prog Mater Sci 2007; 52: 1263–1334. [Google Scholar]
- Carter DR, Hayes WC. Compact bone fatigue damage--I. Residual strength and stiffness. J Biomech 1977; 10: 325–337. [DOI] [PubMed] [Google Scholar]
- Lambers FM, Bouman AR, Rimnac CM, Hernandez CJ. Microdamage caused by fatigue loading in human cancellous bone: relationship to reductions in bone biomechanical performance. PLoS ONE 2013; 8: e83662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler MB. Bone fatigue and remodeling in the development of stress fractures. In: Burr DB (ed). Stress Fracture CRC Press: Boca Raton, FL, USA, 2001, p 161–182. [Google Scholar]
- Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech 1985; 18: 189–200. [DOI] [PubMed] [Google Scholar]
- Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone 1998; 23: 275–281. [DOI] [PubMed] [Google Scholar]
- Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985; 18: 189–200. [DOI] [PubMed] [Google Scholar]
- Martin B. A theory of fatigue damage accumulation and repair in cortical bone. J Orthop Res 1992; 10: 818–825. [DOI] [PubMed] [Google Scholar]
- Burr DB, Martin RB. Calculating the probability that microcracks initiate resorption spaces. J Biomech 1993; 26: 613–616. [DOI] [PubMed] [Google Scholar]
- Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 2000; 15: 613–620. [DOI] [PubMed] [Google Scholar]
- Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone 2006; 39: 872–879. [DOI] [PubMed] [Google Scholar]
- Allen MR, Iwata K, Sato M, Burr DB. Raloxifene enhances vertebral mechanical properties independent of bone density. Bone 2006; 39: 1130–1135. [DOI] [PubMed] [Google Scholar]
- Stepan JJ, Burr DB, Pavo I, Sipos A, Michalska D, Li J et al. Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone 2007; 41: 378–385. [DOI] [PubMed] [Google Scholar]
- Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 2000; 15: 60–67. [DOI] [PubMed] [Google Scholar]
- Verborgt O, Tatton NA, Majeska RJ, Schaffler MB. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res 2002; 17: 907–914. [DOI] [PubMed] [Google Scholar]
- Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res 2009; 24: 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 2012; 50: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 2014; 64: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpler M, Wurger T, Roschger P, Zwettler E, Peterlik H, Fratzl P et al. Microcracks and osteoclast resorption activity in vitro. Calcif Tissue Int 2012; 90: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone 2010; 47: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 2000; 27: 687–694. [DOI] [PubMed] [Google Scholar]
- Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone 2001; 29: 185–191. [DOI] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone 2010; 46: 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly E, Meredith DS, Nguyen JT, Gladnick BP, Rebolledo BJ, Shaffer AD et al. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res 2012; 27: 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010; 25: 2267–2294. [DOI] [PubMed] [Google Scholar]
- Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29: 1–23. [DOI] [PubMed] [Google Scholar]