Abstract
Coronary artery disease is a common occurrence in human, and causes enormous social cost. Poncirus fructus (PF), the dried immature fruits of Poncirus trifoliata Rafinesquem, is used in the treatment of womb contraction and dyspepsia, as a prokinetic, and in improving blood circulation. This study was performed to investigate the effects of PF and some of its flavonoids components on the coronary from the pig. The arterial ring was suspended by a pair of stainless steel stirrups in an organ bath. The end of the upper stirrup was connected to an isometric force transducer. A dose-dependent induction of relaxation was observed by both water and 70% ethanol extracts of PF in the porcine coronary artery precontracted with U46619 (100 nM), a stable analogue of the potent vasoconstrictor thromboxane A2. The 70% ethanol extract showed more efficacy than the water extract. Pretreatment of the artery with L-NAME (100 µM), a nitric oxide synthase inhibitor, resulted in a significant reduction in the relaxation induced by PF extract. In addition, ODQ (10 µM), a soluble guanylate cyclase inhibitor, also significantly reduced the effects of PF extracts. Hesperidin, a flavonoid present in PF, induced very weak relaxation of the porcine coronary artery at a high concentration (100 µM), while its aglycone, hesperetin, demonstrated a dose-dependent relaxation. In conclusion, PF extracts induced relaxation in the porcine coronary artery, partially through the nitric oxide-cGMP pathway, and the aglycones of flavonoids might be also involved in the relaxation of the same artery.
Keywords: Porcine coronary artery, Poncirus fructus, hesperidin, hesperetin
Coronary arteries supply oxygen-rich blood to the heart muscles (myocardium). Limited arterial function results in coronary artery disease (CAD). The incidence of CAD is increasing, with many people being afflicted with chronic infections. The results of a study conducted on 1,000 participants in North America reveal that the average incidence rate of coronary artery disease (CAD) is 12.5 in Caucasian men and 10.6 in African-American men [1]. In addition, according to the heart disease and stroke statistics of the American Heart Association, 770,000 Americans suffered from first-time coronary attacks while 430,000 suffered from a repeat attack during 2008. Every year, 190,000 people were newly afflicted with additional silent acute myocardial infarction [2].
Poncirus fructus (PF) is the dried, immature fruit of the Poncirus trifoliata Rafinesque. PF grows naturally in the Jeju Island or the Gaduk Island in Korea, and is cultivated in a village in the southern boundaries of the central region. Traditionally, it is used as a prokinetic, and to treat womb contraction, dyspepsia, and improve blood circulation [3]. PF is believed to possess many functional properties: anti-inflammatory [4], anti-allergenic [5], lipid-lowering activity [6,7], inhibition of melanogenesis [8], and suppression of body weight gain [9]. A recent study has reported that the lipid-soluble fraction of the PF extract inhibits small intestinal motility, whereas the water extract fraction caused an increase in the small intestine activity in vitro and in the distal colon activity in vivo in guinea pigs [10]. In addition, it has been reported that the water extract of the PF accelerates the transport of intestinal contents [11], while the hexane extract stimulates rat distal colon [12]. The extracts of PF have been shown to induce relaxation in rat gastric fundus [13]. However, the effects of Poncirus fructus on the arterial vessel have not yet been elucidated.
Flavonoids are secondary metabolites in plants which express diverse bioactivities. Hesperidin, neohesperidin, naringin, and poncirin are the main component flavonoids of the PF [14]. Many flavonoids are known to exert a relaxing effect on the arterial smooth muscle [15,16,17,18,19,20]. Hesperidin has been reported to produce an antihypertensive effect in spontaneously hypertensive rats (SHR). However, the effects of these flavonoids, which are biocomponents of PF, on the coronary artery have been scarcely reported.
The major aim of this study was to investigate the possible relaxing effects of PF on porcine coronary artery. In addition, we attempted to determine if the flavonoids contained in PF [21,22] were responsible for this relaxing action.
Materials and Methods
Materials
PF was purchased from an oriental drug store in Yangnyeong market (Daegu, Korea). A sample (100 g) of PF was boiled in 1 L of distilled water at 100℃ for 2 hours. The water extract was filtered and concentrated in vacuo. Finally, the powder was obtained by lyophilization. The 70% ethanol extract was also obtained using the same method.
Three flavonoids that are abundant in PF were used in this study: poncirin, hesperetin, and hesperidin. Hesperetin is an aglycone of hesperidin. All flavonoids were purchased from Sigma-Aldrich (St. Louis, MO, USA).
In order to determine the mechanism of PF action, the following compounds were used: 9,11-dideoxy-9a,11a-methanoepoxy-prostaglandin F2a (U46619, Cayman, Ann Arbor, MI, USA), a stable analog of thromboxane A2; Nitro-L-arginine methyl ester (L-NAME; Sigma-Aldrich), a nitric oxide synthase inhibitor; and 1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; Calbiochem-Novabiochem, La jolla, CA, USA), a soluble guanylate cyclase inhibitor. ODQ was prepared in dimethyl sulfoxide, while the other compounds were diluted in distilled water. In order to obtain the desired concentrations, physiological salt solutions (PSS) were used.
Animals and tissue preparation
The hearts were obtained from twenty-four landrace pigs (3 months) in the local abattoir and transported to the laboratory in cool oxygenated Krebs solution (physiololical salt solutions; PSS) within 30 minutes. The Krebs solution was prepared with NaCl (118 g), KCl (4.7 g), MgCl2 (1.2 g), CaCl2 (2.5 g), KH2PO4 (1.2 g), NaHCO3 (25.0 g), and glucose (10.0 g). The distal portion of the left anterior descending coronary artery (outer diameter, 1-2 mm) was dissected from the heart in PSS. The fat and connective tissues were removed from the coronary artery under the dissecting microscope. The coronary artery was cut into rings (5 mm length). Care was taken to preserve the endothelium (no scratches). The Institutional Animal Care and Use Committee of Kyungpook National University approved the protocols for the animal study (KNU 2012-8).
Measurement of isometric tension
The prepared tissue was suspended by two stainless steel stirrups inan organ bath (volume 10mL) maintained at 37℃. The solution was aerated with 95% O2 and 5% CO2. The end of the upper stirrup was connected to the transducer (FT-03; Grass-Telefactor, West Warwick, RI, USA). The transducer signal was processed using the Powerlab data acquisition software supplemented with Labchart (v.7.0, ADInstruments, Castlehill, Australia). In order to measure the relaxation ability, PF extracts and other drugs were administered after the contraction reached a plateau level when supplemented with U46619 (100 nM), a stable analogue of thromboxane A2, a potent vasoconstrictor.
Statistical analysis
All values are expressed as mean±standard error of mean. The statistical significance was determined using the Student's t-test. A P-value <0.05 was considered to have statistical significance.
Results
Effect of the PF water and 70% ethanol extracts on porcine coronary artery
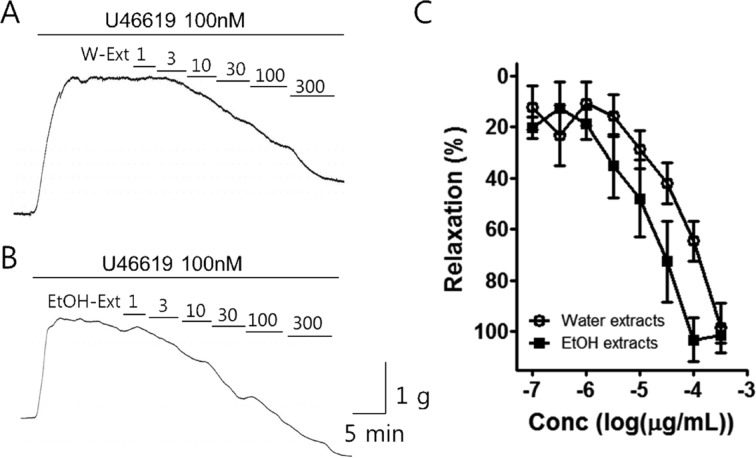
The porcine coronary artery was contracted by U46619 (100 nM). When this reached a plateau, the water and 70% ethanol extracts were cumulatively administered into the bath (1-300 µg/mL) (Figure 1). The percentage of relaxation effected by the water extract on the porcine coronary artery was determined to be 13.02±7.97, 16.82±7.18, 30.55±7.77, 42.14±7.80, 66.94±8.11, and 98.57±9.84 at doses 1, 3, 10, 30, 100, and 300 µg/mL, respectively. The percentage of relaxation effected by the 70% ethanol extract on the porcine coronary artery was 18.93±5.75, 35.31±12.47, 47.74±13.0, 72.31±15.10, 98.64±8.82, and 101.33±3.53 at doses 1, 3, 10, 30, 100, and 300 µg/mL, respectively. The potency of the 70% ethanol extract was greater than that of the water extract.
Figure 1. Effect of Poncirus fructus water and 70% ethanol extracts on porcine coronary artery. The PF (A) water and (B) 70% ethanol extract induced dose-dependent relaxation in the porcine coronary artery. (C) The 70% ethanol extract was show to be more potent than the water extract (n=8).
Effects of L-NAME on the effect of PF water extract on porcine coronary artery
The water extract of PF (100 µg/mL) was observed to induce relaxation in the porcine coronary artery. When L-NAME (100 µM), a nitric oxide synthase inhibitor, was administered to this experimental setup, was observed a significant reduction in the relaxation state of the artery (Figure 2). The percentage of relaxation effected by the PF water extract 100 µg/mL on the porcine coronary artery was determined to be 52.79±7.34. The administration of L-NAME to the experimental setup resulted in a decrease in the percentage of relaxation of water extract (100 µg/mL)-treated porcine coronary artery to 21.03±5.92.
Figure 2. Effect of L-NAME on the relaxation activity of PF water extract on porcine coronary artery. Raw tracings expressing the effect of PF water extract on porcine coronary artery in the (A) absence or (B) presence of L-NAME, a nitric oxide synthase inhibitor. (C) In the presence of L-NAME, the effect of the water extract was significantly reduced. Mean±SEM, *P<0.05, n=5.
Effects of L-NAME on the activity of PF ethanol extract on porcine coronary artery
Administration of the PF ethanol extract (100 µg/mL) induced relaxation in the porcine coronary artery. L-NAME (100 µM), a nitric oxide synthase inhibitor, caused a significant reduction in the PF ethanol extract-induced relaxation (Figure 3). The percentage of relaxation induced by PF ethanol extract (100 µg/mL) on the porcine coronary artery before and after administration of L-NAME was 101.86±17.28 and 58.59±21.93, respectively.
Figure 3. Effect of L-NAME on the relaxation activity of PF ethanol extract on porcine coronary artery. Raw tracings displaying the effects PF of ethanol extract on porcine coronary artery in the (A) absence or (B) presence of L-NAME. (C) L-NAME significantly inhibited the effect of the 70% ethanol extract. Mean±SEM, *P<0.05, n=5.
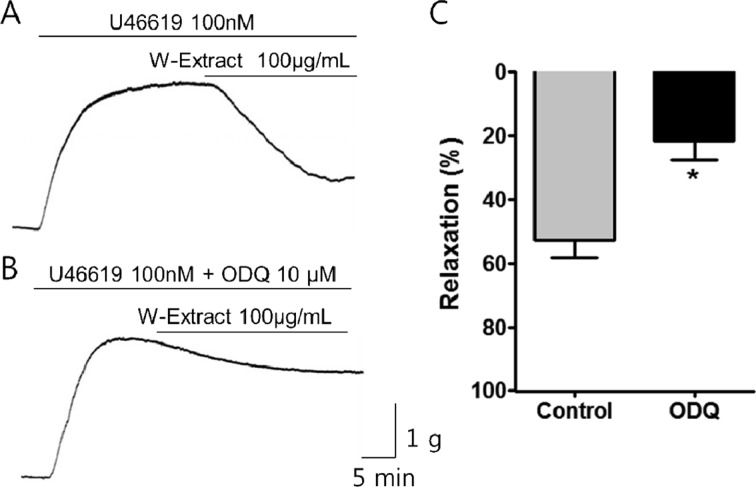
Effects of ODQ on PF water extract activity on porcine coronary artery
The administration of ODQ (10 µM), a guanylate cyclase inhibitor, induced a significant reduction in the PF water extract-induced relaxation of porcine artery (Figure 4). The percentage of relaxation in porcine coronary artery subjected to PF water extract (100 µg/mL) before and after ODQ administration was determined to be 55.79±7.34 and 22.49±6.30, respectively.
Figure 4. Effect of ODQ on the relaxation activity of PF water extract on porcine coronary artery. Raw tracings showing the effects of PF water extract on porcine coronary artery in the (A) absence or (B) presence of ODQ, a guanylate cyclase inhibitor. (C) In the presence of ODQ, the effect of the water extract was observed to be significantly reduced. Mean±SEM, *P<0.05, n=5.
Effect of ODQ administration on PF ethanol extract-treated porcine coronary artery
The administration of ODQ (10 µM) resulted in a significant reduction in the PF ethanol extract-treated porcine artery (Figure 5). The percentage of relaxation seen in porcine artery treated with PF ethanol extract (100 µg/mL) before and after ODQ administration was 105.86±17.28 and 59.25±19.69, respectively.
Figure 5. Effect of ODQ on the relaxation activity of the PF ethanol extract on porcine coronary artery. Raw tracings demonstrating the effects of PF ethanol extract on porcine coronary artery in the (A) absence or (B) presence of ODQ. (C) In the presence of ODQ, the effect of the 70% ethanol extract was observed to be significantly reduced. Mean±SEM, *P<0.05, n=5.
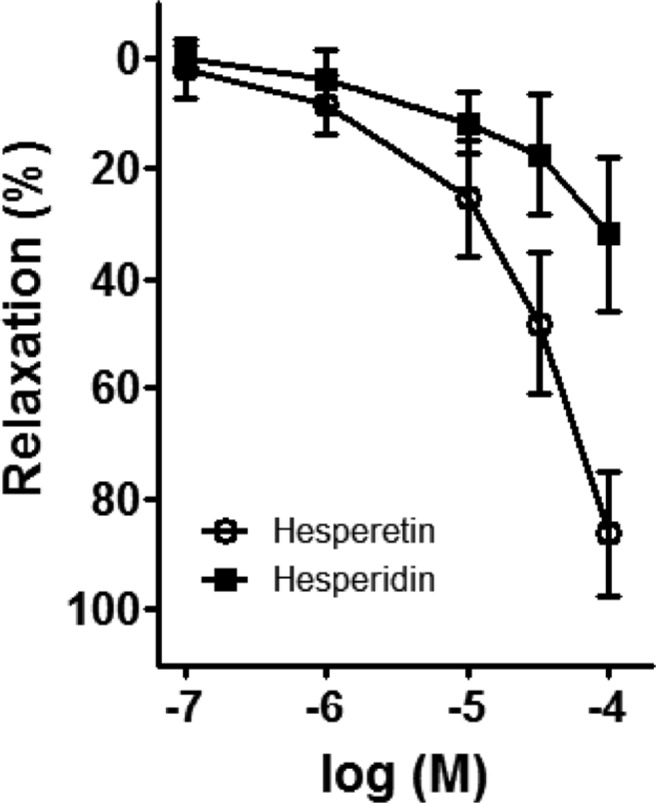
Effect of hesperidin and hesperetin on porcine coronary artery
Hesperidin and hesperetin are known flavonoids present in PF. These were administered into the bath via a cumulative method (0.1-100 µg/mL) (Figure 6). The percentage of relaxation effected by hesperidin on the porcine coronary artery was determined to be 3.08±1.00, 5.46±1.35, 9.74±6.28, 14.00±3.18, 18.81±4.62, and 30.64±7.35, at doses of 0.1, 1, 3, 10, 30, and 100 µM, respectively. The percentage of relaxation of the procine coronary artery induced by hesperetin at doses of 0.1, 1, 3, 10, 30, and 100 µM was determined to be 8.34±1.98, 13.86±3.13, 17.69±9.53, 25.91±13.62, 49.03±13.19, and 82.67±14.77, respectively. The potency of hesperetin was determined to be greater than that of hesperidin.
Figure 6. Effects of hesperidin and hesperetin on porcine coronary artery. The relaxation potency of hesperetin, an aglycone of hesperidin, was greater than that of hesperidin in the porcine coronary artery. Mean±SE, n=5.
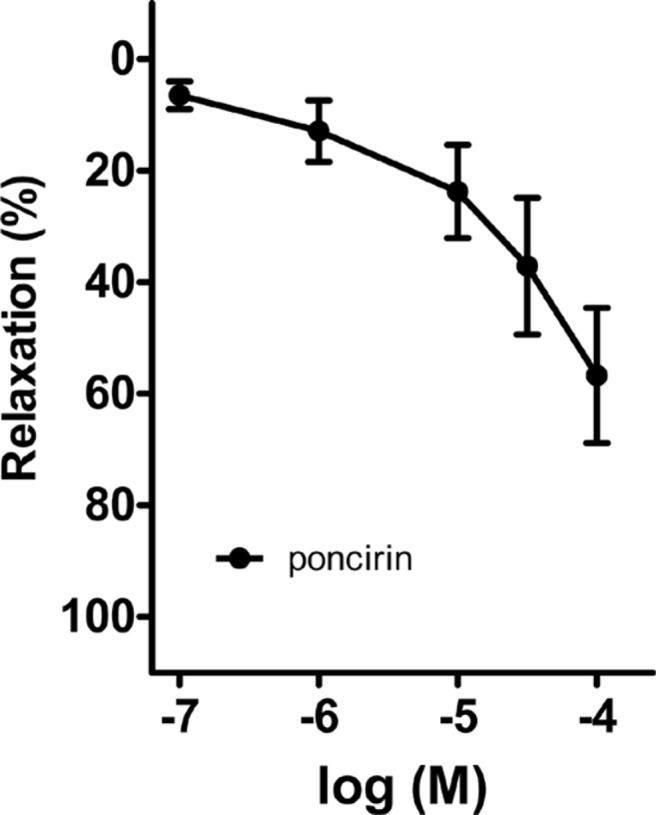
Effect of poncirin on the porcine coronary artery
Poncirin, a flavonoid present in PF, was administered into the bath in a cumulative method (0.1-100 µg/mL) (Figure 7). The percentage of relaxation effected by poncirin at doses of 0.1, 1, 10, 30, and 100 µM, on the porcine coronary artery was determined to be 7.50±2.42, 18.59±5.56, 26.98±8.67, 41.86±12.46, and 57.70±12.02, respectively.
Figure 7. Effect of poncirin on porcine coronary artery. Poncirin-induced relaxation in the porcine coronary artery was pre-contracted by U46619, in a dose-dependent manner. Mean±SE, n=5.
Discussion
Coronary artery is composed of two main branches, the left and right coronary artery. Both vessels rest in the epicardial fat. The left and right coronary arteries are branched into four principal sections: the right coronary artery, left main coronary artery, left anterior descending artery, and left circumflex artery [23]. The vessels supply oxygen-rich blood to the heart muscles; therefore, inappropriate dilation in these vessels can result in angina pectoris or myocardial infarction [24]. Treatment of unstable angina pectoris with nitrates results in improved exercise tolerance, delaying the time to onset of angina [25].
Polyphenols include flavonoids and tannins. Flavonoids are plant pigments which consist of two aromatic rings and an oxygen heterocycle. According to the configuration and state oxidation of the central heterocycle unit, the flavonoids are classed into eight different types: flavones, flavonols, flavonones, chalcones, isoflavones, anthocyanidins, aurones, biflavones, and neoflavonoids. Hesperetin, which is abundantly found in PF, belong to the flavonone group [26].
In recent times, flavonoids have been investigated in the treatment of ischaemic stroke and in reducing chronic vascular disease (CVD) mortality [26,27,28]. Flavonoids are known to possess cardio-protective effects [27,28]. Several studies have demonstrated the effect of dietary flavonoids and polyphenols on human health and disease. In the cardiovascular system, flavonoids influence the endogenous regulation of cholesterol synthesis and are known to scavenge free radicals. They have also been discovered to promote endothelial-derived relaxation factor-induced relaxation in rabbit arteries in vitro [29].
In this study, both the water extract and 70% ethanol extract of PF was observed to induce relaxation in the porcine left descending coronary artery in a dose-dependent manner. The half-maximal inhibitory concentration (IC50) of 70% ethanol extract was approximately 10 µg/mL, and that of water extract was approximately 60 µg/mL. The 70% ethanol extract demonstrated approximately six times greater efficacy than the water extract. This difference between the activities of 70% ethanol and water extract has not been previously reported.
In order to identify the mechanism by which the PF extract induces relaxation, the nitric oxide-cyclic GMP pathway, which was representative of the relaxation mechanism in blood vessels, was tested. The nitric oxide synthase induced nitric oxide synthesis via L-arginine in endothelial cells. The nitric oxide, which migrates into the smooth muscle cell, was observed to bind to and activate guanylyl cyclase. This enzyme catalyzes the dephosphorylation of guanosine triphosphate to cyclic guanosine monophosphate, which relaxes the smooth muscle of the blood vessels.
In this study, the relaxation of porcine artery induced by PF extract was observed to be attenuated by L-NAME and ODQ. Both chemicals were observed to suppress the water and 70% ethanol extract-induced relaxation of coronary arteries. However, the relaxation was not completely suppressed, and was only approximately 50% compared to that effected in the control. These results suggested that the relaxing effect of PF extract was at least partially mediated by the nitric oxide-cGMP pathway. However, the endothelium-dependent mechanism of blood vessel relaxation, regulated by the endothelium-derived hyperpolarizing factor [30,31], could not be completely dismissed. It has been reported that the vasorelaxation effected by dietary flavonoids was mediated by the nitric oxide-cyclic GMP pathway as well as the endothelium-derived hyperpolarizing factor [32].
Hesperidin induced very little relaxation in the porcine coronary artery. However, hesperetin, an aglycone of hesperidin, clearly induced relaxation in the porcine coronary artery. It has been hypothesized that flavonoids, which are large and highly polar molecules, could not be absorbed immediately when ingested via the oral route. The flavonoids are hydrolyzed to aglycones by bacterial enzymes in the lower section of the intestine [33,34], and only then might be partially absorbed or may undergo further biotransformation [35].
Hesperidin is a flavanonol that structurally lacks the C(2)-C(3) double bond in the C-ring. This compound only slightly affects vascular relaxation. Planar flavonoid structures are believed to express potent vasorelaxation effects [36]. The relationship between the structure and activity of flavonoids and their aglycones have been previously reported in antioxidant research [37]; however, their activities in coronary arteries have not been elucidated. In this study, we discovered that the aglycones of PF flavonoids may be involved in effecting relaxation in the porcine coronary artery.
Poncirin, which is present abundantly in PF, was also observed to induce relaxation in the porcine coronary artery. Poncirin, like hesperidin, is also a flavonone and lacks the C(2)-C(3) double bond. However, in this study, this compound effected mild vasodilation in the porcine coronary artery. However, the effect of poncirin on the vessels has not been reported.
The vasorelaxation effects of flavonoids and its mechanism have been reported to be closely associated with the endothelium-derived nitiric oxide pathway [32] and the cyclic adenosine monophosphate/protein kinase A-dependent pathway, and to be partially mediated by a potassium channel, such as the BKca [36]. The antihypertensive effect of flavonoids has also been reported [38], and its ability to induce vasorelaxtion has been associated with the design in chemical structure [36].
In conclusion, the PF extracts have been determined to induce relaxation in the porcine coronary artery. These effects were at least partially induced by the PF flavonoids. The relaxation is partially mediated by the NO-cGMP pathway. The relaxation effects of other pathways, such as the cAMP pathway and the endothelium-derived hyperpolarizing factor, must be investigated in the future. Finally, the aglycones of PF flavonoids could be involved in effecting relaxation in the porcine coronary artery, indicating that the bioactive forms of flavonoids express greater efficacy.
Acknowledgments
This research was supported by the Kyungpook National University Research Fund, 2011.
Footnotes
The authors declare that there is no conflict of interests.
References
- 1.Jones DW, Chambless LE, Folsom AR, Heiss G, Hutchinson RG, Sharrett AR, Szklo M, Taylor HA., Jr Risk factors for coronary heart disease in African Americans: the atherosclerosis risk in communities study, 1987-1997. Arch Intern Med. 2002;162(22):2565–2571. doi: 10.1001/archinte.162.22.2565. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.KAPE. Handbook of the Korea Pharmacopoeia. Seoul: Shinilbooks; 2008. [Google Scholar]
- 4.Park KC, Bae GS, Choi SB, Jo IJ, Gwak TS, S. LG, Park SJ, Song HJ. Protective effect of Poncirus trifoliata and Citrus aurantium extract on acute pancreatitis in mice model. Korean J Herbol. 2012;27(5):9–14. [Google Scholar]
- 5.Lee YM, Kim DK, Kim SH, Shin TY, Kim HM. Antianaphylactic activity of Poncirus trifoliata fruit extract. J Ethnopharmacol. 1996;54(2-3):77–84. doi: 10.1016/s0378-8741(96)01451-1. [DOI] [PubMed] [Google Scholar]
- 6.Ban SS, Yoon HD, Shin OC, Shin YJ, Park CS, Park JH, Seo BI. The Effects of Artemisiae Capillaris, Ponciri Fructus and Cartaegi Fructus in Obese Rats Induced by High Fat Diet. Korean J Herbol. 2006;21(3):55–67. [Google Scholar]
- 7.Ham IH, Lee UC, Lee BH, Choi HY. Lipid Lowering activity of Ponciri Fructus and Aurantii Fructus Immaturus on hyperlipemia rats induced by Triton WR-1339. Korean J Herbol. 2007;22(3):109–116. [Google Scholar]
- 8.Son AR, Choi JY, Kim JA, Cho SH, Xu GH, Park SH, Chung SR, Chung TC, Jahng YD, Son JK, Lee SH. Isolation of Melanogenesis Inhibitors from Ponciri Fructus. Korean J Pharmacogn. 2005;36(1):1–8. [Google Scholar]
- 9.Shim WS, Back H, Seo EK, Lee HT, Shim CK. Long-term administration of an aqueous extract of dried, immature fruit of Poncirus trifoliata (L.) Raf. suppresses body weight gain in rats. J Ethnopharmacol. 2009;126(2):294–299. doi: 10.1016/j.jep.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Lim JH, Kim HS, Choi EJ, Shim CK, Park HJ. Effects of Poncirus Fructus on gastrointestinal motility in guinea pig. Korean J Neurogastroenterol Motil. 2008;14(1):7–17. [Google Scholar]
- 11.Lee HT, Seo EK, Chung SJ, Shim CK. Prokinetic activity of an aqueous extract from dried immature fruit of Poncirus trifoliata (L.) Raf. J Ethnopharmacol. 2005;102(2):131–136. doi: 10.1016/j.jep.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Choi KH, Jeong SI, Hwang BS, Lee JH, Ryoo HK, Lee S, Choi BK, Jung KY. Hexane extract of Poncirus trifoliata (L.) Raf. stimulates the motility of rat distal colon. J Ethnopharmacol. 2010;127(3):718–724. doi: 10.1016/j.jep.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Kim TW. Effects of Poncirus Fructus and Aurantii Fructus Immaturus on the gastric Fundus Motility. J Vet Clin. 2013;30(1):1–4. [Google Scholar]
- 14.Kim CM, Shin MK, Ahn DG, Lee KS. Chungyak Daesajun. Seoul: Jungdam Publisher; 1997. [Google Scholar]
- 15.Ajay M, Gilani AU, Mustafa MR. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003;74(5):603–612. doi: 10.1016/j.lfs.2003.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Chan EC, Pannangpetch P, Woodman OL. Relaxation to flavones and flavonols in rat isolated thoracic aorta: mechanism of action and structure-activity relationships. J Cardiovasc Pharmacol. 2000;35(2):326–333. doi: 10.1097/00005344-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Fan YF, Chen ZW, Guo Y, Wang QH, Song B. Cellular mechanisms underlying Hyperin-induced relaxation of rat basilar artery. Fitoterapia. 2011;82(4):626–631. doi: 10.1016/j.fitote.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Flesch M, Schwarz A, Böhm M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998;275(4 Pt 2):H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Vizcaíno F, Ibarra M, Cogolludo AL, Duarte J, Zaragozá-Arnáez F, Moreno L, López-López G, Tamargo J. Endotheliumindependent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002;302(1):66–72. doi: 10.1124/jpet.302.1.66. [DOI] [PubMed] [Google Scholar]
- 20.Roghani M, Baluchnejadmojarad T, Vaez-Mahdavi MR, Roghani-Dehkordi F. Mechanisms underlying quercetin-induced vasorelaxation in aorta of subchronic diabetic rats: an in vitro study. Vascul Pharmacol. 2004;42(1):31–35. doi: 10.1016/j.vph.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 21.KFDA. The Korea Pharmacopoeia. Seoul: KFDA; 2008. [Google Scholar]
- 22.Nugroho A, Park MG, Jin SE, Choi JS, Park HJ. Quantitative Analsysis of Flavanone Glycosides and Peroxynitrite Scavenging Effect of the Five Oriental Medicinal Drugs (Aurantii nobilis Pericarpium, Citrii unshiu Pericarpium, Citrii unshiu Semen, Aurantii Fructus, Poncirii Fructus) Korean J Pharmacogn. 2009;40(4):370–375. [Google Scholar]
- 23.Malagò R, Pezzato A, Barbiani C, Alfonsi U, Nicolì L, Caliari G, Pozzi Mucelli R. Coronary artery anatomy and variants. Pediatr Radiol. 2011;41(12):1505–1515. doi: 10.1007/s00247-011-2218-9. [DOI] [PubMed] [Google Scholar]
- 24.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 25.Jawad E, Arora R. Chronic stable angina pectoris. Dis Mon. 2008;54(9):671–689. doi: 10.1016/j.disamonth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Toufektsian MC, de Lorgeril M, Nagy N, Salen P, Donati MB, Giordano L, Mock HP, Peterek S, Matros A, Petroni K, Pilu R, Rotilio D, Tonelli C, de Leiris J, Boucher F, Martin C. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J Nutr. 2008;138(4):747–752. doi: 10.1093/jn/138.4.747. [DOI] [PubMed] [Google Scholar]
- 27.Cassidy A, Rimm EB, O'Reilly EJ, Logroscino G, Kay C, Chiuve SE, Rexrode KM. Dietary flavonoids and risk of stroke in women. Stroke. 2012;43(4):946–951. doi: 10.1161/STROKEAHA.111.637835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2008;100(4):890–895. doi: 10.1017/S0007114508945694. [DOI] [PubMed] [Google Scholar]
- 29.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96(2-3):67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 30.Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459(6):863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 31.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117(4):139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 32.Khoo NK, White CR, Pozzo-Miller L, Zhou F, Constance C, Inoue T, Patel RP, Parks DA. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic Biol Med. 2010;49(3):339–347. doi: 10.1016/j.freeradbiomed.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bokkenheuser VD, Shackleton CH, Winter J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem J. 1987;248(3):953–956. doi: 10.1042/bj2480953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffiths LA, Barrow A. Metabolism of flavonoid compounds in germ-free rats. Biochem J. 1972;130(4):1161–1162. doi: 10.1042/bj1301161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J Nutr. 2005;135(1):48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, Che CM, Man RY. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68(8):1179–1188. doi: 10.1016/j.phytochem.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep. 2009;61(1):67–75. doi: 10.1016/s1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]