Abstract
Aim
Short term administration of benzodiazepines (BZD) was found to prolong reaction time (RT) in experimental studies. However, studies on long term BZD use did not always adjust for important confounders and showed inconsistent results. We aimed to identify a possible relationship between long term BZD use and RT in BZD users in this large cross-sectional, observational study.
Methods
The RTs of non-users (n = 2404) were compared with low (n = 288), intermediate (n = 74), and high dose BZD users (n = 57) in the Netherlands Study of Depression and Anxiety (NESDA). RTs were obtained from the Implicit Association Test. Analyses were adjusted for sociodemographic characteristics, health indicators, severity of psychopathology and antidepressant use.
Results
Of the NESDA participants, 419 subjects (14.8%) used BZDs. A higher dose of BZDs was associated with prolonged RTs (P = 0.01). When comparing the different dose groups, the high dose group, but not the low and medium dose groups, had significantly longer RTs than the non-users.
Conclusions
Tolerance for the RT prolonging effect of relatively high doses of BZDs does not seem to develop. As prolonged RTs can have adverse consequences in daily life, BZDs should be prescribed conservatively at the lowest possible dose.
Keywords: anxiety, attention, benzodiazepines, psychomotor speed, reaction time, side effects
What is Already Known about this Subject
Short term benzodiazepine (BZD) use prolongs reaction time (RT).
Although the prevalence of long term BZD use is high, it in unclear whether RT is still affected in chronic use.
What this Study Adds
High dose BZD use is associated with prolonged RTs in chronic users (independent of psychopathology).
Tolerance for the RT prolonging effect of BZDs does not seem to develop at high doses in chronic users.
Introduction
As the prevalence of long term benzodiazepine (BZD) use is high 1, the accompanying side effects are an important research topic. Reaction time (RT) impairments are common in short term BZD use 2 and even seem to remain in chronic use 3. Choice RT tasks (CRTTs), where different responses are to be sorted to one of several stimuli as fast as possible, are an objective means to detect RT impairment due to the use of BZDs 4,5.
Previous research on the association between BZD use and RT (as measured by CRTTs) mainly consisted of small randomized trials, which compared the short term effects of BZD administration with placebo. In most of these studies, BZD administration prolonged RTs for a duration up to 6 weeks 6–11. Only two small studies did not find prolonged RTs after BZD intake 12,13.
The few studies on the association between longer term BZD use and RTs reported inconsistent results. One cross-sectional, observational study found longer RTs in chronic users than in non-users, but did not investigate if this effect was confounded by psychopathology 14. Two studies did not report differential RTs among BZD users and non-users 15,16. When an extra dose of 20 mg oxazepam was administered, RT increased in 18 BZD-naive participants, but not in 18 long term BZD users, suggesting that tolerance to the effects of BZDs on RT may have developed 16.
The inconsistent results regarding chronic BZD use may be caused by the lack of correction for established confounders such as psychopathology 14,16, physical health 14,15 and antidepressant use 14. Further, differences in sample selection (healthy subjects vs. subjects with psychopathology) may have led to the discrepancies. In order to determine whether the effects of BZDs on RT remain in long term BZD use, we analyzed the association between BZD use and RT as measured by the Implicit Association Test in 2823 participants of the Netherlands Study of Depression and Anxiety (NESDA) and corrected for important confounders.
Methods
Subjects
Subjects participated in the baseline assessment of the NESDA 17. The NESDA recruited 2981 individuals aged 18–65 years with and without symptoms of depressive and/or anxiety disorders from different health care settings 17. Lifetime diagnoses were defined as current or past diagnosis of a depressive or anxiety disorder as assessed by the DSM-IV Composite International Diagnostic Interview (CIDI, WHO version 2.1). The baseline assessment included written questionnaires, an oral interview and the implicit association test (IAT) computer task 17. The study protocol was approved by the ethics review board of each participating centre, and all subjects signed an informed consent.
Subjects without IAT data (n = 129), those with unusually long RTs (>10 s, n = 5) or missing values on BZD dose (n = 6) or BZD users without a lifetime diagnosis of depression or anxiety (n = 18) were excluded. After exclusion, 2823 subjects (94.7%) remained for our analyses. Of this group, 419 (14.8%) subjects used BZDs. Subjects who conducted the IAT were not statistically different from those who did not in terms of BZD use in general, used dose of BZDs, gender, education, and severity of depression and anxiety. However, subjects without IAT data were significantly older (P = 0.002).
Measures
BZD use
BZD use was registered by observation of drug containers brought to the interview (73.4%) or self-reports. BZDs were classified as Anatomical Therapeutic Code (ATC)-coded groups N05BA, N05CD, and N03AE01 and the non-BZD hypnotics zopiclone and zolpidem (ATC code N05CF) 18. The daily BZD dose was computed according to the coding system of the ATC and defined daily dose (DDD) system 19. The mean daily dose was calculated by dividing individual daily doses of BZDs by the corresponding DDD. For subjects using BZDs other than diazepam, an equivalent dose was calculated 20. The DDD was categorized into three groups: (i) daily dose ≤0.5 DDD (low dose), (ii) daily dose >0.5 but <1 DDD (intermediate dose), and (iii) daily dose >1 DDD (high dose). BZD users completed the BZD Dependence Self-Report Questionnaire (Bendep-SRQ) as a measure of dependence severity 21,22.
Implicit association test
The IAT is a computerized RT task which measures the strength of implicit associations 23. However, we did not use the IAT to measure implicit associations, but solely to measure RTs in a CRTT. To avoid the interference of implicit associations, we only used four single concept blocks of the IAT (Table S1). Stimulus words from two categories (e.g. anxious or calm) appeared in mixed order in the middle of a computer screen. Participants were instructed to sort the stimulus words as fast as possible to one of the two categories by pressing either a left response key (‘Q’) or a right response key (‘P’) on the keyboard. The RT of a trial was defined as the time from the appearance of a stimulus word until the correct response key was pressed 24. In the NESDA study, two IATs were included, a ‘depression IAT’ and an ‘anxiety IAT’ 25. In the anxiety IAT, subjects needed to sort words (such as nervous or relaxed) into the categories ‘anxious’ and ‘calm’. In the depression IAT, subjects needed to sort words (such as meaningless or valuable) in the categories ‘depressed’ and ‘elated’ 25.
Covariates
As sociodemographic characteristics (gender, age, education), health indicators (alcohol use, chronic disease), psychopathology (severity of anxiety and depression) and antidepressant use were found to be associated with RTs and BZD use 5,26,27, these variables were included as covariates in our analyses. Additionally, the total number of mistakes made during the analyzed IAT blocks was taken into account.
Sociodemographic characteristics were reported during the baseline interview. For regular alcohol use, the mean number of alcoholic consumptions per day was computed. The number of chronic somatic conditions was ascertained by self-report and dichotomized into presence of one or more chronic somatic conditions (yes/no). The severity of generalized anxiety and panic symptoms was assessed with the Beck Anxiety Inventory (BAI) 28. The severity of depressive symptoms was measured by the cognitive/mood scale of the Inventory of Depressive Symptomatology Self Report (IDS-SR) 29. Antidepressant use was subdivided into selective serotonin reuptake inhibitors (SSRIs, ATC code N06AB), tricyclic antidepressants (TCAs, N06AA) and selective serotonin and norepinephrine re-uptake inhibitors (N06AF, N06AX). The mean daily dose of antidepressant use was calculated and categorized into three groups.
Statistical analyses
Sample characteristics were expressed by percentages for categorical variables, by means for continuous, normally distributed variables and by medians for continuous, non-normally distributed variables. RTs were transformed into their negative inverse (−1/RT) due to their positively skewed distributions, yielding a normal distribution 30. The negative inverse of the blocks 2, 5, 8, and 11 of the IAT were averaged to diminish the influence of implicit or a preference for responses with the dominant hand. To correct for the learning effect, z-scores were calculated for each block (using −1/RT transformed values). These were averaged into one single score per subject. A higher z-score indicated a longer RT and thus a prolonged response. Group differences between non-users, low dose users, intermediate dose users and high dose users on RTs were analyzed by analysis of covariance. Post-hoc tests on individual group differences were performed using the Fisher Least Significant Difference test. The analysis was corrected for sociodemographic characteristics, duration of BZD use, health indicators, severity of psychopathology, duration of BZD use, daily dose of antidepressant use and number of mistakes made in the IAT. Analysis for trend was conducted. Linear regression analyses were used to examine associations between characteristics of BZD use as separate independent variables and RT in BZD users only after adjustment for all covariates.
Results
Characteristics of the study population
Table 1 shows the sample characteristics of the 2823 included participants, of which 419 subjects (14.8%) had used BZDs in the past month. Subjects with a low daily dose were more often female (72.2%) than the non-users, intermediate and high dose groups. The average age was lower in the non-users (40.9 years) and increased with each BZD dose. Non-users had lower BAI (median = 8.0) and IDS (median = 6.0) scores than all BZD user groups. The mean RT for the group as a whole was 0.96 s. It was shortest in the non-user group and increased with each dose group. All groups had a median number of three mistakes in the four included blocks.
Table 1.
Characteristics of the study group according to BZD dose category (n = 2823)
| No BZD | BZD low dose ≤0.5 DDD | BZD intermediate dose >0.5–1 DDD | BZD high dose >1 DDD | ||
|---|---|---|---|---|---|
| n = 2404 | n = 288 | n = 74 | n = 57 | P value | |
| Sociodemographics | |||||
| Female gender (%) | 1584 (65.9) | 208 (72.2) | 42 (56.8) | 32 (56.1) | 0.02 |
| Age (years) mean (SD) | 40.9 (13.3) | 45.1 (11.9) | 46.6 (11.1) | 48.8 (9.1) | <0.001 |
| Education level (years) median (IQR) | 12.0 (10.0; 15.0) | 11.0 (10.0; 15.0) | 10.0 (9.0; 15.0) | 10.0 (9.0; 11.5) | <0.001 |
| Health indicators | |||||
| Non/mild drinker (%) | 1470 (61.1) | 189 (65.6) | 55 (74.3) | 41 (71.9) | 0.09 |
| Moderate drinker (%) | 534 (22.2) | 52 (18.1) | 11 (14.9) | 7 (12.3) | |
| Heavy drinker (%) | 400 (16.6) | 47 (16.3) | 8 (10.8) | 9 (15.8) | |
| Somatic disease (%) | 1259 (52.4) | 186 (64.6) | 50 (67.6) | 44 (77.2) | <0.001 |
| Psychopathology | |||||
| BAI, median (IQR) | 8.0 (3.0; 16.0) | 19.0 (9.0; 27.0) | 24.0 (13.8; 30.5) | 21.0 (12.1; 31.5) | <0.001 |
| IDS-SR mc, median (IQR) | 6.0 (2.0; 9.0) | 9.0 (5.0; 12.0) | 10.0 (8.0; 13.0) | 11.0 (8.5; 14.0) | <0.001 |
| Antidepressant use | |||||
| No AD use (%) | 1920 (79.9) | 159 (55.2) | 24 (32.4) | 16 (28.1) | <0.001 |
| AD low dose <0.5 DDD (%) | 58 (2.4) | 18 (6.3) | 3 (4.1) | 2 (3.5) | |
| AD intermediate dose 0.5–1 DDD (%) | 251 (10.4) | 50 (17.4) | 22 (29.7) | 15 (26.3) | |
| AD high dose >1 DDD (%) | 175 (7.3) | 61 (21.2) | 25 (33.8) | 24 (42.1) | |
| Reaction time | |||||
| RT (s), mean (95% CI) | 0.94 (0.93; 0.96) | 1.02 (0.98; 1.06) | 1.11 (1.05; 1.18) | 1.19 (1.12; 1.28) | <0.001* |
| BZD use (%) | |||||
| Duration (months) median (IQR) | N/A | 24.0 (5.0; 93.0) | 12.0 (3.0; 60.0)a | 36.0 (8.5; 96.0) | 0.04 |
| Type | 0.24 | ||||
| Short acting (t1/2 < 24 h, %) | N/A | 238 (82.6) | 60 (81.1) | 40 (70.2) | 0.093 |
| Long acting (t1/2 > 24 h, %) | N/A | 50 (17.4) | 14 (18.9) | 17 (29.8) | |
| BZD dependence (BENDEP-SRQ) | |||||
| Problematic use, median (IQR) | N/A | 9.1 (8.8; 9.5) | 10.5 (9.7; 11.2) | 11.3 (10.4; 12.2) | <0.001 |
| Preoccupation, median (IQR) | N/A | 12.3 (11.8; 12.8) | 14.4 (13.3; 15.4) | 15.6 (14.5; 16.6) | <0.001 |
| Lack of compliance, median (IQR) | N/A | 6.0 (5.0; 9.0) | 7.5 (5.3; 10.0) | 10.0 (7.8; 11.0) | <0.001 |
RT is the mean RT of 80 trials. 1 DDD is defined as 10 mg diazepam equivalents day−1. P is derived by analysis of variance (anova) for quantitative, normally distributed variables, Kruskal−Wallis test for continuous, non-normally distributed variables and χ2 statistics for categorical variables. Significance is inferred at P < 0.05. AD, antidepressant; BAI, Beck anxiety index; BENDEP-SRQ, Benzodiazepine Dependence Self Report Questionnaire; BZD, benzodiazepines; DDD, defined daily dose; IDS-SR mc, Mood cognition scale of the Inventory of Depressive Symptomatology; IQR, interquartile range; RT, reaction time.
Associations between BZD use and RT
Table 2 shows group differences between non-users and low, intermediate and high dose users on RT. In unadjusted (P for linear trend <0.001) and adjusted analyses (P = 0.01) groups differed significantly on RT. Gender (F = 16.69), age (F = 521.32), education (F = 108.03), number of alcohol drinks consumed (F = 5.27), severity of depression (F = 15.88) and anxiety (F = 14.38) had much higher F values than dose of BZD use (F = 2.35). In contrast, duration of BZD use (F = 0.065), daily dose of TCA (F = 0.008), SSRI (F = 0.05) and other antidepressants (F = 0.13) had much lower F values than daily dose of BZDs. In post hoc tests, high dose BZD users had significantly longer RTs than non-users, while the other dose groups did not differ significantly from non-users. Figure 1 shows the adjusted mean RTs per user group as obtained by multivariate regression analysis. Higher BZD doses were significantly associated with longer RTs (P = 0.01).
Table 2.
Differences between non-users, low dose users, intermediate dose users and high dose users on RT as analyzed in 2823 NESDA participants
| No BZD | Low dose ≤0,5 DDD | Intermediate dose >0.5–1 DDD | High dose >1 DDD | ||
|---|---|---|---|---|---|
| n = 2404 | n = 288 | n = 74 | n = 57 | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P | |
| Unadjusted | 0.95 (0.94, 0.96) | 1.03 (1.00, 1.06) | 1.11 (1.04, 1.18) | 1.19 (1.11, 1.29) | <0.001 |
| Adjusted | 0.96 (0.95, 0.97) | 0.97 (0.95, 0.99) | 0.99 (0.95, 1.05) | 1.03 (0.97, 1.10) | 0.01 |
1 DDD was defined as 10 mg diazepam equivalents day−1. The adjusted analysis was adjusted for sociodemographic characteristics (gender, age, education level), health indicators (alcohol intake and presence of a somatic disease), severity of psychopathology (BAI and IDS-mc), daily dose of used antidepressants, duration of BZD use and number of mistakes made in the IAT. P was obtained by ancova (analysis for linear trend). Significance was inferred at P < 0.05. BZD, benzodiazepines; CI, confidence interval; DDD, defined daily dose; RT, reaction time.
Figure 1.

The adjusted mean values of reaction time (in ms) obtained from the implicit association test according to the dose of BZDs used in 2823 NESDA participants. The size of each square is proportional to the number of participants. Vertical lines indicate standard errors. Analyses were adjusted for sociodemographic variables (i.e. gender, age, education), health indicators (i.e. daily alcohol use, presence of somatic disease), psychopathology (i.e. IDS-mc, BAI), antidepressant use (in four categories) and duration of BZD use. Low dose was defined as ≤5 mg diazepam equivalents day−1, intermediate dose as 5.01–10 mg day−1 and high dose as >10 mg day−1. β coefficients and P values by multivariate linear regression analysis
Associations between characteristics of BZD use and RT
Table 3 reports the results of additional regression analyses on specific associations between the characteristics of BZD use and RT among the BZD users only. After adjustment, a higher daily dose of BZDs was associated with longer RTs (β = 0.096, P = 0.03). When duration of use was added to this regression analysis, the results did not change and the β of duration of use was low (β = −0.0.21) and not significant (P = 0.65). This indicates a possible dose−response effect of BZDs on RTs. Further, problematic use showed a positive association (β = 0.118, P = 0.02) with RT. Figure 2 shows the adjusted mean values of reaction time according to problematic use on the Bendep-SRQ in BZD users only (n = 366). β coefficients and P values were obtained by multivariate linear regression analysis. A higher score on the Bendep-SRQ subscale problematic use was significantly associated with longer RTs (P = 0.02).
Table 3.
Associations between characteristics of BZD use and RT in 419 BZD users
| Characteristics of BZD use | n | Univariate analysis | Adjusted model§ | ||
|---|---|---|---|---|---|
| β | P | β | P | ||
| Dose† | 419 | 0.167 | 0.001 | 0.096 | 0.03 |
| Dose*severity of depression | 419 | −0.203 | 0.22 | 0.084 | 0.57 |
| Dose*severity of anxiety | 419 | −0.207 | 0.18 | 0.106 | 0.44 |
| Duration of BZD use | 419 | 0.114 | 0.02 | 0.036 | 0.40 |
| Type of BZD | 419 | 0.115 | 0.02 | −0.013 | 0.77 |
| Problematic use‡ | 366 | 0.190 | <0.001 | 0.118 | 0.02 |
| Preoccupation‡ | 366 | 0.110 | 0.04 | 0.023 | 0.64 |
| Lack of compliance‡ | 366 | 0.210 | <0.001 | 0.070 | 0.17 |
†Daily dose is entered as a continuous variable. ‡Subscales of the Benzodiazepine Dependence Self Report Questionnaire. §The adjusted models were adjusted for sociodemographics (gender, age, education), health indicators (daily alcohol use, presence of somatic disease), severity of psychopathology (IDS-mc, BAI), and antidepressant use (SSRI, TCA, Other Antidepressants). BZD; benzodiazepines, β; standardized β coefficient by linear regression analyses. Values in bold are significant (P < 0.05).
Figure 2.
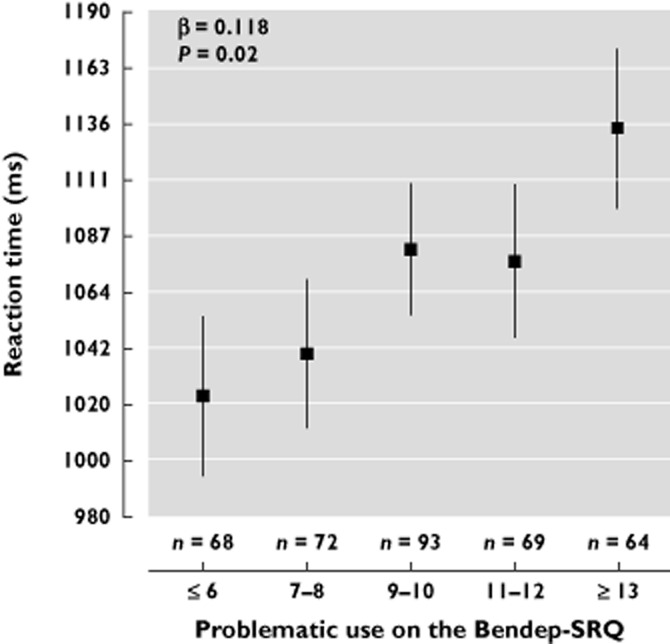
The adjusted mean values of reaction time (in ms) obtained from the implicit association test according to problematic use on the Bendep-SRQ in BZD users only (n = 366). The size of each square is proportional to the number of participants. Vertical lines indicate standard errors. Analyses were adjusted for sociodemographic variables (i.e. gender, age, education), health indicators (i.e. daily alcohol use, presence of somatic disease), psychopathology (i.e. IDS-mc, BAI) and antidepressant use. β coefficients and P values by multivariate linear regression analysis
Discussion
In this cross-sectional, observational cohort study, we investigated the putative association between long term BZD use and RT. High doses of BZDs (>1 DDD), but not lower doses, were associated with prolonged RTs. This indicates that tolerance to the RT prolonging effect of BZDs does not (completely) develop at higher doses of BZDs.
The finding of longer RTs in high dose BZD users was in line with experimental research on short term BZD use and RT 6–11 as well as with an observational study which found longer RTs in anxious, high dose BZD users (1.2–4 DDD) than in healthy non-users 14. However, in the latter study it was unclear, whether prolonged RTs were due to BZD intake or psychopathology.
Still, several studies did not find associations between BZD use and RT in chronic users 15,16. Possibly, BZDs still affect RTs in chronic use, but study design issues led to a lack of significant group differences in these studies (small sample size 15,16, absence of adequate statistical transformations 15,16). Alternatively, the lack of significant associations between BZD use and RT may indicate that tolerance to the prolonging effect of BZDs on RT develops in long term BZD use so that only relatively high doses cause effect. The fact that we did not find an association between duration of use and RT may be due to the majority of BZD users in NESDA being chronic users. The comparison of new users with chronic users might have resulted in different results.
Our study has some limitations. The data are limited by representing only one outcome composed of six individual RT trials. The highest doses in NESDA were still rather moderate doses, so that effects of very high doses could not be investigated. Since there were only four people who took more than 35 mg day−1 and there was heterogeneity in their impairment, it was statistically inappropriate to look at them as a separate category. However, some of the highest impairments were seen with the highest doses and this may have obscured a finer grain interpretation. For this reason, future studies should focus on recruiting enough high dose BZD users. The IAT may not be the most optimal task to measure RT, because the stimulus words were not neutral but related to depression and anxiety and may therefore influence subjects suffering from these illnesses. However, as the effects on RT remained after adjustment for severity of anxiety and depression, this is unlikely. Further, the validity of a CRTT for real life situations, such as driving or working at a machine, is lower than the validity of a simulation task. Despite these limitations, our study makes an important contribution to the literature on BZDs and RT due to the following strengths. NESDA is a large observational, cohort study and includes a large sample of average BZD users with a long duration of use and comorbid psychopathology, so that our findings can be generalized to outpatient BZD users in primary and secondary care. The study size enabled us to adjust for important confounders such as psychopathology. The investigation of various characteristics of BZD use enabled us to determine the aspects of long term BZD use which are associated with RT.
In conclusion, we found increased RTs in high dose BZD users even after adjustment for severity of psychopathology and antidepressant use. This indicates that no complete tolerance to the RT prolonging effect of high BZD doses develops in long term BZD users. Medical doctors should alert their patients of the prolonged RTs associated with high doses of BZDs and possible consequences for everyday tasks where fast reaction is required. This study also underlines the directive to prescribe and use BZDs conservatively, and at the lowest dose possible 31.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
The infrastructure for the NESDA study (http://www.nesda.nl) is funded through the Geestkracht programme of the Netherlands Organization for Health Research and Development (ZonMw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Health Care (IQ Healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos).
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Arrangement of the different Implicit Association Test blocks
References
- 1.College voor zorgverzekeringen. GIPeilingen 2009 Ontwikkelingen genees- en hulpmiddelengebruik. Genees- en hulpmiddelen Informatie Project | September 2010 (31). 1-9-2010.
- 2.Smiley A. Effects of minor tranquilizers and antidepressants on psychomotor performance. J Clin Psychiatry. 1987;48:22–28. [PubMed] [Google Scholar]
- 3.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis. Arch Clin Neuropsychol. 2004;19:437–454. doi: 10.1016/S0887-6177(03)00096-9. [DOI] [PubMed] [Google Scholar]
- 4.Wetherell A. Performance tests. Environ Health Perspect. 1996;104:247–273. doi: 10.1289/ehp.96104s2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosinski RJ. 2010. A Literature Review on Reaction Time. Clemson University,. Retrieved September 2013 from http://bsdc2009.pbworks.com/f/literature+review+-+Clemson+Univ.pdf.
- 6.Fillmore MT, Rush CR, Kelly TH, Hays L. Triazolam impairs inhibitory control of behavior in humans. Exp Clin Psychopharmacol. 2001;9:363–371. doi: 10.1037//1064-1297.9.4.363. [DOI] [PubMed] [Google Scholar]
- 7.Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp Clin Psychopharmacol. 2010;18:1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Neill WM, Hanks GW, Simson P, Fallon MT, Jenkins E, Wesnes K. The cognitive and psychomotor effects of morphine in healthy subjects: a randomized controlled trial of repeated (four) oral doses of dextropropoxyphene, morphine, lorazepam and placebo. Pain. 2000;85:209–215. doi: 10.1016/s0304-3959(99)00274-2. [DOI] [PubMed] [Google Scholar]
- 9.Tiplady B, Hiroz J, Holmes L, Drummond G. Errors in performance testing: a comparison of ethanol and temazepam. J Psychopharmacol. 2003;17:41–49. doi: 10.1177/0269881103017001691. [DOI] [PubMed] [Google Scholar]
- 10.van Ruitenbeek P, Vermeeren A, Smulders FTY, Sambeth A, Riedel WJ. Histamine H1-receptor blockade predominantly impairs sensory processes in human sensorimotor performance. Br J Pharmacol. 2009;157:76–85. doi: 10.1111/j.1476-5381.2008.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLeod DR, Hoehn-Saric R, Labib AS, Greenblatt DJ. Six weeks of diazepam treatment in normal women: effects on psychomotor performance and psychophysiology. J Clin Psychopharmacol. 1988;8:83–99. [PubMed] [Google Scholar]
- 12.Hindmarch I, Parrott AC, Arenillas L. Repeated dose comparison of dichloralphenazone, flunitrazepam and amylobarbitone sodium on some aspects of sleep and early morning behaviour in normal subjects. Br J Clin Pharmacol. 1977;4:229–233. doi: 10.1111/j.1365-2125.1977.tb00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smirne S, Ferini-Strambi L, Pirola R, Tancredi O, Franceschi M, Pinto P, Bareggi SR. Effects of flunitrazepam on cognitive functions. Psychopharmacology (Berl) 1989;98:251–256. doi: 10.1007/BF00444700. [DOI] [PubMed] [Google Scholar]
- 14.Sakol MS, Power KG. The effects of long-term benzodiazepine treatment and graded withdrawal on psychometric performance. Psychopharmacology (Berl) 1988;95:135–138. doi: 10.1007/BF00212782. [DOI] [PubMed] [Google Scholar]
- 15.Schrijvers D, de Bruijn ERA, Maas Y, De Grave C, Sabbe BGC, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44:569–579. doi: 10.1016/j.cortex.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Oude Voshaar RC, Verkes RJ, van Luijtelaar GL, Edelbroek PM, Zitman FG. Effects of additional oxazepam in long-term users of oxazepam. J Clin Psychopharmacol. 2005;25:42–50. doi: 10.1097/01.jcp.0000150219.59056.d0. [DOI] [PubMed] [Google Scholar]
- 17.Penninx BW, Beekman AT, Smit JH, Zitman FG, Nolen WA, Spinhoven P, Cuijpers P, De Jong PJ, Van Marwijk HW, Assendelft WJ, Van Der Meer K, Verhaak P, Wensing M, De Graaf R, Hoogendijk WJ, Ormel J, Van Dyck R NESDA Research Consortium. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manthey L, van Veen T, Stoop J, Giltay E, Penninx BW, Zitman F. Correlates of (inappropriate) benzodiazepine use: the Netherlands Study of Depression and Anxiety (NESDA) Br J Clin Pharmacol. 2011;71:263–272. doi: 10.1111/j.1365-2125.2010.03818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Collaborating Centre for Drug Statistics Methodology. 2009. Guidelines for ATC classification and DDD assignment, 2010. Available at http://www.whocc.no/atc_ddd_publications/guidelines/155.html (last accessed 9 September 2013)
- 20.Zitman FG, Couvee JE. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper-off – report on behalf of the Dutch Chronic Benzodiazepine Working Group. Br J Psychiatry. 2001;178:317–324. doi: 10.1192/bjp.178.4.317. [DOI] [PubMed] [Google Scholar]
- 21.Kan CC, Breteler MH, Timmermans EA, van der Ven AH, Zitman FG. Scalability, reliability, and validity of the benzodiazepine dependence self-report questionnaire in outpatient benzodiazepine users. Compr Psychiatry. 1999;40:283–291. doi: 10.1016/s0010-440x(99)90129-3. [DOI] [PubMed] [Google Scholar]
- 22.Oude Voshaar RC, Mol AJJ, Gorgels WJMJ, Breteler MHM, van Balkom AJLM, van de Lisdonk EH, Kan CC, Zitman FG. Cross-validation, predictive validity, and time course of the benzodiazepine dependence self-report questionnaire in a benzodiazepine discontinuation trial. Compr Psychiatry. 2003;44:247–255. doi: 10.1016/S0010-440X(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 23.Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- 24.Blakemore C, Jenner S. Oxford Companion to the Body. Rothwell, J. C. Reaction time. 2-7-2002. 2-7-2011.
- 25.Glashouwer KA, de Jong PJ. Disorder-specific automatic self-associations in depression and anxiety: results of The Netherlands Study of Depression and Anxiety. Psychol Med. 2010;40:1101–1111. doi: 10.1017/S0033291709991371. [DOI] [PubMed] [Google Scholar]
- 26.Houx PJ, Jolles J. Age-related decline of psychomotor speed: effects of age, brain health, sex, and education. Percept Mot Skills. 1993;76:195–211. doi: 10.2466/pms.1993.76.1.195. [DOI] [PubMed] [Google Scholar]
- 27.Van HR, Teeuw KB, Bakker AB, Bakker A, Leufkens HG. Characteristics of current benzodiazepine users as indicators of differences in physical and mental health. Pharm World Sci. 2000;22:96–101. doi: 10.1023/a:1008749220107. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Brown G, Epstein N, Steer RA. An inventory for measuring clinical anxiety – psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 29.Wardenaar KJ, van Veen T, Giltay EJ, den Hollander-Gijsman M, Penninx BWJH, Zitman FG. The structure and dimensionality of the Inventory of Depressive Symptomatology Self Report (IDS-SR) in patients with depressive disorders and healthy controls. J Affect Disord. 2010;125:146–154. doi: 10.1016/j.jad.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Whelan R. Effective analysis of reaction time data. Psychol Rec. 2008;58:475–482. [Google Scholar]
- 31.Kerngroep Multidisciplinaire Richtlijn Angststoornissen. Richtlijnherziening van de Multidisciplinaire richtlijn Angststoornissen (tweede revisie). Richtlijn voor de diagnostiek, behandeling en begeleiding van volwassen patiënten met een angststoornis. 16-3-2011. Trimbos Instituut.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arrangement of the different Implicit Association Test blocks


