Abstract
Aims
The aims of this study were to develop a population pharmacokinetic (PK) model of ampicillin and sulbactam, to identify patient characteristics influencing the PK, and to explore the relationship between dose regimen and degree of renal impairment with exposure and time above minimum inhibitory concentration (MIC).
Methods
This analysis was performed on PK data for ampicillin and sulbactam and MIC data from a clinical trial in Japanese patients with community acquired pneumonia. Simulations were performed to investigate the effects of different dosing intervals on exposure and time above MIC in various degrees of renal impairment.
Results
The plasma concentrations from 47 patients were adequately described by a two compartment model with simultaneous fit of ampicillin and sulbactam PK data, where creatinine clearance on clearance and body weight on volume in the peripheral compartment were identified as covariates for both drugs. Creatinine clearance contributed to reducing inter-individual variability of clearance by 16%. Mean clearance (inter-individual variability) for ampicillin and sulbactam was estimated to be 10.7 l h−1 (14.8%) and 10.4 l h−1 (15.2%), respectively. The time above MIC for each pathogen was generally > 50% of the treatment period. Simulations for exposure and time above MIC supported currently recommended dose adjustments.
Conclusions
This study provided a PK model for ampicillin and sulbactam, the time above MICs for identified pathogens and associated simulation results. These findings provide useful information and augment evidence for the established dosage regimens in patients with various degrees of renal impairment.
Keywords: ampicillin, pneumonia, population pharmacokinetics, sulbactam, Unasyn
What is already known about this subject
The dose of ampicillin/sulbactam (2:1) up to 12 g daily is approved in many countries around the world, but until recently labelling in Japan had restricted dosing to 6 g daily.
Recently the efficacy and tolerability of the higher 12 g daily dose have been demonstrated in Japanese patients with moderate or severe community-acquired pneumonia.
The pharmacokinetic profile for ampicillin/sulbactam in healthy and renally impaired subjects has been well characterized and recommended dose adjustments due to impaired renal function have been established.
What this study adds
A joint population analysis of ampicillin and sulbactam in Japanese patients with moderate or severe community-acquired pneumonia receiving a high dose (12 g daily) has been performed to investigate the factors influencing the pharmacokinetics in this population.
This study supports the clinical efficacy of the four times daily regimen (12 g daily) demonstrated in the clinical trial using relationships between the pharmacokinetics and minimum inhibitory concentrations and adds evidence for adequacy of the dose adjustment by renal function.
Introduction
Unasyn® is an injectable antimicrobial combination, which contains ampicillin sodium, a β-lactam antibiotic, and sulbactam sodium, a β-lactamase inhibitor, in a dose ratio of 2:1. Ampicillin has potent activity against gram-positive bacteria and gram-negative bacteria such as Escherichia coli, Proteus mirabilis and Hemophilus influenzae. Sulbactam extends the antibacterial spectrum to Proteus vulgaris, Acinetobacter species and Bacteroides species 1,2. The injectable antimicrobial combination has been marketed in over 60 countries, and prescribed for the treatment of various types of infections such as upper and lower respiratory tract infection, renal and urinary tract infection, intraperitoneal infection, genital infection including gonorrhoeal infection, skin and soft tissue infection, and for prophylactic administration during intraperitoneal surgery.
Ampicillin and sulbactam have been shown to have similar pharmacokinetics (PK) with extensive distribution in the extracellular fluids and tissues being achieved for both drugs 3. Ampicillin has been found to be approximately 28% reversibly bound to human serum protein and sulbactam approximately 38% reversibly bound 4. Both drugs are primarily eliminated in urine, principally via glomerular filtration and tubular excretion 3–5. The half-lives of ampicillin and sulbactam are approximately 1 h in healthy subjects 3,6 and 1.6–3.7 h in subjects with abnormal renal function 7. In patients with impairment of renal function, it is recommended to administer the combination drug less frequently depending on their renal function 1.
Antimicrobial activity of β-lactam antibiotics, including ampicillin, is known to be dependent on the time the plasma drug concentrations remain above a minimum inhibitory concentration (MIC) to the antimicrobial agent 8–10. Therefore, it is considered important to choose an appropriate dose and dosing interval that covers the required time above MIC and reduces unnecessary over exposure. The recommended dosage of ampicillin/sulbactam in adult patients with normal renal function is 1.5 to 3 g every 6 h in the US 1. The high dose (12 g daily) is also allowed in many countries including Germany, France, China, Taiwan and South Korea depending on the severity of the infection.
In Japan, the injectable combination was approved in 1994 for indicated bacterial strains of Staphylococcus genus, Escherichia coli, Proteus genus and Haemophilus influenzae, and for indications of pneumonia, pulmonary abscess, peritonitis and cystitis. Until recently, the recommended dosage in adults for pneumonia, pulmonary abscess and peritonitis had been 3 g twice daily, which was lower than the approved high dose in other countries. Recently, a clinical trial was conducted in Japanese patients with moderate or severe community-acquired pneumonia (CAP) to confirm the efficacy and safety of the high dose administration (3 g four times daily) 11. Based on the result, the high dose was approved in 2012. While the PK profile for ampicillin/sulbactam in healthy and renally impaired subjects is well known 3–6,12, there is no published account of high dose ampicillin/sulbactam PK in Japanese patients with CAP.
In this paper, we present the population PK analysis of the data from this clinical study using a simultaneous fit of ampicillin and sulbactam and a covariate analysis to identify patient characteristics influencing the PK. The time above MICs across the range of common pathogenic bacteria identified from these patients are explored and simulations are conducted to examine the impact of renal impairment on the choice of dose regimen.
Methods
Clinical studies and assay methods
This analysis was performed on the PK and MIC data from a multicentre, open label, non-comparative clinical trial in Japanese patients with CAP 11. In brief, patients eligible for the trial were 16 years or older, diagnosed with moderate or severe CAP and requiring in-hospital treatment with antimicrobial agents. Patients with severe renal impairment defined as CLcr < 30 ml min−1 were not allowed to enter the trial. The primary outcomes, % clinical response rate (95% CI) at the end of treatment, the test of cure (7 days after the end of treatment) and follow-up (7 days after the test of cure) were 97.4% (86.5, 99.9), 94.6% (81.8, 99.3) and 94.4% (81.3, 99.3), respectively 11. Based on the study results, clinical efficacy of the high regimen was demonstrated and the regimen was approved in Japan. In this clinical trial, population PK analysis was planned and performed to support the results of the clinical efficacy using the PK−pharmacodynamic (PD) relationships and to help explain relationships between renal function and exposure. The results of this analysis contributed by adding consistent evidence to what it is known about ampicillin/sulbactam PK. In this clinical trial, prospective approval of the clinical trial protocol was obtained by the independent ethics committees and institutional review boards for all study sites. The protocol was submitted to the regulatory agency of Japan, Pharmaceutical and Medical Device Agency (PMDA) in advance. The study was conducted in accordance with GCP principles. Informed consent was obtained from all the subjects in compliance with GCP and the related requirements. The clinical trial was posted on ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT01189487).
Patients who participated in the clinical trial received 30 min intravenous infusions of 3 g ampicillin/sulbactam (2:1 g) every 6 h (four times daily) for 3 days or up to 14 days depending on each patient's condition. During the treatment period, four to five blood samples were taken from each patient to measure plasma ampicillin and sulbactam concentrations simultaneously. Guidance was provided in order for two or three blood samples to be taken within the expected distribution phase (0 to 2.5 h post-dose), with the others being taken within the expected elimination phase (2.5 to 6 h post-dose). There was no restriction placed on the timing of the samples relative to the start of treatment or clock time.
Plasma samples were analyzed for ampicillin and sulbactam using a validated liquid chromatography/tandem mass spectrometry (LC-MS/MS) method at SGS Cephac Europe. Plasma samples were extracted with acetonitrile after adding internal standards, ampicillin D5 and sulbactam D2. The supernatant was injected onto an API-4000 LC-MS/MS system (column: Kinetex PFP, mobile phase: an aqueous acetonitrile solution with formic acid). The precision (CV) was ≤ 6.99% for ampicillin and ≤ 6.19% for sulbactam. The accuracy ranged from −3.10% to 1.07% for ampicillin and −2.40% to 3.20% for sulbactam. The lower and higher limits of quantification for both analytes were 0.100 μg ml−1 and 50.0 μg ml−1, respectively.
To identify individual pathogens and determine their MICs, sputum was collected from each patient before treatment. Micro-organisms in the specimen were isolated and grown in culture, and then identified 13. The MIC for ampicillin/sulbactam was determined for identified micro-organisms by dilution antimicrobial susceptibility tests based on consensus processes 14. All the tests were performed by Mitsubishi Chemical Medience Corporation.
Population pharmacokinetic analysis
The PK of both ampicillin and sulbactam were previously described by a two compartment model 3–6,12. The parameterization with clearance (CL), central volume (V1), inter-compartment clearance (Q), and peripheral volume (V2) was used, though the other models such as one and three compartment models were also tested in advance. Given the prior knowledge of the renal clearance, creatinine clearance (CLcr) based on the Cockcroft & Gault equation 15 was tested as a covariate of CL as a part of base model development. A structural PK model of each drug was developed independently. However the simultaneous fit of the concentration data of both drugs was evaluated by the use of the L2 data item in nonmem 16.
Covariate model
Covariates of interest were tested to develop the final model. Covariates tested were body weight, age, gender, γ-GTP, AST and ALT for CL and body weight and gender for volume of distribution. Since the objective of the covariate modelling was to identify covariates that accounted for unexplained inter-individual variability in the PK parameters, the inclusion of covariates was to be evaluated only for parameters incorporating inter-individual variability.
Continuous variables were tested using a power model. Using the example of CL, the model is expressed as follows:
where CLi(k) represents the model predicted PK parameter for analyte k (1 for ampicillin, 2 for sulbactam) for the ‘typical’ individual i with covariate xi. The CL(k) represents the population central tendency for the individual CLi(k). The median(x) represents the median value for the covariate in the subjects studied and θx represents a scale factor.
The categorical covariates were modelled using the general equation:
where COV = 1 + θx for test group and COV = 1 for reference group.
The stepwise covariate modelling was done using an automated procedure of Perl-speaks-nonmem 17. The automated procedure involved stepwise testing of relationships in a forwards inclusion (ΔOFV of 3.84, P < 0.05, d.f. = 1) and backwards exclusion (ΔOFV of 6.63, P < 0.01, d.f. = 1) procedure. Clinical relevance of the relationship was also considered.
Pharmacokinetic error model
Inter-individual variability in the PK parameters was modelled using multiplicative exponential random effects. For example, CL for the ith individual for analyte k (1 for ampicillin, 2 for sulbactam), CLi(k), has the following form:
where CL(k) is the typical individual (population mean) value of the parameter and ηCLi(k) denotes the individual random effect with mean zero and variance ωCL(k)2.
When the structural PK model of each drug was developed independently, correlation structure for inter-individual random effects (Ω) was investigated for each analyte. For example, inter-individual random effects for CL and V2 for ampicillin (k = 1), ηCLi(1) and ηV2i(1), have the following variance-covariance matrix:
where ωCL(1)×V2(1) represents covariance for random effects of CL and V2 for ampicillin.
When structural PK models of both drugs were developed simultaneously, correlation structure for inter-individual random effects of both drugs were additionally investigated. For example, inter-individual random effects for CL of ampicillin and sulbactam, ηCLi(1) and ηCLi(2), have the following variance-covariance matrix:
where ωCL(1)×CL(2) represents covariance for random effects of CL for ampicillin and sulbactam.
Intra-individual variability was modelled by the log transform both sides approach with an additive error model. An observed plasma concentration for the ith individual at time tij for analyte k, Yij(k), was specified by:
where Fij(k) represents a compartment model for analyte k. The error terms εij(1) and εij(2) were assumed to follow independently a normal distribution with mean zero and common variance σ(1)2 and σ(2)2, respectively. When analyzing the data of both drugs simultaneously with the L2 data item, the error vector εij = {εij(1), εij(2)} formed a variance-covariance matrix Σ.
In the case of different time points or different individuals, the εij(1) and εi'j'(2) (i ≠ i' or j ≠ j') were assumed to be independent (i.e. σ(1)×(2) = 0).
Model qualification
The final model was validated by diagnostic plots including plots of conditional weighted residual (CWRES) 18,19 and a visual predictive check. The visual predictive checks were performed to validate the final model. One thousand data sets with identical design to the original data set were simulated using the final parameter estimates including inter-individual and residual variability without uncertainty in the model parameters. Plots showing the areas covering 95% CIs of the median, the 10th and the 90th percentiles of the simulated profiles were shown together with the median, 10th and 90th percentiles of the observed concentration–time profiles. The η and ε shrinkage were also calculated to evaluate model adequacy 20.
Pharmacodynamic analyses
The MIC for ampicillin/sulbactam was determined when ampicillin/sulbactam solution (dose ratio 2:1) was added to each identified pathogen. The MIC value was reported as ampicillin concentration (μg ml−1) in this study. The time above MIC during the treatment period for each identified pathogen of each individual was calculated using the following equation:
where f·C(t) represents a free fraction of plasma ampicillin at each time point for the individual, MIC is that for the identified pathogen and GAM is a factor fixed to 99. The free fraction f was set to 0.72 1. When f·C(t) > > MIC then 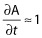 else
else 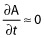 . Integration of this function provides the time above MIC for the free fraction of plasma ampicillin (f t>MIC). The percentage of time above MIC (f t>MIC%) was obtained by dividing f t>MIC by the treatment duration. Treatment duration for each individual was defined as time from initial to last dose of ampicillin/sulbactam. MICs against specific bacteria, which were judged as causative pathogens by investigators, were used to estimate individual f t>MIC. For subjects who had more than one causative pathogen identified the f t>MIC value was reported for each bacterium.
. Integration of this function provides the time above MIC for the free fraction of plasma ampicillin (f t>MIC). The percentage of time above MIC (f t>MIC%) was obtained by dividing f t>MIC by the treatment duration. Treatment duration for each individual was defined as time from initial to last dose of ampicillin/sulbactam. MICs against specific bacteria, which were judged as causative pathogens by investigators, were used to estimate individual f t>MIC. For subjects who had more than one causative pathogen identified the f t>MIC value was reported for each bacterium.
Simulation exercises
Simulations were performed to investigate effects of different dosing intervals (6, 8, 12 and 24 h) on exposure and f t>MIC% for subpopulations with different levels of renal function. The dosing intervals were based on recommendations for different levels of renal impairment from the US label 1. Each subpopulation of 500 subjects was randomly sampled from a uniform distribution of CLcr (CLcr 60 to 90, 30 to < 60, 15 to < 30 or 5 to < 15 ml min−1) and a uniform distribution of body weight, based on the observed range from the current study (31.3 to 78.7 kg).
For each subpopulation, plasma ampicillin and sulbactam concentration−time data were generated utilizing parameter estimates of the final model across intersubject variability. Based on the simulated data, PK parameters and f t>MIC were calculated for different renal function ranges and different dosing intervals. Since the required f t>MIC% for bacteriostasis and bacteriocidal activity for penicillin is reported to be 30% and 50%, respectively 8,9, the probability of achieving f t>MIC% ≥ 30%, 40% or 50% across the population were calculated for different levels of renal impairment and potential dosing intervals.
Software
Plasma ampicillin and sulbactam concentrations vs. time data were analyzed using the non-linear mixed effects modelling methodology as implemented by nonmem Version 6 Level 2.0 (ICON Development Solutions, Ellicott City, MA, USA). nonmem was used to estimate the population parameters, mean and interindividual variability and to identify potential covariates that explain interindividual variability in the parameters. The first order conditional estimation method (FOCE) was used. Both statistical package S-PLUS® Version 7.0 (TIBCO Software Inc, Palo Alto, CA, USA) and R Version 2.5.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for the exploratory analysis and to assist the model building. Perl-speaks-nonmem (PsN) Version 2.3.0 was used for model evaluation and covariate model building.
Results
Patient characteristics
Data from 47 patients with moderate or severe CAP were used for this analysis. Baseline characteristics of the 47 patients are summarized in Table 1. All subjects were Japanese (21 females and 26 males) with a median age (range) of 67 (28−85) years. Elderly patients defined as 65 years or older accounted for 57% of the population (n = 27). Fourteen patients (30%) had lower body weight defined as ≤45 kg. Renal function based on CLcr 21 varied among the patients, where the number of patients with normal renal function (CLcr ≥ 90 ml min−1), mild (60 ml min−1 ≤ CLcr < 90 ml min−1) and moderate (30 ml min−1 ≤ CLcr < 60 ml min−1) renal impairment was 17 (36%), 10 (21%) and 20 (43%), respectively.
Table 1.
Subject demographics for the entire analysis dataset
| Demographics variable | n | Median | Range |
|---|---|---|---|
| Male/female | 26/21 | ||
| Age (years) | 67 | 28–85 | |
| Body weight (kg) | 51.2 | 31.3–78.7 | |
| Body mass index (kg m–2) | 20.4 | 13.7–29.0 | |
| CLcr (ml min–1) | 71.0 | 34.6–176 | |
| Serum creatinine (mg dl–1) | 0.73 | 0.38–1.40 | |
| γ-glutamyl transferase (U l–1) | 32 | 8–240 | |
| Alanine aminotransferase (U l–1) | 19 | 7–82 | |
| Aspartate aminotransferase (U l–1) | 28 | 15–128 | |
Pharmacokinetic analyses
In total, 444 plasma concentrations (222 each for ampicillin and sulbactam) were available for the population PK modelling. Figure 1 shows plasma concentration vs. time profile for ampicillin and sulbactam, respectively. As expected, PK profiles for ampicillin and sulbactam were similar and suggested bi-phasic elimination.
Figure 1.
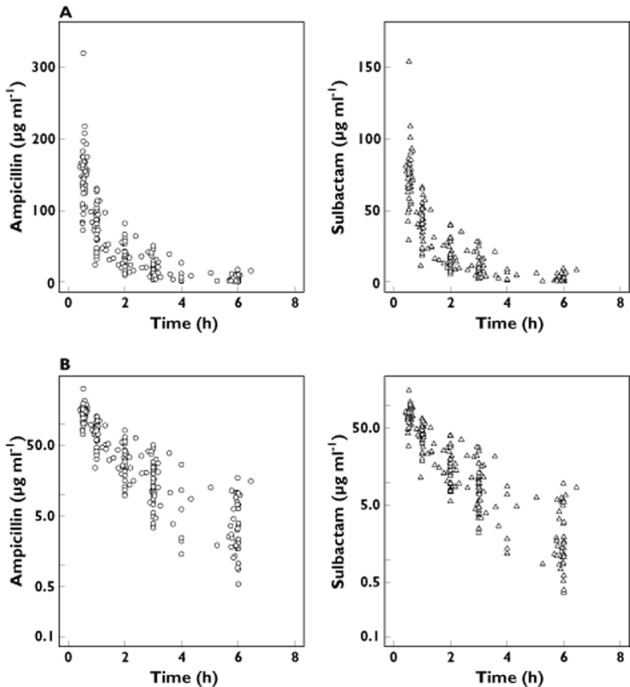
(A) Normal and (B) semilog-scaled observed plasma ampicillin and sulbactam concentrations vs. time profile. The time represents time after the start of the most recent intravenous infusion of ampicillin /sulbactam 3 g four times daily
For ampicillin, a two compartment model resulted in a statistically significant improvement. The three compartment model did not improve the fit in comparison with the two compartment model (ΔOFV = −0.667). For the two compartment model, inter-individual variability for CL and V2 were supported by the data. The correlation between ηCL and ηV2 was not significant (ΔOFV = −0.009). Consistent with the previous knowledge that ampicillin is renally cleared, CLcr was included as a covariate from the base modelling steps (ΔOFV = −53.661). The inter-individual variability was reduced by 16% when adding the effect of CLcr on CL in the base modelling step. For sulbactam, very similar results to those for ampicillin were obtained. Parameter estimates of the base model for each drug are shown in Table 2.
Table 2.
Parameters estimates (RSE%) for the base models for ampicillin and sulbactam and the combined final model
| Parameter | Separate base model | Combined final model | ||
|---|---|---|---|---|
| Estimate | (RSE%) | Estimate | (RSE%) | |
| Ampicillin (1) | ||||
| CL(1) (l h−1) | 10.7 | (3.40) | 10.7 | (3.39) |
| V1(1) (l) | 9.45 | (6.33) | 9.97 | (6.07) |
| Q(1) (l h−1) | 4.78 | (18.3) | 4.14 | (21.8) |
| V2(1) (l) | 4.91 | (10.3) | 4.48 | (9.91) |
| θCLcr on CL | 0.687 | (8.31) | 0.701 | (7.65) |
| θBWT on V2 | – | – | 1.00 | Fix |
| Interindividual variability* | ||||
| CV [ηCL,i(1)] (%) | 15.5 | (13.9) | 14.8 | (15.5) |
| CV [ηV2,i(1)] (%) | 26.5 | (31.5) | 15.2 | (36.2) |
| Intra-individual variability | ||||
| CV [εij(1)] (%) | 23.5 | (14.9) | 24.2§ | (13.5)§ |
| Sulbactam (2) | ||||
| CL(2) (l h−1) | 10.4 | (3.39) | 10.4 | (3.40) |
| V1(2) (l) | 9.77 | (6.72) | 10.2 | (7.04) |
| Q(2) (l h−1) | 4.90 | (22.0) | 4.58 | (28.2) |
| V2(2) (l) | 4.57 | (11.2) | 4.04 | (12.1) |
| θCLcr on CL | 0.667 | (8.92) | 0.701 | (7.65) |
| θBWTon V2 | – | – | 1.00 | Fix |
| Inter-individual variability* | ||||
| CV [ηCL,i(2)] (%) | 15.4 | (14.4) | 15.2 | (15.2) |
| CV [ηV2,i(2)] (%) | 14.7 | (65.7) | 14.8 | (28.3) |
| Intra-individual variability | ||||
| CV [εij(2)] (%) | 22.7 | (15.7) | 23.3§ | (14.4)§ |
| ρ[εij(1), εij(2)]† | – | – | 0.946 | (29.5) |
| ρ[ηCL,i(1), ηCL,i(2)]‡ | – | – | 0.858 | (34.8) |
*The CV and its RSE were calculated based on Tailor approximation of square root of ω2 with SE of ω2. †Correlation coefficient between the intra-individual variability for ampicillin and sulbactam. ‡Correlation coefficient between the random effects on CL for ampicillin and sulbactam. §The CV and its RSE were calculated based on Tailor approximation of square root of σ2 with SE of σ2.
Data from both drugs were integrated and simultaneously modelled using the L2 data item of nonmem. When correlation was allowed for ηCL of ampicillin and sulbactam, the OFV decreased by 53.161 points, whereas correlation between ηV2 of ampicillin and sulbactam was not statistically significant (ΔOFV = −0.673). The effects of CLcr on CL for ampicillin and sulbactam were integrated into one common parameter, because the OFV increased only 0.054 points after the integration.
In the stepwise covariate modelling, covariates of interest were tested using the automated stepwise procedure in PsN. For ampicillin, only the effect of body weight on V2 was significant. Since the estimate was sufficiently close to 1.0 which was considered a standard physiological allometric scaling on volume 21, the fixed scaling was therefore chosen for the final model. Inclusion of any other covariate was not statistically significant. For sulbactam, body weight on V2 was found to be significant in the forwards inclusion step (ΔOFV = −4.358), whereas it was not included in the backwards exclusion process. Considering the clinical relevance between body weight and distribution volume, body weight on V2 was ultimately included as a physiological allometric scaling parameter for sulbactam. The final model for both ampicillin and sulbactam is given below (k = 1 for ampicillin or 2 for sulbactam).
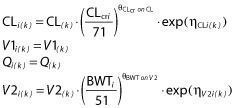 |
Parameter estimates of the combined final model are shown in Table 2. The inter-individual variability was relatively small (14.8 to 15.2%) and, as expected, inter-individual variability for CL of ampicillin and sulbactam was highly correlated (ρ = 0.858). The intra-individual variability was also highly correlated (ρ = 0.946), likely due to the fact that the same sample was used to determine the concentration of each drug.
There was a low to moderate degree of shrinkage in the inter-individual variability. The η shrinkage was 7.8% and 7.1% for CL of ampicillin and sulbactam, respectively and 38% and 37% for V2 of ampicillin and sulbactam, respectively. The degree of shrinkage in the intra-individual variability (ε shrinkage) was low, 10% for both ampicillin and sulbactam. The η shrinkage of V2 for ampicillin and sulbactam was relatively high. It has been reported previously that when shrinkage is present (usually greater than 20% to 30%), model diagnostics should be based not on empirical Bayesian estimation but on simulation-based diagnostics 20. In this analysis, the simulation-based diagnostics (visual predictive check) showed adequacy of the model as described below. Diagnostic plots are presented in Figure 2. The plots of observed plasma concentrations (DV) vs. PRED and IPRED indicated central tendency to the identity line (DV = PRED or IPRED) and no major bias was observed. In addition, plots of CWRES vs. PRED or TIME did not show any systematic trend. A visual predictive check was performed by providing plots of predicted and observed concentrations for ampicillin/sulbactam vs. time based on the final combined model (Figure 3). The observed concentrations at 10th, 50th and 90th percentile points were within the respective predicted 95% intervals. On the whole, the final model adequately described the actual ampicillin/sulbactam PK profile.
Figure 2.
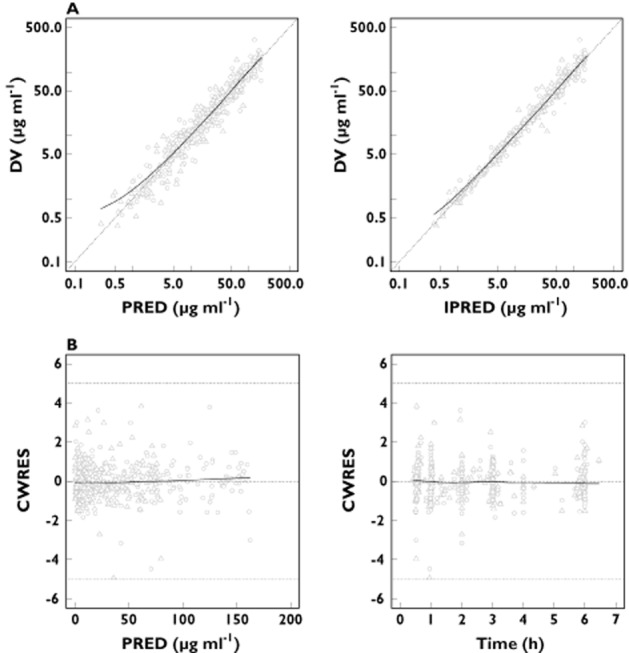
(A) Scatter plots of observed plasma concentrations (DV) vs. population predicted concentrations (PRED) and DV vs. individual predicted concentrations (IPRED) for the final model. Each dotted line represents the concordance line (Y = X). (B) Scatter plots of residuals normalized by the SD of the data (conditional weighted residuals [CWRES]) vs. PRED and CWRES vs. time after the last dose for the final model. Dotted lines represent CWRES = 0 or ± 5. Note: circles and triangles represent ampicillin and sulbactam, respectively. Each solid line indicates a non-parametric regression with robust local linear fit
Figure 3.
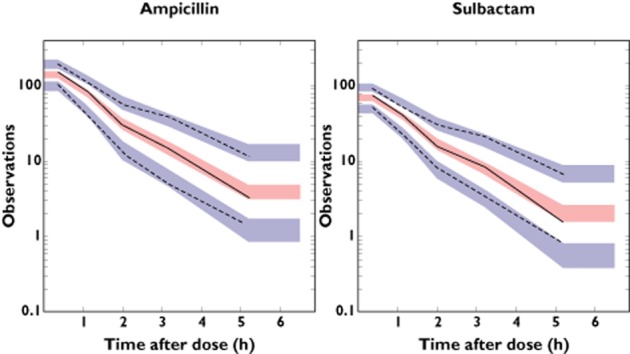
Visual predictive check plots representing concentration for ampicillin (left panel) and sulbactam (right panel) (μg ml−1) vs. time after the most recent administration (h). Each panel shows observed 10, 50 and 90th percentile points (black lines) and model predicted 95% CIs for 10, 50 and 90th percentile points (blue and red areas)
Pharmacodynamic analyses
Causal pathogens were identified for 23 of 47 patients prior to treatment. Eight different kinds of pathogens were identified, most were S. pneumonia, H. influenza or M. catarrhalis. The MICs were determined for all the identified pathogens (n = 32). Table 3 shows mean (SD) for f t>MIC% for each pathogen. In all the cases, these means were usually > 50% of the treatment period, which is generally accepted as a good threshold for clinical efficacy 8–10.
Table 3.
Summary of identified pathogens, MICs and time above MICs
| Identified bacteria | Number of patients | MIC (μg ml−1)* | f t>MIC%† | ||
|---|---|---|---|---|---|
| S. pneumonia | 11 | 0.06 | 0.06–2.00 | 98.1 | (6.46) |
| H. influenza | 8 | 0.50 | 0.12–4.00 | 84.7 | (23.4) |
| M. catarrhalis | 8 | 0.12 | 0.06–0.12 | 100 | (0.00) |
| E. coli | 1 | 4.00 | NA | 94.0 | (NA) |
| K. oxytoca | 1 | 16.00 | NA | 44.7 | (NA) |
| S. aureus | 1 | 0.12 | NA | 100 | (NA) |
| K. pneumonia | 1 | 4.00 | NA | 100 | (NA) |
| E. aerogenes | 1 | 4.00 | NA | 78.5 | (NA) |
NA = not applicable. *Median (range) as ampicillin concentration. †Mean (SD) fraction of time above MIC for treatment period as unbound plasma ampicillin concentration assuming the free fraction was 0.72.
Simulation results
The typical Cmax, AUC and t1/2 for ampicillin and sulbactam following 30 min intravenous infusions of 3 g ampicillin/sulbactam at different recommended dosing intervals in accordance with CLcr are shown in Table 4. The predicted ranges for Cmax and AUC across CLcr ranges of 60 to 90, 30 to <60, 15 to <30 and 5 to <15 ml min−1 for the four times daily, three times daily, twice daily and once daily regimens, were similar.
Table 4.
Predicted range of Cmax, AUC, and t1/2 for ampicillin and sulbactam following 30 min intravenous infusions of 3 g ampicillin/sulbactam in a typical individual with different CLcr and different dosing intervals
| CLcr category (ml min−1) | Dosing interval | Ampicillin | Sulbactam | ||||
|---|---|---|---|---|---|---|---|
| Cmax (μg ml−1) | AUC(0,48 h) (μg ml−1 h) | t1/2 (h) | Cmax (μg ml−1) | AUC(0,48 h) (μg ml−1 h) | t1/2 (h) | ||
| 60–90 | Four times daily | 139–151 | 1260–1670 | 1.20–1.42 | 68.6–74.2 | 650–861 | 1.09–1.33 |
| 30–<60 | Four times daily | 151–173 | 1690–2690 | 1.43–2.02 | 74.4–85.1 | 872–1380 | 1.34–1.96 |
| 30–<60 | Three times daily | 149–166 | 1270–2030 | 1.43–2.02 | 73.3–81.5 | 655–1050 | 1.34–1.96 |
| 15–<30 | Twice daily | 162–176 | 1400–2190 | 2.06–3.06 | 79.5–86.4 | 718–1120 | 2.00–3.03 |
| 5–<15 | Once daily | 170–185 | 1160–2310 | 3.20–6.27 | 83.1–90.7 | 599–1190 | 3.16–6.28 |
Note: The predicted range represents Cmax, AUC or t1/2 values for a typical individual (51 kg body weight) with maximum or minimum CLcr of each category.
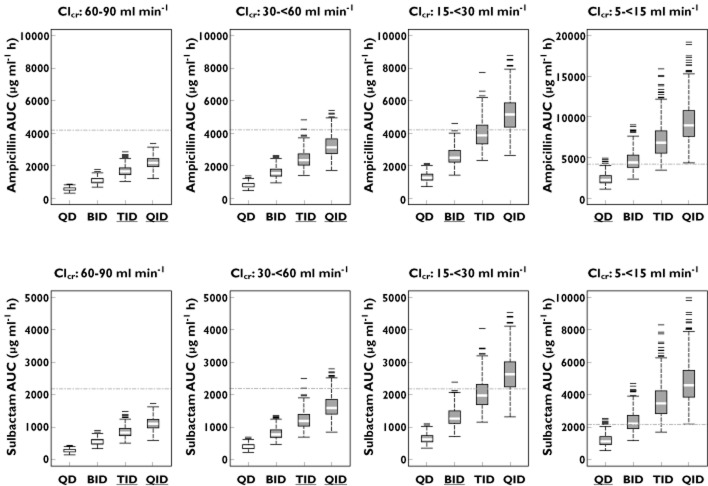
The results of the population based simulation over a 3 day treatment duration (minimal treatment duration specified in the clinical trial) are shown in Figures 4 and 5. Figure 4 shows the simulated distributions for AUC across the dosing intervals compared with the highest individual AUC observed in the clinical trial (empirical Bayesian estimate). The recommended dosing intervals for CLcr ranges of 30 to <60, 15 to <30 and 5 to <15 ml min−1 resulted in AUC distributions, which in comparison with shorter dosing intervals, did not significantly exceed the highest individual AUC observed in the clinical trial. For 60 to 90 ml min−1, the AUC distribution following the four times daily dosing was significantly lower than the highest individual AUC observed in the clinical trial.
Figure 4.
Box plots of simulated AUC for ampicillin (upper panels) and sulbactam (lower panels) following multiple 30 min intravenous infusions of 3 g ampicillin/sulbactam for 3 days of treatment by different renal functions with different dosing intervals. The dotted line represents the highest observed AUC (empirical Bayesian estimate) following intravenous administrations of ampicillin/sulbactam 3 g four times daily in CAP patients. Note: dosing intervals with under bar (in x-axis) represent recommended dosing intervals. QD once daily dosing, BID twice daily dosing, TID three times daily dosing, QID four times daily dosing
Figure 5.
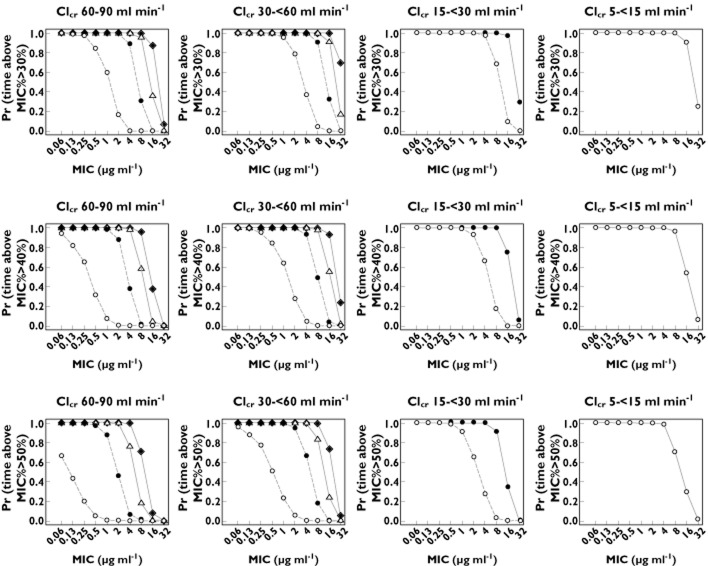
Probability of fraction of time above MIC for treatment period as unbound plasma ampicillin concentration (f t>MIC%) ≥ 30, 40 and 50% following multiple 30 min intravenous infusions of 3 g ampicillin/sulbactam for 3 days of treatment. The lines represent the probability by each dosing interval (○ once daily, • twice daily, △ three times daily, ♦ four times daily). The solid lines represent recommended dosing intervals
Figure 5 shows that the probability of achieving f t>MIC % ≥ 30, 40 or 50% increases as the dosing interval is shorten. Simulations indicated that the high dose (ampicillin/sulbactam 3 g four times daily) provided the highest probability of exceeding the f t>MIC% across the range of causative pathogen MIC (0.06 to 16.0 μg ml−1). When the dosing interval was reduced to three times daily, twice daily and once daily, for CLcr ranges of 30 to <60, 15 to <30 and 5 to <15 ml min−1, respectively, the probability-MIC curves were similar to those achieved with four times daily for patients with CLcr of 60 to 90 ml min−1. For example, all the regimens (four times daily, three times daily, twice daily and once daily dosing for CLcr ranges of 60 to 90, 30 to <60, 15 to <30, and 5 to <15 ml min−1) provided at least 80% probability of exceeding f t>MIC% equal to 30% for an MIC of 16 μg ml−1 over a 3 day treatment period. Similarly, all the regimens provided at least 80% probability of exceeding f t>MIC% equal to 50% for an MIC of 4 to 8 μg ml−1 over a 3 day treatment period.
Discussion
The present analysis has demonstrated that the PK following 30 min infusions of the high dose (3 g ampicillin/sulbactam four times daily) is adequately described by a two compartment model with simultaneous fit of ampicillin and sulbactam PK data, where CLcr on CL and body weight on V2 were identified as covariates for both drugs. It is important to mention that even though body weight was not identified as a covariate on CL in our analysis, it is indirectly included through CLcr, since body weight is used to estimate CLcr through the Cockcroft & Gault equation.
Noguchi et al. 5 reviewed the PK of ampicillin and sulbactam in healthy volunteers. Total clearance was reported to range from 17.8 to 20.9 and 12.1 to 16.0 l h−1 for ampicillin and sulbactam, respectively. The steady-state volumes of distribution ranged from 14.2 to 15.1 and 12.2 to 16.3 l for ampicillin and sulbactam, respectively (based on a body weight of 51 kg). In the present analysis, the population parameter for CL (CLcr = 120 ml min−1) was estimated to be 15.5 and 15.0 l h−1 for ampicillin and sulbactam, respectively. Population mean values of V1 plus V2 were estimated to be 14.5 and 14.2 l, respectively. The mean ampicillin CL of this patient population was lower when compared with that of healthy volunteers. The difference might be due to the difference in renal function between the two populations. However, the difference was small and the other parameter values for ampicillin and sulbactam were similar. The PK model established in this analysis was generally consistent with PK models previously reported in healthy volunteers.
The strong influence of renal function on exposure to these drugs is consistent with prior knowledge. The PK of a single dose of 2 g ampicillin and 1 g sulbactam in subjects with reduced renal function has been previously reported 7. The mean (SD) AUC in subjects with CLcr 30 to 60 ml min−1 (n = 6) was 217 (73.5) and 121 (45.0) μg ml−1 h for ampicillin and sulbactam, respectively. The mean (SD) of AUC for empirical Bayesian estimates from this analysis with the same level of renal impairment (n = 20) was estimated to be 262 (44.7) and 135 (25.3) μg ml−1 h for ampicillin and sulbactam, respectively. Similarly, the mean (SD) AUC in subjects with CLcr 7 to 30 ml min−1 (n = 4, mean CLcr 21.8 ml min−1) was previously reported to be 380 (59.6) and 262 (77.3) μg ml−1 h, respectively. Although the present population did not include patients with CLcr below 30 ml min−1, the predicted typical values of AUC for CLcr equal to 21.8 ml min−1 were 428 and 220 μg ml−1 h for ampicillin and sulbactam, respectively. The model-based results were therefore considered sufficiently similar to the previously reported data in subjects with lower renal functions, to allow extrapolation of this model to patients with severe renal impairment.
The Cockcroft & Gault equation as a surrogate for renal function is reported to have proven very useful due to its simplicity and relative accuracy 22. However, the equation has limitations. For example, it overestimates GFR 23, it is difficult to obtain an accurate estimate at serum creatinine <1 mg dl−1 24, and bias of the equation is influenced by body weight and BMI 25. Although there are other methods available to estimate kidney function, like the Modification of Diet in Renal Disease (MDRD) study equation, this also has limitations. The MDRD equation needs to be multiplied by each patient's ratio of body surface area/1.73 for PK studies to guide dosage adjustment for individual patients 22. In addition, the equation is recommended to be used for reporting the specific value only if the estimated GFR is less than 60 ml min−1 1.73 m–2 26. While there are some benefits and limitations for each equation, consistency of the measuring method to previous studies should also be considered. With regard to ampicillin and sulbactam, effects of renal impairment on exposure had been historically assessed by using CLcr as a surrogate for renal function. It is therefore considered adequate to evaluate the effect of renal function on the PK using an estimated CLcr of the Cockcroft & Gault equation.
The good estimated f t>MIC% coverage for the majority of the identified pathogens in the clinical trial was consistent with the good reported clinical response in the treatment of the CAP with 3 g ampicillin/sulbactam (2:1 g) four times daily in this population 11. Of the 23 patients whose pathogen(s) were identified, only one patient was considered to have an ineffective clinical response 11. The causative pathogens (MIC μg ml−1) in this patient were H. influenza (0.50), M. catarrhalis (0.12), S. aureus (0.12) and S. pneumonia (0.06). The f t>MIC% for the pathogens was almost 100%, and therefore under exposure can be ruled out as the reason for the ineffective response. As shown in Table 3, most of the MICs for identified pathogens ranged from 0.06 to 4.00 μg ml−1, with the highest MIC 16.0 μg ml−1 found in one patient, whose causal pathogen was K. oxytoca. Although the f t>MIC % for the patient (44.7%) was borderline for bactericidal activity (50%), the response in this patient was determined as being clinically effective.
The recommended dosage in the US label 1 for CLcr ≥ 30, 15 to 29, and 5 to 14 ml min−1 1.73 m−2, for which value from the Cockcroft & Gault equation may be substituted, is 1.5 to 3.0 g every 6 or 8 h, every 12 h and every 24 h, respectively. The population based simulation indicated that the simulated AUC distributions were generally below the highest observed exposure from the clinical trial, when the dosing interval was adjusted in accordance with the recommendation of the US label. It was also shown that this dose adjustment provided a similar probability of achieving bacteriostatic (f t>MIC% ≥ 30%) and bactericidal (f t>MIC% ≥ 50%) activity in comparison with four times daily dosing in patients with mild reductions in GFR (CLcr 60 to 90 ml min−1). In particular, the simulations indicated that the recommended regimens provided at least 80% probability of patients achieving exposure coverage associated with bactericidal and bacteriostatic activity for pathogens with MICs of up 4 to 8 μg ml−1 and 16 μg ml−1 over a 3 day treatment period, respectively.
In some cases of our simulation, probability of f t>MIC% > 30, 40 or 50% is identical between the three times daily and four times daily dosing regimens for certain MIC values. For example, probability of f t>MIC% > 50% in patients with CLcr 60–90 ml min−1 (bottom left panel of Figure 5) was identical between three times daily and four times daily at MIC ≤ 2 μg ml−1. Meanwhile, the difference appears at MIC ≥ 4 μg ml−1. Based on EUCAST (European Committee on Antimicrobial Susceptibility Testing) reference 27, microorganisms with MIC < 8.0 μg ml−1 are generally susceptible to ampicillin/sulbactam. At the threshold (MIC = 8.0 μg ml−1) in patients with CLcr 60–90 ml min−1, the simulated probabilities of f t>MIC > 50% in three times daily and four times daily were 18% and 71%, respectively. It is suggested that the probability of f t>MIC% > 30, 40 or 50% by the dosage regimen (three times daily or four times daily) is likely similar at low MIC values, but different at high MIC values.
The maximum observed AUC from the actual study to set an arbitrary ‘upper’ exposure level was used to assess the potential of unnecessary over exposure. This level is higher than that which would be achieved in patients with normal renal function (> 90 ml min−1) given 64% of the population had mild to moderate reduction in GFR (<90 ml min−1), but we consider it a reasonable initial guide given the good observed toleration profile in the patient population 11.
Our simulation results assume no impact of renal function on protein binding. However, given that both sulbactam and ampicillin are relatively unbound, a change here should not drastically change our results. Similarly, it should be noted that despite good comparability with the established literature, our predictions for CLcr below 30 ml min−1 still depend on extrapolation, so caution is required even with once daily dosing in these patients.
Despite these promising clinical trial results and subsequent simulations, the clinical effectiveness and toleration of a high dose ampicillin/sulbactam (3 g four times daily) depends on the disease, the pathogen and the infection site. These predictions should therefore only be used as a guide to inform standard clinical management of patients with CAP being treated with this product. However the same approach as that presented here can be used to determine time above MIC for other drugs where the effectiveness is related to the time of exposure and in other diseases.
In conclusion, this study, using a population modelling approach, provided PK models for ampicillin and sulbactam, the time above MICs for identified pathogens, and associated simulation results. These findings provide useful information and augment evidence for the established dosage regimens in patients with various degrees of renal impairment.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare the studies described herein were funded by Pfizer Japan Inc. Elena Soto and Scott Marshall are employees of Pfizer Inc. Satoshi Shoji, Yoshiro Tomono and Chieko Muto are employees of Pfizer Japan Inc. All authors had support from Pfizer Japan Inc. and Pfizer Inc. for the submitted work. There are no other relationships or activities that could appear to have influenced the submitted work.
List of principal investigators (study centre)
Masaharu Kinoshita (Nagata Hospital), Toru Rikimaru (Fukuoka Sanno Hospital), Hiroyuki Taniguchi (Tosei General Hospital), Naoki Miyao (Nippon Koukan Hospital), Sekiya Koyama (National Hospital Organization Matsumoto Medical Center Chushin Matsumoto Hospital), Yutaka Nishigaki (National Hospital Organization Asahikawa Medical Center), Tsutomu Shinohara (National Hospital Organization Kochi National Hospital), Eri Hagiwara (Kanagawa Cardiovascular and Respiratory Center), Hirohisa Ichikawa (KKR Takamatsu Hospital), Toyomitsu Sawai (National Hospital Organization Ureshino Medical Center), Masafumi Miyajima (National Hospital Organization Kumamoto Saishyunsou Hospital), Atsuhiko Tada (National Hospital Organization Minami-Okayama Medical Center), Masahiro Shirai (National Hospital Organization Tenryu National Hospital), Hiroshi Yamamoto (National Hospital Organization Hokkaido Medical Center), Yoshiro Mochiduki (National Hospital Organization Himeji Medical Center), Masahiro Aoshima (Sekishinkai Sayama Hospital), Hiroshi Takahashi (Saka General Hospital), Kouko Hidaka (National Hospital Organization Kokura Medical Center), Hiroyuki Muranaka (Saiseikai Kumamoto Hospital), Hiroshi Mukae (University of Occupational and Environmental Health), Yoshihiro Yamamoto (Nagasaki University School of Medicine), Kiyoyasu Fukushima (Japanese Red Cross Nagasaki Genbaku Isahaya Hospital).
Acknowledgments
We thank Stuart Pearce of Clinical Informatics & Innovation, Pfizer Inc. and contributors who provided the dataset used for this population PK analysis, and Yuichi Yamamoto of Clinical Pharmacology, Pfizer Japan Inc. who provided information about the assay method.
We gratefully acknowledge the contributions of trial participants, principal investigators listed below, and all of the medical personnel for the clinical trial used for this population pharmacokinetic−pharmacodynamic analysis. We also acknowledge the contributions of Toshihide Ito, Rio Itamura, Yoshiomi Nakazuru, and Yasuko Kimura who are employees of Pfizer Japan Inc. for the clinical trial conduct.
References
- 1.USP. 2012. UNASYN® (ampicillin sodium/sulbactam sodium), Jan.
- 2.Levin AS. Multiresistant Acinetobacter infections: a role for sulbactam combinations in overcoming an emerging worldwide problem. Clin Microbiol Infect. 2002;8:144–153. doi: 10.1046/j.1469-0691.2002.00415.x. [DOI] [PubMed] [Google Scholar]
- 3.Ripa S, Ferrante L, Prenna M. Pharmacokinetics of sulbactam/ampicillin in humans after intravenous and intramuscular injection. Chemotherapy. 1990;36:185–192. doi: 10.1159/000238765. [DOI] [PubMed] [Google Scholar]
- 4.Foulds G. Pharmacokinetics of sulbactam/ampicillin in humans: a review. Rev Infect Dis. 1986;8:S503–511. doi: 10.1093/clinids/8.supplement_5.503. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi JK, Gill MA. Sulbactam: a β-lactamase inhibitor. Clin Pharm. 1988;7:37–51. [PubMed] [Google Scholar]
- 6.Benson JM, Nahata MC. Sulbactam/ampicillin, a new beta-lactam inhibitor /beta-lactam antibiotic combination. Drug Intell Clin Pharm. 1988;22:534–541. doi: 10.1177/106002808802200702. [DOI] [PubMed] [Google Scholar]
- 7.Blum RA, Kohli RK, Harrison NJ, Schentag JJ. Pharmacokinetics of ampicillin (2.0 grams) and sulbactam (1.0 gram) coadministered to subjects with normal and abnormal renal function and with end-stage renal disease on hemodialysis. Antimicrob Agents Chemother. 1989;33:1470–1476. doi: 10.1128/aac.33.9.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs MR. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin Microbiol Infect. 2001;7:589–596. doi: 10.1046/j.1198-743x.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- 9.Drusano GL. Pharmacokinetic optimisation of β-lactams for the treatment of ventilator-associated pneumonia. Eur Respir Rev. 2007;16:45–49. [Google Scholar]
- 10.Drusano GL. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clinical infectious diseases. 2003;36:S42–50. doi: 10.1086/344653. [DOI] [PubMed] [Google Scholar]
- 11.Pfizer Inc. A multicenter, unblinded, non-comparative study of Unasyn-S 12 g/day evaluating the safety and efficacy in Japanese adult subjects with community acquired pneumonia. Bethesda (MD): National Library of Medicine (US); 2000. ClinicalTrials.gov [Internet]. Available at http://clinicaltrials.gov/ct2/show/results/NCT01189487 (last accessed 4 February 2013). NLM Identifier: NCT01189487. [Google Scholar]
- 12.Shiba K, Saito A, Shimada J, Omori M, Yamaji T, Houjou T, Kaji M, Okuda S, Hori S, Miyahara T, Ueda Y. Clinical studies on sulbactam-ampicillin. Chemotherapy. 1988;36:149–159. [Google Scholar]
- 13.Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Landry ML. Manual of Clinical Microbiology. ninth edn. Washington, DC: ASM Press; 2007. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2012. : Available at http://www.clsi.org/ (accessed 25 July 2012)
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Beal SL, Sheiner LB, Boeckmann AJ, editors. NONMEM Users Guides. Ellicott City, MD: ICON Development Solutions; 1989. –2006. [Google Scholar]
- 17.Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN) – a perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res. 2007;24:2187–2197. doi: 10.1007/s11095-007-9361-x. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82:17–20. doi: 10.1038/sj.clpt.6100241. [DOI] [PubMed] [Google Scholar]
- 20.Savic RM, Karlsson MO. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 22.Lalonde RL, Wagner JA. Drug development perspective on pharmacokinetic studies of new drugs in patients with renal impairment. Clin Pharmacol Ther. 2009;86:557–561. doi: 10.1038/clpt.2009.182. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greence T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Winter ME. Basic Clinical Pharmacokinetics. 3rd edn. Washington, DC: Applied therapeutics Inc. Sixth Printing; 1999. pp. 93–103. [Google Scholar]
- 25.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function – measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 27.The European Committee on Antimicrobial Susceptibility Testing – EUCAST. Available at http://www.eucast.org/ (accessed 19 November 2012) [DOI] [PubMed]