Abstract
Due to socioeconomic factors, more couples are choosing to delay conception than ever. Increasing average maternal and paternal age in developed countries over the past 40 years has raised the question of how aging affects reproductive success of males and females. Since oxidative stress in the male reproductive tract increases with age, we investigated the impact of advanced paternal age on the integrity of sperm nucleus and reproductive success of males by using a Prdx6−/− mouse model. We compared sperm motility, cytoplasmic droplet retention sperm chromatin quality and reproductive outcomes of young (2-month-old), adult (8-month-old), and old (20-month-old) Prdx6−/− males with their age-matched wild type (WT) controls. Absence of PRDX6 caused age-dependent impairment of sperm motility and sperm maturation and increased sperm DNA fragmentation and oxidation as well as decreased sperm DNA compaction and protamination. Litter size, total number of litters and total number of pups per male were significantly lower in Prdx6−/− males compared to WT controls. These abnormal reproductive outcomes were severely affected by age in Prdx6−/− males. In conclusion, the advanced paternal age affects sperm chromatin integrity and fertility more severely in the absence of PRDX6, suggesting a protective role of PRDX6 in age-associated decline in the sperm quality and fertility in mice.
Keywords: PRDX6, Paternal age, Sperm chromatin, Oxidative stress, Male infertility, Reactive oxygen species
Abbreviations: 8-OHdG, 8-hydroxy-deoxyguanosine; APE1, apurinic endonuclease 1; CDR, cytoplasmic droplet retention; CMA3, chromomycin A3; Cys, cysteine residue; DFI, DNA fragmentation index; DTT, dithiothreitol; GPX4, glutathione peroxidase 4; GSH, reduced glutathione; H2O2, hydrogen peroxide; HDS, high DNA stainability; mBBr, monobromobimane; nGPX4, nuclear glutathione peroxidase 4; OGG1, 8-oxoguanine glycosylase; PRDX1, peroxiredoxin 1; PRDX6, peroxiredoxin 6; Prdx6−/−, peroxiredoxin null; ROS, reactive oxygen species; SCSA, sperm chromatin structure assay; WT, wild type; XRRC1, X-ray repair complementing defective repair in Chinese hamster cells 1
Graphical abstract
Highlights
-
•
Absence of PRDX6 promotes increased levels of 8-OHdG in mouse spermatozoa.
-
•
Sperm DNA damage and impairment of sperm maturation and motility is exacerbated by the absence of PRDX6 in aging males.
-
•
Prdx6−/− fathers are subfertile producing smaller litters and pups with low birth weight compared to wild-type fathers.
-
•
PRDX6 is important to protect paternal DNA and sperm functions during aging.
Introduction
Delayed parenthood due to socio-economic factors is becoming an increasingly widespread phenomenon in industrialized countries [1,2]. Paternal age has been continuously rising since 1980 [1]. Although we know more about the effects of maternal age on reproductive outcomes, little is understood about the effects of advanced paternal age on sperm quality, reproductive outcomes and offspring health.
Epidemiologic studies have indicated that advancing paternal age at childbearing is linked to several genetic disorders, including autism spectrum disorders [3,4], schizophrenia [5,6], and bipolar disorder [7], as well as psychiatric and academic morbidity problems [8]. Recent genomic studies have reported that the age of the fathers at conception is an important factor in determining the number of de novo mutations in children [9].
There is growing evidence that advancing paternal age is associated with an increased frequency of certain genetic and chromosomal defects in spermatozoa [10–12]. Clinical studies and animal models have shown that the quality of semen changes with advancing age, leading to decreased motility and abnormal morphology of sperm, decreased semen volume, and altered pregnancy outcome [13–16]. These studies provide strong evidence that spermatozoa produced in old males differ from those of young ones, and are more sensitive to the oxidative stress associated with paternal age [17,18]. Higher levels of double-stranded DNA breaks were reported in older men [19], and a gradual age-related upward trend has been proposed for DNA damage since the DNA fragmentation index (DFI) increased more than double in 60-year old compared to 20-year old men [20,21].
The integrity of the sperm nucleus is a critical issue in male fertility; several studies have shown that increased DNA fragmentation in spermatozoa is associated with decreased fertility, infertility or problems in the health of the offspring [22–24]. Moreover, the evidence for increased sperm DNA damage with advancing paternal age is well documented [25–27].
Peroxiredoxins (PRDXs) are newly discovered antioxidant enzymes with a wide distribution among species [28]. PRDX6 is a novel antioxidant enzyme that contains one cysteine residue in its active site (1-Cys PRDX) [29] and has glutathione peroxidase and Ca2+-independent phospholipase A2 activities [30] that protects cells from oxidative stress-mediated damage [30]. PRDX6 is the most abundant PRDX and is present in all compartments of human spermatozoa [31,32]. Spermatozoa from infertile men have lower levels of PRDX1 and PRDX6 associated with low sperm motility and high DNA damage [33]. Moreover, we showed that PRDX6 was the only PRDX member reacting with low amounts of hydrogen peroxide (H2O2) in the range needed for sperm capacitation [31].
We recently showed that PRDX6 is required to maintain sperm quality and function in mice; 2-month old Prdx6−/− males had low sperm motility, reduced fertility, increased levels of oxidative stress biomarkers (protein carbonylation, lipid peroxidation, and DNA oxidation), and higher post-translational protein modifications (S-glutathionylation and nitrosylation) than the wild type males [34]. Since oxidative stress is increased during aging, the aim of the present study was to determine the impact of aging on reproductive outcomes and sperm quality in Prdx6−/− male mice.
Materials and methods
Reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), except for monobromobimane (mBBr), purchased from Calbiochem, San Diego, CA, USA. Biotinylated horse anti-mouse IgG was bought from Vector Laboratories (Burlington, ON, Canada). Alexa Fluor 555 conjugate of streptavidin and Prolong Antifade were purchased from Molecular Probes (Eugene, OR, USA). The anti-8-OHdG antibody was purchased from StressMarq Biosciences Inc (Victoria, BC, Canada). Donkey anti-rabbit immunoglobulin IgG and goat anti-mouse IgG antibodies (both conjugated with horseradish peroxidase) were provided by Cederlane Laboratories Ltd (Hornby, Canada). Nitrocellulose membranes (pore size, 0.22 µm) were bought from Osmonics Inc (Westborough, MA, USA) and the enhanced chemiluminescence kit Lumi-Light from Roche Molecular Biochemicals (Laval, QC Canada). Immunodetection of blotted proteins was performed with radiographic films obtained from Fuji (Minami-Ashigara, Japan). Other chemicals used were of at least reagent grade.
Animals
Prdx6−/− mice were produced at the Royal Victoria Hospital Animal Facility from breeder pairs generously donated by Dr. Aron Fisher (University of Pennsylvania) [35]. The Prdx6−/− mouse model has been generated by Dr. Ye Shih Ho in collaboration with the laboratory of Dr. Aron Fisher at the University of Pennsylvania on a mixed background [35]. The mice were subsequently backcrossed to >99.9% genetic identity for C57Bl/6 as determined by microsatellite analysis performed by the Jackson laboratory [36]. Age groups were selected as 2 months, 8 months and 20 months, and each age group consisted of Prdx6−/− mice (n=10) and wild type (C57BL/6J) (n=10) for each age. Mice were maintained on a 14-h light/10-h dark cycle and provided with food and water ad libitum. All procedures were carried out in accordance with the regulations of the Canadian Council for Animal Care (CACC) and were approved by the Animal Care Committees of McGill University and the McGill University Health Centre. Mice of all ages were euthanized at the end of 6-month long mating experiments.
Mating experiments
All three age groups of Prdx6−/− males and their littermate wild type controls (n=10) to be paired with 8 week old wild type females as duos, and monitored for litter numbers, litter size, sex of the pups and total number of pups per male for 6 months. Reproductive health of the offspring of these animals was also monitored, at 2 months of age; each male offspring (n=4) was paired with a young wild type female for 2 months. Litter frequency, litter size, number of litters and pups were evaluated. Pups body weight at fixed 21-day for weaning time (normal time for WT pups) as well as extra days to achieve weaning weight needed for pups sired by Prdx6−/− fathers were recorded.
Organs and sperm samples
The testes, epididymides, seminal vesicles and other reproductive accessory glands were removed immediately after euthanasia. Body and reproductive organs (testes, epididymes, seminal vesicles and prostate) weights were recorded. Cauda epididymes were placed in phosphate-buffered saline (PBS) (1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl, pH 7.0) and pierced with 27G needle 5 times to allow spermatozoa to swim-out for 10 min at 37 °C. Spermatozoa were further diluted to 5×106 cells/ml in Biggers, Whitten, and Whittingham (BWW) medium composed of 91.5 mM NaCl, 4.6 mM KCl, 1.7 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 5.6 mM d-glucose, 0.27 mM sodium pyruvate, 44 mM sodium lactate, and 20 mM HEPES.
Sperm motility and cytoplasmic droplet retention
Sperm motility (total and progressive) and cytoplasmic droplet retention determination were performed promptly, while keeping the spermatozoa at 37 °C. Evaluation of sperm motility parameters was conducted using a computer-assisted sperm analysis system (CASA) with Sperm Vision HR software version 1.01 (Minitube, Ingersoll, ON, Canada). A total of 200 spermatozoa were examined for each sample. Samples were analyzed for percent motility as well as progressive motility (average path velocity >25 µM/s).
The presence of cytoplasmic droplets, the leftovers of cytoplasm, was determined using the method established by Syntin and Robaire [16]. Motility images that had been recorded with CASA were still-frozen and spermatozoa were assessed for the presence or absence of cytoplasmic droplets. Minimum of 100 spermatozoa (in duplicate) per animal were evaluated.
Sperm chromatin structure integrity
Sperm DNA fragmentation and DNA compaction were determined by differential flow-cytometry-based assays using a MACSQuant Analyzer flow cytometry (Miltenyi Biotec, Inc., Auburn, CA, USA). A total of 10,000 spermatozoa were analyzed per assay.
Sperm DNA fragmentation
The susceptibility of sperm DNA to acid-induced denaturation was assessed by the Sperm Chromatin Structure Assay (SCSA) [37,38]. The flow cytometer is equipped with a 585/625 nm filter and a laser tuned to 488-nm line excitation. The raw data were processed using WinList Software (Verity Software, Topsham, ME, USA). The extent of DNA denaturation was expressed as the percentage of DNA fragmentation (DNA fragmentation index, DFI) and DNA compaction was assessed by calculating the High DNA stainability (HDS).
Sperm DNA oxidation
The levels of 8-hydroxy-deoxyguanosine (8-OHdG) were determined by immunocytochemistry. Sperm suspension was smeared onto superfrost plus slides (Fisher Scientific, Montreal, QC, Canada). Air-dried cells were permeabilized with 100% methanol as done before [31]. Cells were rehydrated with PBS supplemented with Triton-X100 (PBS-T), and blocked with 5% goat serum in PBS-T for 30 min at 20 °C. Slides were washed in PBS-T and incubated overnight at 4 °C with anti-8-OHdG antibody (1:100) antibody. After a wash, cells were incubated with biotinylated secondary antibody horse anti-mouse IgG (1:2000) for 1 h at 20 °C. Then, streptavidin conjugated to alexa fluor 555 (1:500) was applied to slides and smears were mounted with ProLong Antifade (Molecular Probes, Eugene, OR, US), and coverslip. Fluorescent signals were examined under an epifluorescence microscope (Zeiss Axiophot, Germany). All images were captured with a digital camera (Retiga 1300, QImaging, Burnaby, BC, Canada) and digitized with Northern Eclipse digital imaging software, version 6.0 (Empix Imaging, Mississauga, ON, Canada). No distinct signals were seen when the primary antibodies were omitted (negative control). Results were presented as % of cells with positive signal.
Chromomycin A3 (CMA3) labeling
The level of protamination was determined on the basis of the accessibility of the fluorochrome, Chromomycin A3 (CMA3), to bind to protamine free sites in the sperm DNA [39]. Aliquots of epididymal spermatozoa (5×106 ml−1) were incubated with 0.25 mg/ml CMA3 in McIlvaine buffer (0.1 M citric acid, 0.2 M Na2HPO4, 10 mM MgCl2; pH 7.0) and incubated for 20 min at 25 °C in the dark. Results were presented as percentage of cells with CMA3 labeling.
Monobromobimane (mBBr) thiol labeling assay
The presence of free thiols in sperm nucleus was determined by labeling them with mBBr as previously described [39] with modifications. Briefly, spermatozoa were incubated in the presence or absence of 1 mM dithiothreitol (DTT). After washed with PBS, the samples were incubated in the dark with 0.5 mM mBBr for 10 min at 37 °C. Spermatozoa were then washed in PBS, sonicated on ice to separate heads from tails, and stored at 4 °C in the dark until analysis. Results were expressed as the percentage of free thiols determined from the mean fluorescence of spermatozoa incubated without DTT and the fluorescence of DTT-treated cells.
Statistical analysis
Normal distribution of data was confirmed using Shapiro–Wilk or Lilliefors tests. Data were analyzed using two-way analysis of variance (ANOVA) (for age and genotype), followed by post hoc Bonferroni test. Differences with a p value of <0.05% were regarded as significant. Results are expressed as mean±SEM. Statistical analyses were performed using the Sigma Systat 13 (Systat software Inc.).
Results
Fertility in aging Prdx6−/− males is severely affected by aging
Given that Prdx6−/− males at 2 months of age had reduced number of litters and pups (Fig. 1), we investigated whether advanced aging has an impact on fertility outcomes of Prdx6−/− males. After 6 month-long mating experiments with young WT females, Prdx6−/− males from 8 and 20 months of age groups produced significantly lower number of litters per male compared to age-matched WT controls (Fig. 1A). We observed a significant decrease in the number of pups per male in Prdx6−/− mice at all ages when compared to WT controls (Fig. 1B).
Fig. 1.
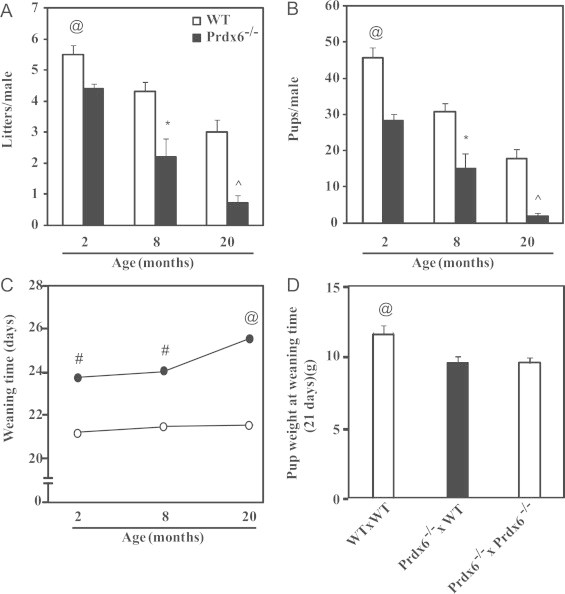
Reproductive outcomes of aging males. Number of litters (A), of pups (B), weaning time of pups sired by WT or Prdx6−/− males (C) and weight at fixed weaning time of 21 days (D) of pups sired by 20-month old WT males mated with young WT females (WT×WT), 20-month old Prd×6−/− males mated with young WT (Prd×6−/−×WT) or Prdx6−/− (Prdx6−/−×Prdx6−/−) females. @ and ^ Mean the highest and lowest values, respectively; # and * Mean higher or lower than their respective age-matched group. N=10 for each group and genotype.
Pups sired by Prdx6−/− males required more time than those from WT age-matched controls to reach the appropriate weight to be weaned (Fig. 1C). This increased time was significantly higher in pups sired by 20-month old Prdx6−/− mice compared to younger knockout fathers. Interestingly, the weight of pups is influenced by the 20-month old Prdx6−/− fathers since hetero or homozygous pups had similar weight at 21 days but lower than that of pups sired by 20-month old WT fathers (Fig. 1D).
Epididymal sperm maturation and motility are impaired in Prdx6−/− during aging
Prdx6−/− epididymes have lower weight than WT controls and other organ weights were similar in all groups (data not shown). At 2 months of age, a significant percentage of spermatozoa from Prdx6−/− mice had higher cytoplasmic droplet retention (CDR) when compared to wild type (Fig. 2) and this proportion was increased during aging, showing the oldest males the highest values of CDR.
Fig. 2.
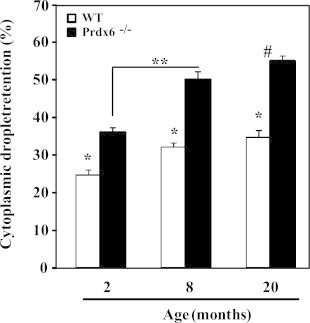
Percentage of cytoplasmic droplet retention in Prdx6−/− and WT epididymal spermatozoa. *Means lower than the respective age-matched group, **Means significantly different (p<0.001). # Means the highest value. N=10 for each group and genotype.
Aging decreased sperm motility in WT and Prdx6−/− males (Fig. 3); however, total and progressive sperm motility in Prdx6−/− mice was significantly lower at all ages compared to age-matched WT controls.
Fig. 3.
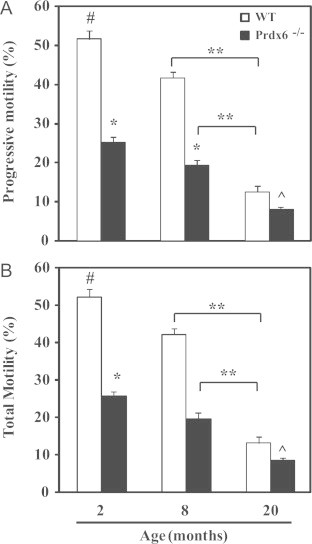
Sperm motility in aging PRDX6−/− and WT mice. # and ^ Mean the highest and lowest values, respectively; *means lower than the other age-matched group and ** Means different (p<0.001). N=10 for each group and genotype.
Prdx6 is required for integrity of sperm chromatin in old Prdx6−/− mice
We next assessed the integrity of sperm chromatin in aging Prdx6−/− males and their age-matched WT controls. An age-dependent increase in sperm DNA fragmentation (DFI) was observed in both groups of males. Starting from 2 months of age, DFI in spermatozoa from Prdx6-null mice was significantly higher than their age-matched WT controls (Fig. 4). By 20 months of age, the highest values of DFI were observed in Prdx6−/− males. Similarly, sperm DNA oxidation (8-OHdG) was increased due to age in both groups (Fig. 5). Young Prdx6−/− (2-month old) had 8-OHdG values that were more than double to those from age-matched WT controls. Moreover, these values were higher at all ages in Prdx6−/− mice compared to the age-matched WT controls (Fig. 5B).
Fig. 4.
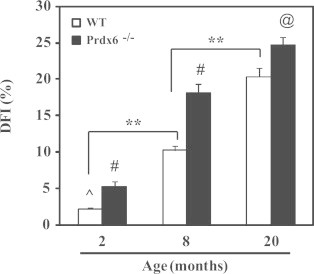
Sperm DNA fragmentation in aging Prdx6−/− and WT mice. @ and ^ Mean the highest and lowest values, respectively. # Means higher than the other age-matched group and ** Means different (p<0.001). N=10 for each group and genotype.
Fig. 5.
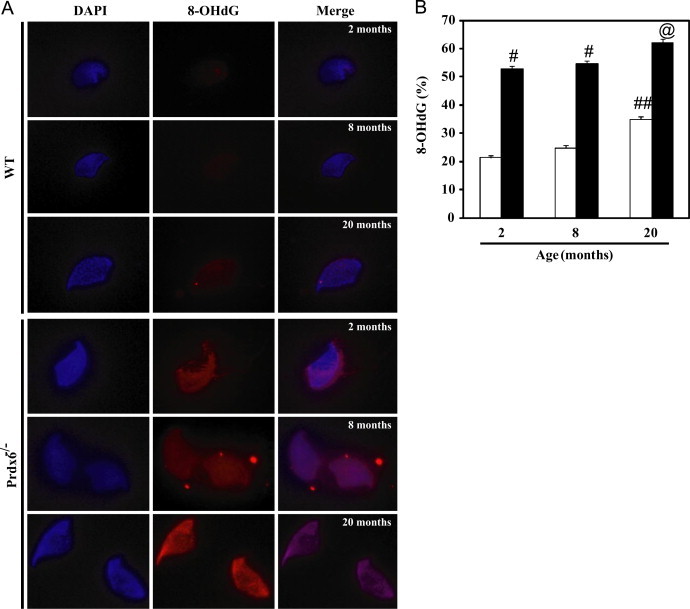
Sperm DNA oxidation in aging Prdx6−/− and WT mice. (A) Immunocytochemistry showing 8-OHdG labeling in mouse spermatozoa. Cauda epididymis spermatozoa were permeabilized with methanol and incubated overnight with the anti-8-OHdG antibody and then with the biotin-labeled anti-mouse antibody followed by streptavidin conjugated to Alexa Fluor 555. All pictures were taken at 1000× magnification using the same exposure time. No labeling was observed with secondary antibody alone (data not shown). (B) Percentage of spermatozoa showing DNA oxidation (8-OHdG labeling). # Means higher than the other age-matched group and ## means higher than the other age groups of WT males (p<0.001). N=10 for each group and genotype.
Sperm DNA compaction was severely affected in Prdx6−/− males (Fig. 6). The three parameters used to characterize sperm DNA compaction (HDS, level of protamination; CMA3 labeling and percentage of nuclear free thiols) were significantly higher in Prdx6−/− spermatozoa compared to those from WT controls at all ages. Interestingly, only the oldest WT males showed significant increase of these parameters compared to the other WT groups. Noteworthy, the lower sperm DNA compaction was evidenced also by the greater nuclear area identified by DAPI stain in Prdx6−/− and WT older mice compared to young WT animals (Fig. 5A).
Fig. 6.
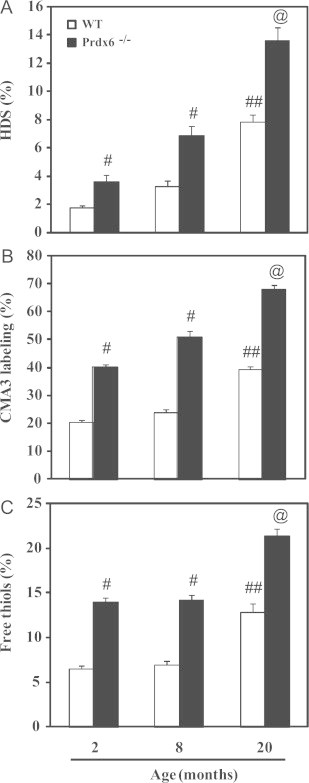
Sperm DNA compaction and level of protamination and of free thiols.@ Means the highest value, # means higher than the other age-matched group and ## means higher than the other age groups of WT males (p<0.001). N=10 for each group and genotype.
Discussion
We demonstrate for the first time the impairment of sperm function, sperm chromatin quality and fertility outcomes of Prdx6−/− mice during aging. These findings support an important role of Prdx6 in the protection of spermatozoa against the age-related damage that translates into low reproductive success and may affect offspring’s health.
One of the hypotheses for the effect of the loss of PRDX6 is that there is an ongoing oxidative stress during aging. Indeed, increased levels of reactive oxygen species (ROS) and a decrease in antioxidant enzymes have been reported in aging male rats [40–42]. This aging-associated oxidative stress promotes sperm DNA oxidation and abnormal sperm chromatin in these animals [17,43]. The increased levels of 8-OHdG and the abnormal sperm chromatin structure in aging male mice which are exacerbated by the lack of PRDX6 indicates the presence of oxidative stress in the aging testes of mice and suggests that PRDX6 is important to scavenge ROS produced during the aging process. This conclusion is of interest since previous reports have indicated the presence of high levels of GSH peroxidase type form (GPX4) in testis and suggested that this is the primary anti-oxidant enzyme in that organ [44]. GPX4 is essential during spermiogenesis to form part of the mitochondrial sheath by its interacting with hydroperoxides [45,46]. The mitochondrial GPX4 knockout male mice are infertile having morphologically abnormal spermatozoa [47]. However, the role of mGPX4 as an antioxidant in the mature spermatozoa is negligible since this enzyme is tightly bound in mitochondria and its activity in vitro can only be obtained after solubilization with high concentrations of dithiothreitol (0.1 M) in the presence of guanidine [45]. The nuclear isoform of GPX4 (nGPX4) is also found in spermatozoa, but it is not essential for sperm function since nGPX4 knockout males are fertile [48]. Spermatozoa from Prdx6−/− (Fig. 6C) or nGPX4−/− mice [48] showed lower sperm DNA compaction with high levels of nuclear free thiols compared to WT controls, indicating a possible cross-talk between PRDX6 and nGPX4 to maintain sperm chromatin stability by acting as protamine thiol peroxidases responsible for the formation of cross-linked protamine disulfides during sperm maturation [49]. However, the subfertility was observed only in Prdx6−/− males, suggesting a major role of PRDX6 in maintaining normal sperm chromatin structure. Moreover, GPX4 is not sufficient to maintain normal age-related antioxidant function while PRDX6 plays an important antioxidant role in the testis and spermatozoa protecting paternal DNA; PRDX6 has been shown previously to be active in spermatozoa and to protect against oxidative stress [31,33].
Salomon et al. [42], reported that an oxidative stress is observed in testis of 24-month old rats due to a decrease in catalase, 2-Cys PRDXs, and GPX as well as superoxide dismutase activities [42]. These enzymes can serve to maintain low intracellular superoxide and H2O2 concentrations but are not able to reduce phospholipid hydroperoxides in order to quench the chain reaction of lipid peroxidation or to repair peroxidized cell membranes. GPX4 and PRDX6 are the only enzymes that have been reported with the ability to reduce phospholipid hydroperoxides at significant rates [50]. The enzyme(s) responsible for the observed GPX activity in the previous study [42] was not determined but could have been PRDX6 since it as well as the classical GSH peroxidases (including GPX4) utilize the glutathione/glutathione reductase system for their activity [51]. Even though these other antioxidant enzymes display significantly lower activity in aged testes, the greater oxidative stress-associated effect in our Prdx6−/− compared to age-matched WT males highlights the importance of this PRDX in the protection of the spermatozoon during aging.
The damaged paternal DNA is associated with a variety of abnormal reproductive outcomes [52,53]. The abnormal reproductive outcomes found in aging Prdx6−/− males (Fig. 1) could be produced by defects in the paternal genome promoted by the aging-related oxidative stress observed (Fig. 2B). It is known that an increase in DNA damage such as aneuploidies and increased mutation frequency during spermatogenesis occur in old compared to young mice [54,55]. This increase in DNA damage is due to an altered base excision repair activity due to a reduction of apurinic endonuclease in old male mice [56]. Similarly, old male rats show a significant reduction of apurinic endonuclease 1 (APE1) and X-ray repair complementing defective repair in Chinese hamster cells 1 (XRRC1; a scaffolding enzyme involved in the stabilization of the base excision repair pathway) in pachytene spermatocytes, thus producing increased levels of 8-OHdG compared to young animals [43]. Moreover, the spermatozoon does not have a complete set of enzymes to perform an appropriate DNA repair. Certainly, downstream base excision repair enzymes APE1 and XRRC1 are absent in human spermatozoa [57]. Therefore, although DNA glycosylases such as OGG1 are present in spermatozoa [57] and can remove 8-OHdG, the abasic site cannot be repaired and the DNA remains damaged.
It is then important to prevent as much as possible the oxidative damage of paternal DNA to assure a healthy progeny; in this model the absence of PRDX6 generates extensive DNA oxidation which is exacerbated by age. This DNA oxidation may promote a high rate of DNA mutations because 8-OHdG induces DNA base mutations such as G>T/C>A transversions [58] that can account for the reduction of fertility observed in old Prdx6−/− males. Therefore, the increased levels of 8-OHdG in spermatozoa observed in PRDX6−/− males suggest the importance of PRDX6 in the protection of paternal DNA against oxidative stress particularly during aging.
PRDX6 is present in the human sperm head particularly in the perinuclear theca [31]. The fact that sperm chromatin is severely affected in Prdx6−/− males compared to WT controls (Figs. 4 and 5) and the location of PRDX6 in the sperm head suggest a role of this antioxidant enzyme in the protection of the paternal genome against ROS. Although the intimate relationship between PRDX6 and sperm DNA is still yet to be established, the fact that PRDX6 contains a lysine-rich C-terminus may allow this interaction. It is known that The Plasmodium falciparum nuclear peroxiredoxin, a 1-Cys PRDX, is a genome wide chromatin associated nuclear peroxiredoxin that binds to DNA by its lysine rich C-terminus, thus protecting the parasite genome against oxidative stress [59].
An interesting finding was the delay of weaning time of pups sired by PRDX6−/− males and exacerbated by aging (Fig. 1C) due to a low weight at the time that WT pups were ready to be weaned (Fig. 1D). We can consider that the damaged paternal genome in Prdx6−/− mice triggers substantial development impairment of these pups generating low birth weight. Moreover, this effect on pup weight is clearly due to the damaged paternal DNA carried by Prdx6−/− fathers regardless of the genotype of the mothers. This result is in accordance with studies in humans where low birth weight odds increased with advanced paternal age [60].
In a recent study, couples where only the man is smoker and diabetic (both conditions associated with oxidative stress that impact negatively on spermatozoa [61,62]) have children with low birth weight [63]. It is possible that a higher rate of DNA mutations is present in the paternal genome of aging PRDX6−/− mice due to the accumulation of 8-OHdG, capable of inducing DNA base transversions as mentioned above [58] that are inherited by the offspring [64]. Since spermatozoa lack complete base excision repair mechanism [57], these accumulated mutations maybe the cause for the reduced fertility of PRDX6−/− males. Oxidative stress is known to produce epigenetic instability promoting the suppression of tumor suppressor genes and thus promoting hepatocarcinogenesis [65]. It is plausible that the oxidative stress generated by the absence of PRDX6 in the knockout males promotes epigenetic instability and consequent dysregulation of gene expression, therefore affecting the embryo development that will translate into pups born with low weight and augmented weaning time.
It has been recognized that the aging male is at risk of transmitting multiple genetic and chromosomal defects to his child [4,20]. Although the oocyte has the capacity to repair some degree of paternal DNA damage [66], most of the sperm DNA damage persists after fertilization and DNA synthesis in the embryo is incapable of repairing the damaged paternal DNA [67].
The effect of the aging-associated oxidative stress could be due to a direct detrimental action on germ cells that will become spermatozoa during spermatogenesis, but also due to an impairment of epididymal maturation. Cytoplasmic droplet retention is a well known indicator of abnormal epididymal epithelium function; increased number of spermatozoa with CDR is associated with infertility [68]. Our results confirm previous studies where increased CDR was observed in aging Brown, Norway male rats [16]. Whereas the presence of a high percentage of cytoplasmic droplets in spermatozoa is associated with a failure of spermatozoa to mature properly and thus may account for the reduced litter size, this abnormal reproductive outcome can also be ascribed to malfunctioning spermatozoa displaying severe motility impairment (Fig. 3). However, what it is clear from these results is that the absence of PRDX6 promotes a greater impairment of sperm maturation and motility. We previously showed that PRDX6−/− spermatozoa have increased levels of redox-dependent modifications such as S-glutathionylation and carbonylation [34]. Redox-dependent modifications are associated with low sperm motility and inability to achieve fertilizing ability as reported in human spermatozoa [69]. Thus, the inability of aging PRDX6−/− males to produce similar numbers of pups as the age-matched WT controls could be due to the impaired motility and fertilizing ability produced by increased levels of redox-dependent modified proteins involved in the motility machinery and the sperm capacitation process.
In conclusion, we demonstrated that PRDX6 plays a significant role in the protection of spermatozoa against the aging-associated oxidative stress. Sperm chromatin and the functionality of spermatozoa is impaired by oxidative stress during spermatogenesis and/or epididymal maturation and the absence of PRDX6 exacerbated the damaged inflicted on the paternal genome generating a reduced number of pups in the aging knockout males. In times where fatherhood is delayed due to social and economic factors, it is important to minimize the aging-associated oxidative stress to assure healthy offspring from older fathers.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR). BO was supported by the Fonds de recherche du Québec Nature et Technologies (FRQNT). Support for the generation of prdx6−/− mice was provided by the National Heart, Lung and Blood Institute of the National Institutes of Health (USA).
References
- 1.Martin J.A., Hamilton B.E., Ventura S.J., Osterman M.J., Mathews T.J. Births: final data for 2011. Natl. Vital Statist. Rep. From Centers Dis. Contr. Prev. Natl. Center Health Statist. Natl. Vital Statist. Syst. 2013;62(1):1–70. 24974591 [PubMed] [Google Scholar]
- 2.Lutz W. Fertility rates and future population trends: will Europe’s birth rate recover or continue to decline? International Journal of Andrology. 2006;29(1):25–33. doi: 10.1111/j.1365-2605.2005.00639.x. 16466521 [DOI] [PubMed] [Google Scholar]
- 3.Frans E.M., Sandin S., Reichenberg A., Långström N., Lichtenstein P., McGrath J.J., Hultman C.M. Autism risk across generations: A population-based study of advancing grandpaternal and paternal age. J.A.M.A. Psychiatry. 2013;70(5):516–521. doi: 10.1001/jamapsychiatry.2013.1180. 23553111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hultman C.M., Sandin S., Levine S.Z., Lichtenstein P., Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry. 2011;16(12):1203–1212. doi: 10.1038/mp.2010.121. 21116277 [DOI] [PubMed] [Google Scholar]
- 5.Frans E.M., McGrath J.J., Sandin S., Lichtenstein P., Reichenberg A., Långström N., Hultman C.M. Advanced paternal and grandpaternal age and schizophrenia: A three-generation perspective. Schizophr. Res. 2011;133(1–3):120–124. doi: 10.1016/j.schres.2011.09.027. 22000939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sipos A., Rasmussen F., Harrison G., Tynelius P., Lewis G., Leon D.A., Gunnell D. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004;329(7474):1070. doi: 10.1136/bmj.38243.672396.55. 15501901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frans E.M., Sandin S., Reichenberg A., Lichtenstein P., Långström N., Hultman C.M. Advancing paternal age and bipolar disorder. Arch. Gen. Psychiatry. 2008;65(9):1034–1040. doi: 10.1001/archpsyc.65.9.1034. 18762589 [DOI] [PubMed] [Google Scholar]
- 8.D’Onofrio B.M., Rickert M.E., Frans E., Kuja-Halkola R., Almqvist C., Sjölander A., Larsson H., Lichtenstein P. Paternal age at childbearing and offspring psychiatric and academic morbidity. J.A.M.A. Psychiatry. 2014;71(4):432–438. doi: 10.1001/jamapsychiatry.2013.4525. 24577047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong A., Frigge M.L., Masson G., Besenbacher S., Sulem P., Magnusson G., Gudjonsson S.A., Sigurdsson A., Jonasdottir A., Jonasdottir A., Wong W.S., Sigurdsson G., Walters G.B., Steinberg S., Helgason H., Thorleifsson G., Gudbjartsson D.F., Helgason A., Magnusson O.T., Thorsteinsdottir U. Rate of de novo mutations and the importance of father/’s age to disease risk. Nature. 2012;488(7412):471–475. doi: 10.1038/nature11396. 22914163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q., Martin R.H. Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet. Cell Genet. 2000;90(3–4):219–226. doi: 10.1159/000056773. 11124518 [DOI] [PubMed] [Google Scholar]
- 11.Bosch M., Rajmil O., Egozcue J., Templado C. Linear increase of structural and numerical chromosome 9 abnormalities in human sperm regarding age. Eur. J. Hum. Genet. 2003;11(10):754–759. doi: 10.1038/sj.ejhg.5201049. 14512965 [DOI] [PubMed] [Google Scholar]
- 12.Sloter E.D., Marchetti F., Eskenazi B., Weldon R.H., Nath J., Cabreros D., Wyrobek A.J. Frequency of human sperm carrying structural aberrations of chromosome 1 increases with advancing age. Fertil. Steril. 2007;87(5):1077–1086. doi: 10.1016/j.fertnstert.2006.08.112. 17433321 [DOI] [PubMed] [Google Scholar]
- 13.Jung A., Schuppe H.C., Schill W.B. Comparison of semen quality in older and younger men attending an andrology clinic. Andrologia. 2002;34(2):116–122. doi: 10.1046/j.0303-4569.2001.00487.x. 11966579 [DOI] [PubMed] [Google Scholar]
- 14.Kidd S.A., Eskenazi B., Wyrobek A.J. Effects of male age on semen quality and fertility: a review of the literature. Fertil. Steril. 2001;75(2):237–248. doi: 10.1016/s0015-0282(00)01679-4. 11172821 [DOI] [PubMed] [Google Scholar]
- 15.Serre V., Robaire B. Paternal age affects fertility and progeny outcome in the Brown Norway rat. Fertil. Steril. 1998;70(4):625–631. doi: 10.1016/s0015-0282(98)00259-3. 9797088 [DOI] [PubMed] [Google Scholar]
- 16.Syntin P., Robaire B. Sperm structural and motility changes during aging in the Brown Norway rat. Journal of Andrology. 2001;22(2):235–244. 11229797 [PubMed] [Google Scholar]
- 17.Zubkova E.V., Wade M., Robaire B. Changes in spermatozoal chromatin packaging and susceptibility to oxidative challenge during aging. Fertil. Steril. 2005;84(Suppl. 2):1191–1198. doi: 10.1016/j.fertnstert.2005.04.044. 16210011 [DOI] [PubMed] [Google Scholar]
- 18.Smith T.B., De Iuliis G.N., Lord T., Aitken R.J. The senescence-accelerated mouse prone 8 as a model for oxidative stress and impaired DNA repair in the male germ line. Reproduction. 2013;146(3):253–262. doi: 10.1530/REP-13-0186. 23813448 [DOI] [PubMed] [Google Scholar]
- 19.Singh N.P., Muller C.H., Berger R.E. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil. Steril. 2003;80(6):1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. 14667878 [DOI] [PubMed] [Google Scholar]
- 20.Wyrobek A.J., Eskenazi B., Young S., Arnheim N., Tiemann-Boege I., Jabs E.W., Glaser R.L., Pearson F.S., Evenson D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(25):9601–9606. doi: 10.1073/pnas.0506468103. 16766665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chianese C., Brilli S., Krausz C. Genomic changes in spermatozoa of the aging male. In: Baldi E., Muratori M., editors. Genetic Damage in Human Spermatozoa. Springer; New York: 2014. pp. 13–26. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadi A., Ng S.C. Fertilizing ability of DNA-damaged spermatozoa. J. Exp. Zool. 1999;284(6):696–704. doi: 10.1002/(sici)1097-010x(19991101)284:6<696::aid-jez11>3.0.co;2-e. 10531556 [DOI] [PubMed] [Google Scholar]
- 23.Høst E., Lindenberg S., Smidt-jensen S. The role of DNA strand breaks in human spermatozoa used for IVF and ICSI. Acta Obstet. Gynecol. Scand. 2000;79(7):559–563. 10929955 [PubMed] [Google Scholar]
- 24.Bungum M., Spanò M., Humaidan P., Eleuteri P., Rescia M., Giwercman A. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Human Reproduction. 2008;23(1):4–10. doi: 10.1093/humrep/dem353. 17986484 [DOI] [PubMed] [Google Scholar]
- 25.Plastira K., Msaouel P., Angelopoulou R., Zanioti K., Plastiras A., Pothos A., Bolaris S., Paparisteidis N., Mantas D. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J. Assist. Reprod. Genet. 2007;24(10):437–443. doi: 10.1007/s10815-007-9162-5. 17768675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belloc S., Benkhalifa M., Junca A.M., Dumont M., Bacrie P.C., Ménézo Y. Paternal age and sperm DNA decay: discrepancy between chromomycin and aniline blue staining. Reprod. Biomed. Online. 2009;19(2):264–269. doi: 10.1016/s1472-6483(10)60083-1. 19712565 [DOI] [PubMed] [Google Scholar]
- 27.Schmid T.E., Grant P.G., Marchetti F., Weldon R.H., Eskenazi B., Wyrobek A.J. Elemental composition of human semen is associated with motility and genomic sperm defects among older men. Human Reproduction. 2013;28(1):274–282. doi: 10.1093/humrep/des321. 23042799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee S.G., Chae H.Z., Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. 15917183 [DOI] [PubMed] [Google Scholar]
- 29.Manevich Y., Sweitzer T., Pak J.H., Feinstein S.I., Muzykantov V., Fisher A.B. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11599–11604. doi: 10.1073/pnas.182384499. 12193653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox Signal. 2011;15(3):831–844. doi: 10.1089/ars.2010.3412. 20919932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Flaherty C., de Souza A.R. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol. Reprod. 2011;84(2):238–247. doi: 10.1095/biolreprod.110.085712. 20864641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Flaherty C., Hales B.F., Chan P., Robaire B. Impact of chemotherapeutics and advanced testicular cancer or Hodgkin lymphoma on sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010;94(4):1374–1379. doi: 10.1016/j.fertnstert.2009.05.068. 19591994 [DOI] [PubMed] [Google Scholar]
- 33.Gong S., San Gabriel M.C., Zini A., Chan P., O’Flaherty C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. Journal of Andrology. 2012;33(6):1342–1351. doi: 10.2164/jandrol.111.016162. 22492841 [DOI] [PubMed] [Google Scholar]
- 34.Ozkosem B., O’Flaherty C. Detrimental effects of oxidative stress on spermatozoa lacking peroxiredoxin 6. Free Radic. Biol. Med. 2012;53:S86. [Google Scholar]
- 35.Mo Y., Feinstein S.I., Manevich Y., Zhang Q., Lu L., Ho Y.S., Fisher A.B. 1-Cys peroxiredoxin knock-out mice express mRNA but not protein for a highly related intronless gene. F.E.B.S. Lett. 2003;555(2):192–198. doi: 10.1016/s0014-5793(03)01199-2. 14644414 [DOI] [PubMed] [Google Scholar]
- 36.Liu G., Feinstein S.I., Wang Y., Dodia C., Fisher D., Yu K., Ho Y.S., Fisher A.B. Comparison of glutathione peroxidase 1 and peroxiredoxin 6 in protection against oxidative stress in the mouse lung. Free Radic. Biol. Med. 2010;49(7):1172–1181. doi: 10.1016/j.freeradbiomed.2010.07.002. 20627125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sailer B.L., Sarkar L.J., Bjordahl J.A., Jost L.K., Evenson D.P. Effects of heat stress on mouse testicular cells and sperm chromatin structure. Journal of Andrology. 1997;18(3):294–301. 9203058 [PubMed] [Google Scholar]
- 38.Evenson D.P., Larson K.L., Jost L.K. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. Journal of Andrology. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. 11780920 [DOI] [PubMed] [Google Scholar]
- 39.O’Flaherty C., Vaisheva F., Hales B.F., Chan P., Robaire B. Characterization of sperm chromatin quality in testicular cancer and Hodgkin’s lymphoma patients prior to chemotherapy. Human Reproduction. 2008;23(5):1044–1052. doi: 10.1093/humrep/den081. 18346994 [DOI] [PubMed] [Google Scholar]
- 40.Weir C.P., Robaire B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. Journal of Andrology. 2007;28(2):229–240. doi: 10.2164/jandrol.106.001362. 17021340 [DOI] [PubMed] [Google Scholar]
- 41.Zubkova E.V., Robaire B. Effect of glutathione depletion on antioxidant enzymes in the epididymis, seminal vesicles, and liver and on spermatozoa motility in the aging Brown Norway rat. Biol. Reprod. 2004;71(3):1002–1008. doi: 10.1095/biolreprod.104.028373. 15151930 [DOI] [PubMed] [Google Scholar]
- 42.Salomon T.B., Hackenhaar F.S., Almeida A.C., Schüller A.K., Gil Alabarse P.V., Ehrenbrink G., Benfato M.S. Oxidative stress in testis of animals during aging with and without reproductive activity. Exp. Gerontol. 2013;48(9):940–946. doi: 10.1016/j.exger.2013.06.010. 23834967 [DOI] [PubMed] [Google Scholar]
- 43.Paul C., Nagano M., Robaire B. Aging results in differential regulation of DNA repair pathways in pachytene spermatocytes in the brown Norway rat. Biol. Reprod. 2011;85(6):1269–1278. doi: 10.1095/biolreprod.111.094219. 21865553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerriero G., Trocchia S., Abdel-Gawad F.K., Ciarcia G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 2014;5:56. doi: 10.3389/fendo.2014.00056. 24795696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ursini F., Heim S., Kiess M., Maiorino M., Roveri A., Wissing J., Flohé L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285(5432):1393–1396. doi: 10.1126/science.285.5432.1393. 10464096 [DOI] [PubMed] [Google Scholar]
- 46.Foresta C., Flohé L., Garolla A., Roveri A., Ursini F., Maiorino M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002;67(3):967–971. doi: 10.1095/biolreprod.102.003822. 12193409 [DOI] [PubMed] [Google Scholar]
- 47.Schneider M., Förster H., Boersma A., Seiler A., Wehnes H., Sinowatz F., Neumüller C., Deutsch M.J., Walch A., Hrabé de Angelis M., Wurst W., Ursini F., Roveri A., Maleszewski M., Maiorino M., Conrad M. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. F.A.S.E.B. J. 2009;23(9):3233–3242. doi: 10.1096/fj.09-132795. 19417079 [DOI] [PubMed] [Google Scholar]
- 48.Conrad M., Moreno S.G., Sinowatz F., Ursini F., Kölle S., Roveri A., Brielmeier M., Wurst W., Maiorino M., Bornkamm G.W. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol. Cell. Biol. 2005;25(17):7637–7644. doi: 10.1128/MCB.25.17.7637-7644.2005. 16107710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeifer H., Conrad M., Roethlein D., Kyriakopoulos A., Brielmeier M., Bornkamm G.W., Behne D. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. F.A.S.E.B. J. 2001;15(7):1236–1238. 11344099 [PubMed] [Google Scholar]
- 50.Fisher A.B., Dodia C., Manevich Y., Chen J.W., Feinstein S.I. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J. Biol. Chem. 1999;274(30):21326–21334. doi: 10.1074/jbc.274.30.21326. 10409692 [DOI] [PubMed] [Google Scholar]
- 51.Manevich Y., Feinstein S.I., Fisher A.B. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with piGST. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(11):3780–3785. doi: 10.1073/pnas.0400181101. 15004285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst. Biol. Reprod. Med. 2011;57(1–2):78–85. doi: 10.3109/19396368.2010.515704. 21208147 [DOI] [PubMed] [Google Scholar]
- 53.Lewis S.E., John Aitken R., Conner S.J., Iuliis G.D., Evenson D.P., Henkel R., Giwercman A., Gharagozloo P. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod. Biomed. Online. 2013;27(4):325–337. doi: 10.1016/j.rbmo.2013.06.014. 23948450 [DOI] [PubMed] [Google Scholar]
- 54.Walter C.A., Intano G.W., McCarrey J.R., McMahan C.A., Walter R.B. Mutation frequency declines during spermatogenesis in young mice but increases in old mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(17):10015–10019. doi: 10.1073/pnas.95.17.10015. 9707592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowe X., Collins B., Allen J., Titenko-Holland N., Breneman J., van Beek M., Bishop J., Wyrobek A.J. Aneuploidies and micronuclei in the germ cells of male mice of advanced age. Mutat. Res. 1995;338:59–76. doi: 10.1016/0921-8734(95)00012-u. 7565883 [DOI] [PubMed] [Google Scholar]
- 56.Intano G.W., McMahan C.A., McCarrey J.R., Walter R.B., McKenna A.E., Matsumoto Y., MacInnes M.A., Chen D.J., Walter C.A. Base excision repair is limited by different proteins in Male germ cell nuclear extracts prepared from young and old mice. Mol. Cell. Biol. 2002;22(7):2410–2418. doi: 10.1128/MCB.22.7.2410-2418.2002. 11884623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith T.B., Dun M.D., Smith N.D., Curry B.J., Connaughton H.S., Aitken R.J. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J. Cell Sci. 2013;126(6):1488–1497. doi: 10.1242/jcs.121657. 23378024 [DOI] [PubMed] [Google Scholar]
- 58.Kino K., Sugiyama H. GC − >CG transversion mutation might be caused by 8-oxoguanine oxidation product. Nucleic Acids Symp. Ser. 2000;139–140(44):139–140. doi: 10.1093/nass/44.1.139. 12903307 [DOI] [PubMed] [Google Scholar]
- 59.Richard D., Bartfai R., Volz J., Ralph S.A., Muller S., Stunnenberg H.G., Cowman A.F. A genome-wide chromatin-associated nuclear peroxiredoxin from the malaria parasite Plasmodium falciparum. J. Biol. Chem. 2011;286(13):11746–11755. doi: 10.1074/jbc.M110.198499. 21282103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah P.S., Knowledge Synthesis Group on Determinants of Preterm/Low Birthweight Births Paternal factors and low birthweight, preterm, and small for gestational age births: a systematic review. Am. J. Obstet. Gynecol. 2010;202(2):103–123. doi: 10.1016/j.ajog.2009.08.026. 20113689 [DOI] [PubMed] [Google Scholar]
- 61.Amaral S., Oliveira P.J., Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr. Diabetes Rev. 2008;4(1):46–54. doi: 10.2174/157339908783502398. 18220695 [DOI] [PubMed] [Google Scholar]
- 62.Linschooten J.O., Laubenthal J., Cemeli E., Baumgartner A., Anderson D., Sipinen V.E., Brunborg G., Haenen G.R., Fthenou E., Briedé J.J., van Schooten F.J., Godschalk R.W. Incomplete protection of genetic integrity of mature spermatozoa against oxidative stress. Reprod. Toxicol. 2011;32(1):106–111. doi: 10.1016/j.reprotox.2011.05.004. 21621604 [DOI] [PubMed] [Google Scholar]
- 63.Hillman S., Peebles D.M., Williams D.J. Paternal metabolic and cardiovascular risk factors for fetal growth restriction: a case-control study. Diabetes Care. 2013;36(6):1675–1680. doi: 10.2337/dc12-1280. 23315598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohno M., Sakumi K., Fukumura R., Furuichi M., Iwasaki Y., Hokama M., Ikemura T., Tsuzuki T., Gondo Y., Nakabeppu Y. 8-oxoguanine causes spontaneous de novo germline mutations in mice. Sci. Rep. 2014;4:4689. doi: 10.1038/srep04689. 24732879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishida N., Kudo M. Oxidative stress and epigenetic instability in human hepatocarcinogenesis. Dig. Dis. 2013;31(5–6):447–453. doi: 10.1159/000355243. 24281019 [DOI] [PubMed] [Google Scholar]
- 66.Marchetti F., Essers J., Kanaar R., Wyrobek A.J. Disruption of maternal DNA repair increases sperm-derived chromosomal aberrations. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(45):17725–17729. doi: 10.1073/pnas.0705257104. 17978187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamauchi Y., Riel J.M., Ward M.A. Paternal DNA Damage resulting from various sperm treatments persists after fertilization and is similar before and after DNA replication. Journal of Andrology. 2012;33(2):229–238. doi: 10.2164/jandrol.111.013532. 21546611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dadoune J.P. Ultrastructural abnormalities of human spermatozoa. Human Reproduction. 1988;3(3):311–318. doi: 10.1093/oxfordjournals.humrep.a136701. 3286678 [DOI] [PubMed] [Google Scholar]
- 69.Morielli T., O’Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction. 2015;149(1):113–123. doi: 10.1530/REP-14-0240. 25385721 [DOI] [PMC free article] [PubMed] [Google Scholar]


