Summary
Membrane skeletal protein 4.1R is the prototypical member of a family of four highly paralogous proteins that include 4.1G, 4.1N and 4.1B. Two isoforms of 4.1R (4.1R135 and 4.1R80) as well as 4.1G are expressed in erythroblasts during terminal differentiation, but only 4.1R80 is present in mature erythrocytes. While the function of 4.1R isoforms in erythroid cells has been well characterized, there is little or no information on the function of 4.1G in these cells. In the present study, we performed detailed characterization of the interaction of 4.1G with various erythroid membrane proteins and the regulation of these interactions by calcium-saturated calmodulin. Like both isoforms of 4.1R, 4.1G bound to band 3, glycophorin C, CD44, p55 and calmodulin. While both 4.1G and 4.1R135 interact with similar affinity with CD44 and p55, there are significant differences in the affinity of their interaction with band 3 and glycophorin C. This difference in affinity is related to the non-conserved N-terminal headpiece region of the two proteins that is upstream of the 30kDa membrane binding domain that harbors the binding sites for the various membrane proteins. The headpiece region of 4.1G also contains a high affinity calcium-dependent calmodulin-binding site that plays a key role in modulating its interaction with various membrane proteins. We suggest that expression of the two paralogs of protein 4.1 with different affinities for band 3 and glycophorin C is likely to play a role in assembly of these two membrane proteins during terminal erythroid differentiation.
Keywords: protein 4.1R, protein 4.1G, membrane proteins, calcium, calmodulin
INTRODUCTION
Protein 4.1R [1] is the prototypical member of a family of four highly paralogous proteins that include 4.1G (Generally expressed) [2], 4.1N (Neuronal type) [3], and 4.1B (Brain type) [4]. There are two major isoforms of 4.1R, the 135kDa isofrom (4.1R135) and the 80kDa isoform (4.1R80) that lacks the head piece (HP) region [5, 6]. We previously reported that both 4.1R135 and 4.1R80 isoforms interact with cytoplasmic domains of glycophorin C (GPC) [7], band 3 [8] and CD44 [9], and with p55 [10, 11] and that the 30kDa domain of the proteins is responsible for these interactions. Interestingly, we noted that the interaction of 4.1R135 with GPC was an order of magnitude weaker than that of 4.1R80, while its interaction with band 3 was an order of magnitude stronger [12], indicating possible regulation of the interactions by the HP region. 4.1G and both isoforms of 4.1R are expressed in erythroblasts during terminal differentiation [13] but only 4.1R80 is present in mature erythrocytes. While the function of 4.1R isoforms in erythroid cells has been well characterized [12, 14], there is little or no information on the function of 4.1G in these cells. The primay structure of 4.1G is very similar to that of 4.1R135 (Figure 1). The primary amino acid sequence of the 30kDa FERM (Four-one, Ezrin, Radixin, Moesin) domain of 4.1G is 71% identical to that of 4.1R. 4.1G is therefore predicted to bind to many of 4.1R binding partners described above. In contrast to the high conservation of the 30kDa domains, the amino acid sequence identity of the headpiece (HP) region of 4.1G and 4.1R135 is quite low (35%). We hypothesize that the HP region may confer some distinct binding properties to the 30kDa domain of 4.1G.
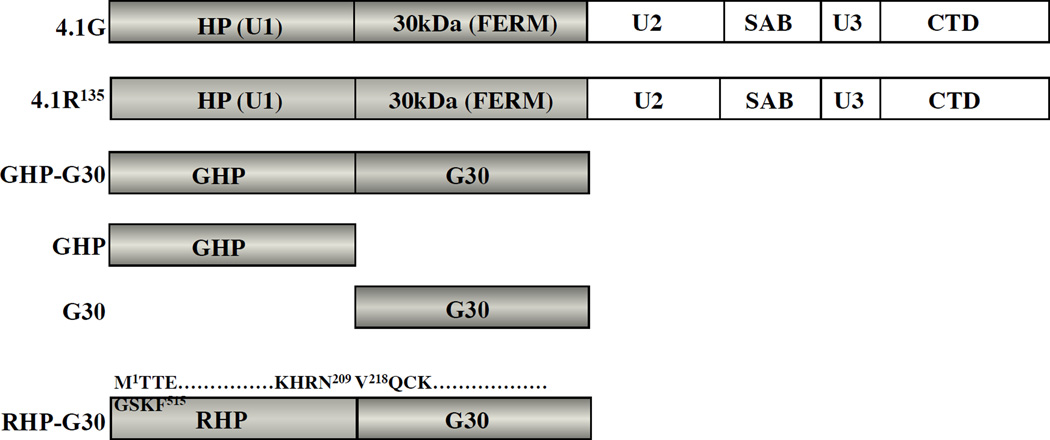
Figure 1. Structure of 4.1G, 4.1R and various 4.1G constructs used in this study.
4.1R135 consists of a 209 amino acids headpiece region (HP), a 298 amino acids 30kDa domain (FERM domain), a 10kDa spectrin-actin binding domain (SAB), and a 22/24kDa carboxy-terminal domain (CTD). The headpiece is also referred to as unique region 1 (U1) whereas the 16kDa domain between the 30kDa domain and the SAB domain and the domain between the SAB domain and the CTD domain are referred to as unique region 2 (U2) and 3 (U3), respectively. The apparent molecular weights of SAB and CTD are 10kDa and 22/24kDa based on the migration of fragments obtained after α-chymotryptic digestion of 4.1R80 [1]. The accession number for human 4.1R135 is P11171. The primary structure of 4.1G is registered under accession No. AAC16923. The amino acid sequence identity between 4.1R135 and 4.1G HPs (RHP and GHP) and between 30kDa domains 4.1R135 and 4.1G (G30) is displayed (35% and 71%, respectively). Various domains of 4.1R135 and 4.1G used in binding assays are depicted including a chimera protein corresponding to 4.1R135 HP and 4.1G 30kDa domain, RHP-G30. The amino acid sequences at the boundary of each domain of this chimera protein are displayed, the amino acid numbering for each domain referring to the protein of origin.
Calmodulin (CaM) binds to both isoforms of 4.1R. While its binding to 4.1R80 was Ca2+-independent [15], its binding to 4.1R135 was strongly Ca2+-dependent [12]. Ca2+ saturated CaM completely inhibited the binding of 4.1R135 to GPC and markedly reduced the affinity of its binding to band 3 [12]. The 4.1R135 has three CaM binding sites: a Ca2+-dependent site in the HP region and two sites in the 30kDa domain [12, 14, 15]. The two CaM binding sites in the 30kDa domain are conserved in 4.1G and 4.1R. The sequence motif S76RGLSRLFSSFLKRPKS in the HP of 4.1R135 is responsible for Ca2+-dependent CaM binding [16]. There is a similar sequence, S71RGISRFIPPWLKKQKS in the HP of 4.1G. The question thus arises as to whether CaM binds to all three site(s) and which site(s) would be essential for the Ca2+-dependent regulation of 4.1G binding to membrane proteins through CaM binding. In the present study, we performed detailed characterization of the interaction of various erythroid membrane proteins with 4.1G and the regulation of these interactions by Ca2+ and CaM.
MATERIALS AND METHODS
Reagents
The pET31b(+) bacterial expression vector was purchased from Novagen Inc. (Madison, WI). pGEX-4T2 and pGEX-6P2 bacterial expression vectors, glutathione Sepharose 4B, CaM Sepharose 4B, heparin Sepharose CL-6B, Q Sepharose, Sephacryl S-200 and Sephacryl S-300 were purchased from GE Healthcare Ltd. (Buckinghamshire, England). Restriction enzymes were purchased from New England BioLabs Inc. (Ipswich, MA, US). Serum-Free Expansion Medium (SFEM), fetal bovine serum (FBS), stem cell factor (SCF), IL-3 and erythropoietin (EPO) were purchased from Stemcell Technologies Inc., Vancouver, BC, Canada. All other reagents were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan) and Sigma-Aldrich Co. (St. Louis, MO, USA) unless noted otherwise. IAsys® cuvettes coated with aminosilane were supplied by Affinity Sensors (Cambridge, UK). CaM was purified as previously described [9].
Antibodies
Polyclonal antibodies against 4.1G, 4.1R exon2 and 4.1R exon 13 were raised in rabbit using His-tag recombinant 4.1G headpiece, synthetic 4.1R exon2 and exon13 peptides as antigens. The antibodies were affinity purified on Sulfolink Coupling Gel (Thermo Fisher Scientific Inc., South Logan, UT, USA). The horseradish peroxidase conjugated anti-rabbit immunoglobulins used for immuno-blot were purchased from DAKO (Glostrup, Denmark). The specificity of each antibody was confirmed by western blotting on recombinant full-length protein 4.1 isoforms, on tissues taken from the cognate knockout mice as negative controls [17].
Computational analysis of structures of HP and 30kDa domain
The disorder probability for the structure of each domain was calculated using the PrDOS software package (http://prdos.hgc.jp) [18]. Their secondary structure was predicted by the PAPIA software package (http://mbs.cbrc.jp/papia/). The 3D structure of 4.1G 30kDa domain was predicted by multiple alignment using the Modeller™ software (http://salilab.org/modeller/) based on the structure of the 30kD domains of 4.1R (1GG3A) and 4.1B (2HE7). The 50 potential models obtained were ranked based on evaluation function [19]. The secondary structure and corresponding amino acid sequenec of FERM domins are drawn using software package DSSP (http://swift.cmbi.ru.nl/gv/dssp/) [20].
Preparation of recombinant human 4.1G and 4.1R proteins
Recombinant proteins corresponding to various combinations of head-piece region of 4.1G (GHP), 4.1G 30kDa domain (G30) and 4.1R 30kDa domain (R30) were expressed as glutathione-S-transferase (GST)-fusion proteins in Escherichia coli BL21(DE3). They included recombinant proteins corresponding to HP and 30kDa domains of 4.1G (GHP-G30) and to the chimera protein RHP-G30 (Figure 1). Preparation of recombinant 4.1R135 was described previously [12]. After sonication, bacterial lysates were loaded on to a glutathione affinity column to purify GST fusion proteins. Recombinant proteins were eluted from the column after cleavage of the GST tag with thrombin as previously described [9]. After desalting, proteins were further purified on a heparin Sepharose equilibrated with 50mM Tris-HCl pH7.5 containing 200 mM NaCl, 1 mM EDTA and 1 mM 2-ME and Sephacryl S-300 for GHP-G30 and Sephacryl S-200 for G30 to remove contaminants and breakdown products. Sephacryl S-300 and Sephacryl S-200 were equilibrated with 50 mM Tris-HCl pH7.5 containing 500 mM NaCl, 1 mM EDTA, 1 mM 2-ME, 2 mM NaF and 1% glycerol (Buffer A). The retention time is recorded by Akta Prime™ Plus (GE Healthcare Ltd., Buckinghamshire, England). The purity of recombinant proteins was assessed by SDS-PAGE and Western blot analysis. Preparations of p55 and the cytoplasmic domains of band 3 (band 3cyt), GPC (GPCcyt), and CD44 (CD44cyt), have been previously described [9, 11, 15]. Protein concentrations were determined as previously described [9].
Cloning of HP, 30kDa domain and chimera constructs
Human RHP, R30, GHP and G30 were cloned using 5’-NsiI-XhoI-3’ sites into pET31b(+) vector or 5’-EcoRI-XhoI-3’ sites into pGEX-4T2 vector. Full length human 4.1G was cloned using 5’-EcoRI-SalI-3’ sites into pGEX-6P2 vector (the internal SalI site in human 4.1G coding sequence being mutated prior to cloning without altering the amino acid sequence of the protein). A chimera protein corresponding to RHP and G30 (RHP-G30) was generated by the “sewing method”. Briefly, the RHP was amplified with a forward primer creating an EcoRI site (underlined) upstream of the ATG initiation site of 4.1R shown in bold (primer A: 5’-TCCAGGAATTCCCATGACAACAGAGAAGAGTTTAGTGACTGAGGC-3’) and a reverse primer containing the end of RHP and the beginning of the G30 (shown in italics) (primer B’: 5’-TACTAAGAGGGTCACTTTACACTGGACGTTCCTGTGTTTT CTGATTGGTTTTTGGGAAG-3’). In a similar fashion, G30 was amplified with a forward primer complementary to the reverse primer B’ described above (primer B: 5’-TCCCAAAAACCAATCAGAAAACACAGGAACGTCCAGTGTAAAGTGACCCTCTTAGAT-3’) and a reverse primer corresponding to the end of the G30 coding sequence followed by a stop codon shown in bold and a XhoI site underlined (primer C: 5’-GTTCCGCTCGAGTCAAAATTTGGACCCCAAGGTCAGGAACTTG-3’). The two PCR products were then mixed in equal amounts and subjected to a second amplification using primers A and C. The ~1.5kb chimera PCR product was digested EcoRI-XhoI and cloned into pGEX-4T2 vector. All constructs were checked by DNA sequencing (Elim BioPharmaceuticals Inc., Hayward, CA, USA) prior to expression in bacteria or mammalian cell transfection. The accession numbers for nucleotide sequences of human 4.1R and 4.1G are BC039079 and AF027299, respectively.
Preparation of IOVs and binding assay
Inside-out-vesicles depleted of all peripheral proteins (IOVs) were prepared according to Danilov et al. [21] with minor modifications [22]. The pelleting assay was described previously [12]. Briefly, various concentrations of G30 and GHP-G30 were incubated for 14 hrs at 5°C with IOVs in TBS (50 mM Tris-HCl, pH7.5, containing 150 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 2 mM NaF and protease inhibitors (Complete®, Roche Diagnostics GmbH, Mannheim, Germany)). The mixture of G30 and IOVs were laid on a 150 µl of 3.3% sucrose cushion equilibrated in TBS. The sample was centrifuged at 60,000 rpm for 20 min at 4°C in a TL-100 ultracentrifuge using a TLA-100 rotor (Beckman Coulter Inc., Fullerton, CA, USA). The supernatant and pellet were collected, treated with sample buffer and subjected to SDS-PAGE, 12% and 8.4% gel for G30 and GHP-G30, respectively. Proteins bound to IOVs were visualized on Coomasie Brilliant Blue G250 (CBB, Gelcode Blue®, Pierce, Rockford, IL, U.S.A.) stained gels. Bound GHP-G30 to IOVs was analyzed by immuno-blot. The photometric intensity (PI) of bound proteins were analyzed with ChemiDoc XRS Plus® (Bio-Rad Laboratories Inc., Hercules, CA, USA). The PI values of corresponding bands were plotted againt concentrations of added proteins. An apparent dissociation constant at equilibrium estimated from pelleting assay is represented as K’. The results represnted as mean ± satndard deviation calculated from three different measurements.
Purification of non-tagged full length 4.1G
Bacteria expressing full length recombinant human 4.1G were sonicated in 50 mM Tris-HCl, pH8.0, 200 mM NaCl, 1 mM EDTA, 2 mM EGTA, 1 mM 2-ME, 1 mM benzamidine, 1 mM PMSF and 1 mM diisopropylfluorophosphate on ice. The bacterial lysate was desalted stepwise by saturation with 35% to 50% ammonium sulfate. The 35%-50% fraction containing 4.1G was dialyzed against 50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM 2-ME (Buffer B) then loaded onto a Q-Sepharose column (1x29 cm, flow rate 0.7 ml/min) pre-equilibrated with Buffer B. Protein elution, monitored by absorbance at 280 nm (A280), was achieved with a linear gradient of NaCl (0–500 mM) in Buffer B. The fractions enriched in 4.1G (eluted with 320 mM NaCl) were further purified on a CaM-Sepharose column to remove the ~50kDa contaminant protein. .
Binding analysis using a CaM Sepharose 4B
Full length 4.1G and GHP were dialyzed against 50 mM Tris-HCl, pH7.5, 100 mM NaCl, and 1 mM CaCl2 (Buffer C). Protein samples were loaded onto a 1.6 × 8 cm CaM Sepharose 4B column pre-equilibrated with Buffer C and run at a flow rate of 0.2 ml/min. The column was washed with an excess of Buffer C until the A280 went back to baseline level. Bound proteins were eluted with Buffer C supplemented with 500 mM NaCl or 50 mM Tris-HCl, pH7.5, 100 mM NaCl, and 5 mM EGTA.
Resonant mirror detection binding assays
Protein-protein interactions and protein-peptide interactions were measured using the IAsys® based on the resonant mirror detection method (Affinity Sensors, Cambridge, UK) [23]. In the following, the protein or peptide immobilized on the cuvette is referred to as the "ligand" while the protein added in solution to the cuvette is referred to as the "analyte". Protein and peptide immobilization on aminosilane cuvettes has been previously described [9]. All binding assays were carried out at 25°C under constant stirring. In experiments designed to quantify the effects of Ca2+ and CaM on 4.1G and chimera protein binding profiles, full length recombinant 4.1G (50 nM~1 µM) was pre-incubated with 5 µM CaM in 50 mM Tris-HCl, pH 7.5, 100 mM NaCl (Buffer D) and either 0.1 mM EGTA or 1.1 mM CaCl2 and 1.0 mM EGTA at 25°C for 30 min prior to binding assays with immobilized GPCcyt, band 3cyt, CD44cyt, and p55 [9, 11, 15].
The kinetic analysis and the stoichiometry of analyte binding to ligand were calculated according to previously published equations [9, 24]. In brief, a dissociation constant (termed "K(D)") was calculated as K(D) = kd / ka where ka is the association rate constant and kd is the dissociation rate constant. The K(D) was obtained from the means of 3–5 measurements for ka and kd. The K(D) was confirmed by Scatchard plotting using the maximum binding value (Bmax) and the added molar concentrations of analyte. The Bmax was calculated from the binding profile using the software package Fastfit® version 2.1. The method for estimation of binding ratios has been previously described [15]. The Bmax of GHP-G30 represented as arc seconds was obtained from the Scatchard plot as previously described [15]. The amount of immobilized CaM on the aminosilane cuvette was determined as the difference of arc seconds between bis(sulfosuccinimidyl)suberate and CaM under equilibrium conditions. The stoichiometry of GHP-G30 binding to CaM was calculated according to the following equation described in the Method Guide of the IAsys® system; Stoichiometry of GHP-G30 : CaM = (Bmax of GHP-G30/58,892) : (amount of immobilized CaM on aminosilane cuvette/16,705), where 58,892 and 16,705 are apparent molecular weights (Da) of GHP-G30 and CaM, respectively. The cuvettes were re-used after cleaning with 20 mM HCl. Original binding curves could be replicated after HCl washes implying that the washing procedure did not denature the bound ligands.
In vitro culture of erythroblasts
CD34+ hematopoietic stem cell (HSC) precursor cells were purified from cord blood by percoll separation followed by CD34 MicroBead® kit (Miltenyi Biotec Inc., CA, USA). Cells were cultured using two-phase culture system with modifications. In the first phase (day 0–6), cells (105/ml) were cultured for three days in Serum-Free Expansion Medium (SFEM) supplemented with 10% FBS in the presence of SCF (50 ng/ml), IL-3 (10 ng/ml), EPO (1 U/ml), α-thioglycerol (60 µM) and penicillin (100 units/ml)/streptomycin (100 µg/ml). On day 4, cells were diluted to a density of 105/ml with fresh medium and the culture was continued for another three days. In the second phase (day 7–13), cells were cultured at 105/ml in SFEM medium supplemented with 30% FBS in the presence of EPO, α-thioglycerol and penicillin-streptomycin. Cellular morphology was assessed by cytospin on a daily basis followed by May-Grünwald Giemsa staining and light microscopy. The vast majority of the cells were proerythroblasts on day 7 and orthochromatic erythroblasts on day 13.
Calculation of molecular weight and isoelectric point
Theoretical molecular weights and isoelectric point were calculated based on peptide amino acid sequence using the software package DNASIS® (Hitachi, Tokyo, Japan).
RESULTS
4.1G interaction with 4.1R binding partners
4.1G interacted in vitro with band 3cyt, GPCcyt, p55 and CD44cyt with K(D)s in the range of ~200 nM (Table 1). Importantly, the binding affinities of 4.1G for band 3cyt and GPCcyt were different from those of 4.1R135. 4.1G interacted with band 3cyt with a much lower affinity (high K(D) value) than did 4.1R135, while it interacted with much higher affinity with GPCcyt. These differences were mainly derived from differences in the association rate constant ka. In contrast, both 4.1G and 4.1R135 interacted with CD44cyt and p55 with similar affinities.
Table 1.
4.1G binding to membrane proteins
| Analyte | Ligand | ka (M−1 s−1) | kd (s−1) | K(D) (nM) |
|---|---|---|---|---|
| 4.1G | band3cyt | 8.0 ± 0.1 × 104 | 1.4 ± 0.3 × 10−2 | 185 ± 23 |
| 4.1R | band3cyt | 3.1 ± 0.2 × 105 | 7.1 ± 0.2 × 10−3 | 23 ± 2 |
| 4.1G | GPCdyt | 5.6 ± 0.1 × 104 | 8.1 ± 0.2 × 10−3 | 144 ± 5 |
| 4.1R | GPCcyt | 8.0 ± 0.2 × 103 | 11 ± 0.1 × 10−2 | 1327 ± 103 |
| 4.1G | p55 | 4.7 ± 0.1 × 104 | 8.5 ± 0.1 × 10−3 | 181 ± 10 |
| 4.1R | p55 | 1.9 ± 0.1 × 104 | 4.7 ± 0.1 × 10−3 | 248 ± 17 |
| 4.1G | CD44cyt | 3.8 ± 0.1 × 104 | 6.6 ± 0.2 × 10−3 | 178 ± 19 |
| 4.1R | CD44cyt | 4.2 ± 0.2 × 104 | 1.5 ± 0.1 × 10−2 | 329 ± 64 |
K(D) for the interactions of 4.1G and 4.1R with band 3cyt, GPCcyt, p55 and CD44cyt are shown. Each analyte (at 50nM to 2 µM) was applied to the listed ligands immobilized on aminosilane cuvettes as described under “Materials and Methods”. From the binding curves obtained by the resonant mirror detection method, K(D) were determined using the software package FAST-Fit®. ka, kd and K(D) were calculated from three independent experiments represents (mean ± S.D.).
Binding affinities of G30 and GHP-G30 to these membrane proteins were very similar to those of full length 4.1G, suggesting that 4.1G interacted with its binding partners primarily through the 30kDa domain (G30) and GHP did not affect these binding interactions. This is in marked contrast to interactions of 30kDa domain of 4.1R (R30) which were significantly affected by RHP [14], indicating that while RHP influences R30 binding to its partners, GHP has little influence on G30 binding to its partners. Interestingly, a recombinant chimera protein consisting of RHP and G30 (RHP-G30) showed similar binding affinities as G30 and GHP-G30 (data not shown) implying significant differences in the structure and function of RHP and GHP. It should be noted that neither GHP nor RHP bound to any of these membrane proteins.
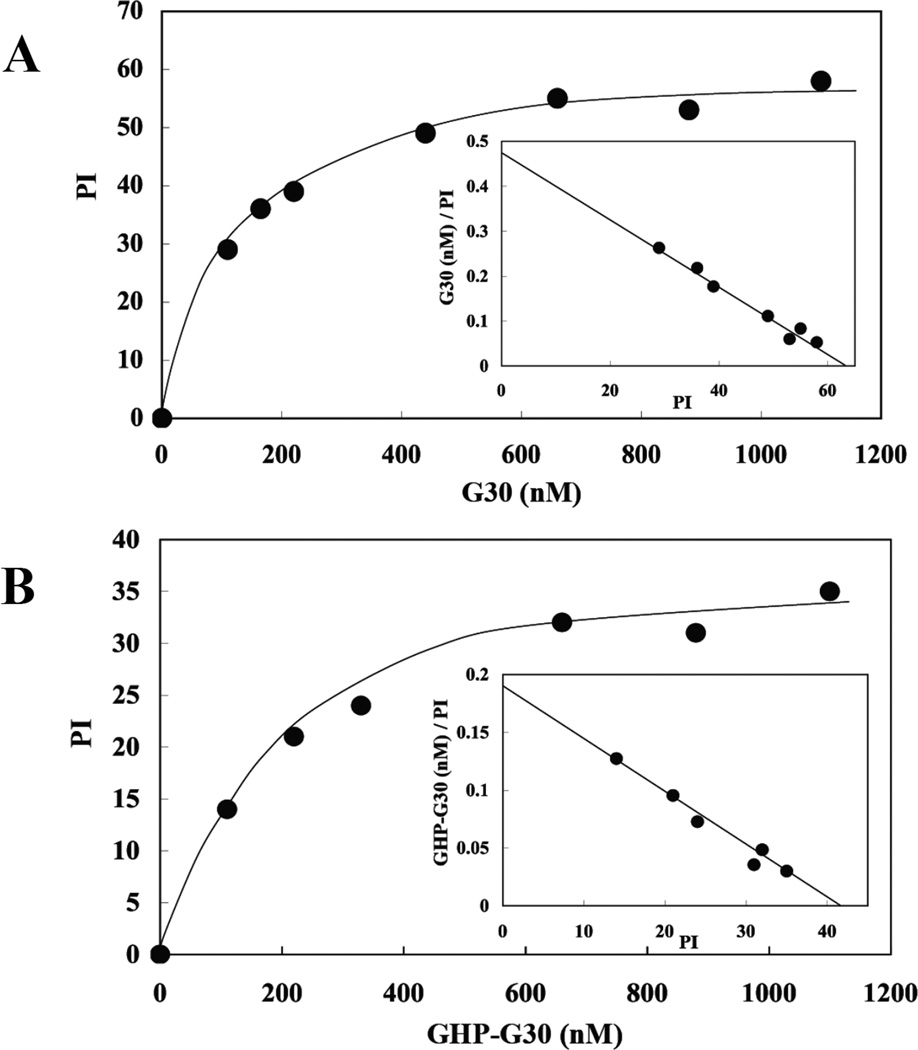
To validate 4.1G binding to intact membranes, we assayed binding of G30 or GHP-G30 to inside out vesicles (IOVs) prepared from erythrocyte membranes. Scatchard plot showed that these proteins bound to IOVs in a saturable manner (Figure 2). The apparent K’ values for G30 and GHP-G30 binding to IOVs were 169±67 nM and 207±49 nM, respectively. These values were similar to those obtained using Resonant Mirror Detection (Table1). These findings demonstrate that 4.1G can bind to transmembrane proteins of erythrocyte membrane through its 30kDa domain.
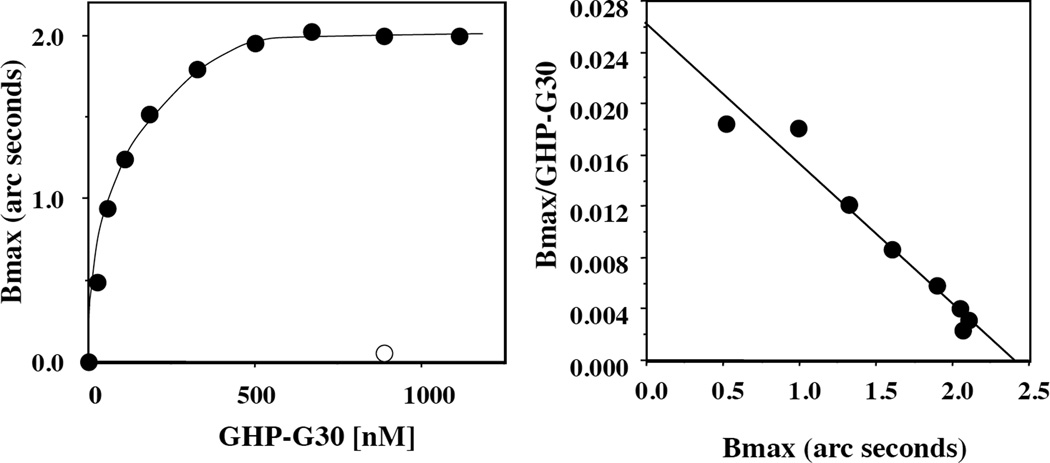
Figure 2. Binding profiles of 4.1G 30kDa domain (G30) and GHP-G30 polypeptide binding to human erythrocyte inside-out-vesicles (IOVs).
G30 and GHP-G30 at different concentrations were incubated with IOVs and the IOVs were subsequently pelleted by centrifugation. The photometric intensity (PI) of IOVs bound G30 (A) and GHP-G30 (B) in the pellet was measured by densitometry. The PI values are plotted as a function of protein concentration and the Scatchard plots are shown in the inserts.
Computational analysis of 4.1G
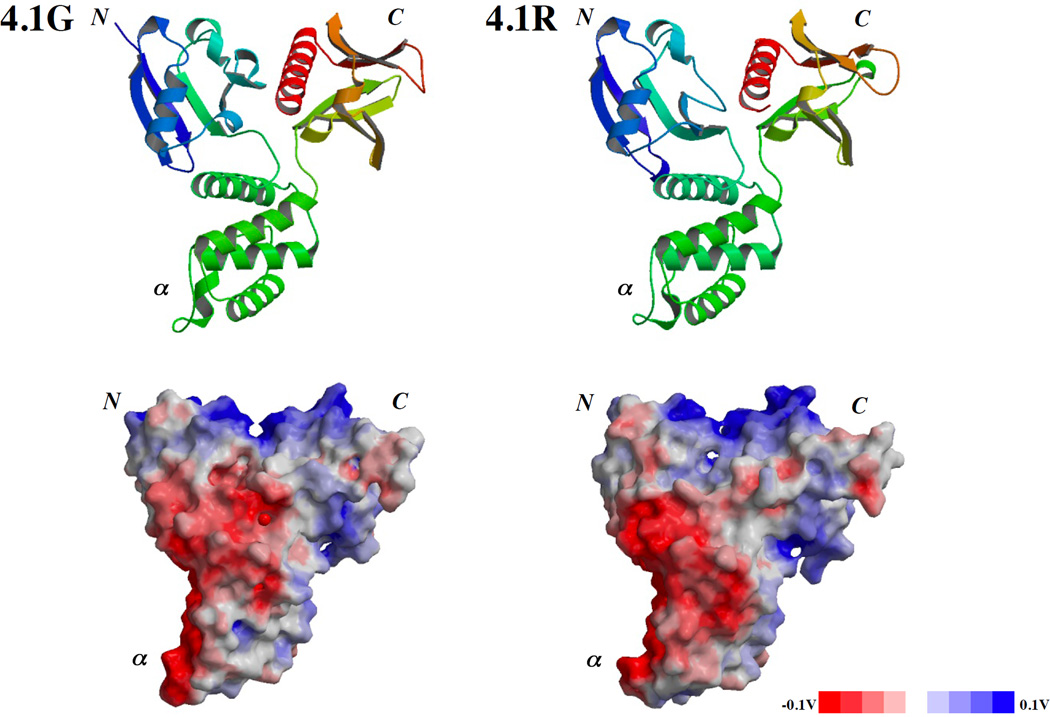
Based on the crystal structures of the 30kDa domain of 4.1R (PDB accession No.1GG3) and of 4.1B (PDB accession No.2HE7) the structure of 4.1G was modeled by Modeller™ software based on the multiple alignment obtained by CLUSTAL W (http://align.genome.jp/) [25] (Figure 3). The secondary structures in the FERM domains are conserved among 4.1G, 4.1R and 4.1B (supplementary Figure S1). The derived structure was very similar to that of 4.1R except a deviation in the N-terminal lobe due to the LFQESPEQ sequence in 4.1G. The results of this computational analysis validated the structural basis for 4.1G binding to previously defined 4.1R binding partners through the 30kDa domain and that the 30kDa domain of 4.1G could interact with CaM in a Ca2+-independent manner as the 30kDa domain of 4.1R.
Figure 3. in silico prediction of 3D structure of 4.1G 30kDa domain.
Predicted ribbon model (upper panel) and surface charge distribution (bottom panel) for 4.1G (left column) based on the defined structure of 4.1R 30kDa domain (upper and lower panels in right column; PDB accession No.1GG3). N, α and C present N-lobe, α-lobe and C-lobe, respectively [26].
Calculation of the disorder probability of each domain was carried out based on PrDOS software analysis, a value greater than 0.5 reflecting a disordered structure, the probability of false prediction reaching only 5%. This analysis predicted a highly disordered structure for the HP region (amino acids 1-209) and, as expected, a highly ordered structure for the 30kDa domain (amino acids 210-507). Of particular note, while the overall HP region adopted a disordered structure, a polypeptide (amino acids 70-80), corresponding to a previously identified Ca2+-dependent CaM binding site [16], exhibited an ordered structure (supplementary Figure S2). SDS-PAGE analysis and size exclusion chromatography (SEC) [see reviews, 26– 28] lent some indirect support for the structural prediction that the HP domain is an unfolded protein. The theoretical molecular weight of GHP is 23kDa but the apparent molecular weight was estimated as 36kDa by SDS-PAGE as shown in Figure 4C. While the theoretical molecular weights of GHP-G30 and G30 are 57kDa and 33kDa, respectively, they migrated as polypeptides of 75kDa and 35kDa, respectively, on SDS-PAGE (supplementary Figure S3). Although we cannot rule out that the acidic nature of GHP could affect its binding to SDS, other acidic proteins with similar isoelectric points (5.3–5.6) such as bovine serum albumin (BSA) or glial fibrillary acidic protein (GFAP) show no increase in apparent molecular weight [29]. Furthermore, SEC analysis, employing Sephacryl S-300, shows that GHP-G30 was eluted between IgG (150kDa) and albumin (68kDa) (supplementary Figure S4), further suggesting that GHP adopts an abnormal shape. Taken together, these findings suggest but do not prove that the GHP is an unfolded domain while G30 is folded. PrDOS-based analysis of full length 4.1G and 4.1R135 also predicted that the 30kDa domain to be the only region in these full length proteins than can adopt an ordered structure (data not shown).
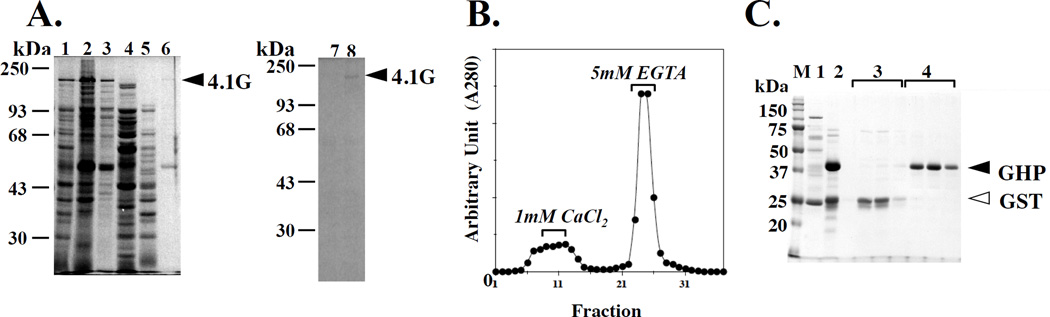
Figure 4. 4.1G and GHP binding to CaM Sepharose 4B.
(A) Monitoring of non-tagged full length 4.1G purification on a 10% SDS-PAGE gel stained with Coomassie Brilliant Blue (See Materials and Methods). lane 1: bacterial lysate, lane 2: 35% saturated ammonium sulfate precipitate, lane 3: fraction after dialysis, lane 4: 50% saturated ammonium sulfate precipitate, lane 5: 50% saturated ammonium sulfate supernatant, lane 6: active fraction eluted from Q Sepharose column, lane 7: flow through fraction from CaM Sepharose 4B column in presence of Ca2+, lane 8: 5mM EGTA elution fraction from CaM-Sepharose 4B. The arrowhead indicates the position of purified full length 4.1G. (B) Elution profile of GST and of GHP from a CaM Sepharose 4B column in the presence of 1 mM CaCl2 and 5 mM EGTA, respectively. Protein elution is monitored by absorbance at 280 nm. (C) GHP binding to a CaM Sepharose 4B column was analyzed by SDS-PAGE (12% gel) stained with Coomassie Brilliant Blue. lane 1: GST-GHP fusion protein purified on a glutathione Sepharose column, lane 2: affinity purified thrombin-treated GST-GHP fusion protein, lanes 3: “1 mM CaCl2” flow through fractions shown in panel B enriched in GST (open arrowhead), lanes 4: 5 mM EGTA elution fractions shown in panel B and enriched in GHP (closed arrowhead). Note that GST as a control did not bind to CaM Sepharose 4B.
4.1G binding to CaM in a Ca2+-dependent manner
We then investigated whether CaM binds to 4.1G in the absence and presence of Ca2+. Both full length 4.1G and GHP bound to a CaM Sepharose 4B column in the presence of Ca2+ and could be eluted with 5 mM EGTA (Figures 4A, 4B and 4C). The K(D) for CaM binding to 4.1G and GHP increased dramatically following chelation of Ca2+ with EGTA (Table 2). These findings established that CaM bound to the HP region of 4.1G in a Ca2+-dependent manner. These data recapitulated previous observations made for CaM binding to RHP and 4.1R135 [12].
Table 2.
Ca 2+-dependence of binding of 4.1G, its fragments and RHP-G30 to CaM
| Analyte | Ligand | ka (M−1 s−1) | kd (s−1) | K(D) (nM) |
|---|---|---|---|---|
| 4.1G | EGTA/CaM | 3.7 ± 0.2 × 103 | 8.3 ± 0.1 × 10−3 | 2245 ± 41 |
| Ca2+/CaM | 9.4 ± 0.2 × 104 | 5.1 ± 0.2 × 10−3 | 54 ± 3 | |
| GHP-G30 | EGTA/CaM | 3.0 ± 0.3 × 103 | 8.8 ± 0.2 × 10−3 | 2967 ± 313 |
| Ca2+/CaM | 1.8 ± 0.1 × 105 | 8.0 ± 0.4 × 10−3 | 44 ± 3 | |
| GHP | EGTA/CaM | 3.7 ± 0.2 × 103 | 8.0 ± 0.1 × 10−2 | 21644 ± 628 |
| Ca2+/CaM | 1.8 ± 0.1 × 105 | 9.0 ± 0.1 × 10−3 | 62 ± 8 | |
| G30 | EGTA/CaM | 8.8 ± 0.3 × 103 | 11 ± 0.1 × 10−3 | 130 ± 8 |
| Ca2+/CaM | 7.8 ± 0.1 × 104 | 1.6 ± 0.1 × 10−2 | 190 ± 16 | |
| RHP-G30 | EGTA/CaM | 4.1 ± 0.1 × 104 | 1.8 ± 0.1 × 10−2 | 441 ± 41 |
| Ca2+/CaM | 1.0 ± 0.1 × 105 | 2.3 ± 0.1 × 10−2 | 213 ± 22 |
K(D) for the interactions of 4.1G, recombinant proteins corresponding to headpiece (HP) and 30kDa membrane binding domain of 4.1G (GHP-G30), headpiece of 4.1G (GHP), 30kDa membrane binding domain of 4.1G (G30) and chimera protein of HP of 4.1R and 30kDa FERM domain of 4.1G (RHP-G30) with CaM immobilized aminosilane cuvettes in the absence (EGTA/CaM) or presence of Ca2+ (Ca2+/CaM) are shown. Each analyte (at 50 nM to 2 µM) was incubated with CaM immobilized on aminosilane cuvettes as described under “Materials and Methods”. From the binding curves obtained by the resonant mirror detection method, K(D) were determined using the software package FAST-Fit ®. ka, kd and K(D) were calculated from three independent experiments represents (mean ± S.D.).
In addition to the CaM binding site in the HP region of 4.1G, two CaM binding sites have been mapped in the 30kDa domain, one of them being Ca2+-independent. Indeed, G30 alone bound to CaM in a Ca2+-independent manner as did R30 alone [14, 15] (Table 2). We therefore probed a recombinant 4.1G expressing both HP region and 30kDa domain (GHP-G30). Recombinant GHP-G30 protein behaved like full length 4.1G, i.e. it bound to a CaM Sepharose 4B column in the presence of Ca2+ and was eluted in the presence of EGTA (data not shown). The K(D) for CaM binding to GHP-G30 increased dramatically in the presence of EGTA as observed for full length 4.1G (Table 2). We also determined the stoichiometry of GHP-G30 binding to CaM using immobilized CaM in IAsys® system assay (Figure 5). The binding ratio was calculated as (2.2/58892) : (0.7/16800) = 1.1:1, where 2.2 (arc seconds) is Bmax of GHP-G30, 0.7 (arc seconds) is immobilized CaM concentration, and 58,892 Da and 16,705 Da are the molecular weights of GHP-G30 and CaM, respectively. The fact that the stoichiometry of binding of GHP-G30 and CaM is ~ 1 to 1, implies that only one of the three potential CaM binding sites in GHP-G30 is functional in 4.1G. It is to be noted that the chimeric protein of RHP-G30 (where GHP is replaced with RHP of GHP-G30) completely lost its ability to regulate G30 binding to CaM by Ca2+ (Table 2). This observation strongly supported the importance of GHP on the Ca2+-dependence of CaM binding to 4.1G.
Figure 5. Analysis of GHP-G30 binding to CaM by Resonant Mirror Detection (RMD).
CaM was immobilized on an aminosilane cuvette. The binding assay was carried out in the presence of 1 mM Ca2+. The Bmax (arc seconds) at each GHP-G30 concentration was calculated using the software package Fastfit® and Bmax is plotted against the ratio Bmax/concentration of added GHP-G30 (Bmax/GHP-G30). The maximum binding (Bmax) at the crossing point on the X-axis was 2.4. The half maximum binding occurred at 87nM of GHP-G30, an affinity that is similar to the K(D) value calculated from kinetic binding analysis (44nM, Table 2). Open circle presents the Bmax in the absence of Ca2+.
Ca2+/CaM regulation of 4.1G binding to membrane proteins
In light of these observations and our previous studies documenting Ca2+/CaM-dependent regulation of 4.1R binding to membrane proteins through its 30kDa domain [12, we examined the Ca2+/CaM-dependent regulation of 4.1G binding to its various binding partners. As shown in Table 3, Ca2+/CaM binding to the headpiece of 4.1G resulted in complete inhibition of 4.1G binding to band 3cyt, CD44cyt and p55 and a significant increase in the K(D) for 4.1G binding to GPCcyt.
Table 3.
Ca2+/CaM-mediated inhibition of 4.1G interactions with membrane proteins
| Analyte | Ligand | Condition | ka (M−1 s−1) | kd (s−1) | K(D) (nM) |
|---|---|---|---|---|---|
| 4.1G/CaM | band3cyt | EGTA | 5.1 ± 0.2 × 105 | 6.4 ± 0.2 × 10−2 | 127 ± 6 |
| Ca2+ | No Binding | No Binding | No Binding* | ||
| GPCcyt | EGTA | 8.4 ± 0.2 × 104 | 1.3 ± 0.2 × 10−2 | 155 ± 22 | |
| Ca2+ | 1.2 ± 0.1 × 104 | 1.5 ± 0.1 × 10−2 | 1251 ± 21 | ||
| p55 | EGTA | 11 ± 0.2 × 105 | 2.3 ± 0.2 × 10−2 | 215 ± 43 | |
| Ca2+ | No Binding | No Binding | No Binding | ||
| CD44cyt | EGTA | 7.1 ± 0.2 × 104 | 1.4 ± 0.2 × 10−2 | 197 ± 29 | |
| Ca2+ | No Binding | No Binding | No Binding |
K(D) for the interactions of the complex of 4.1G and CaM (4.1G/CaM) with cytoplasmic domain of band 3 (band 3cyt), that of GPC (GPCcyt), that of CD44 (CD44cyt) or with p55 in the absence (EGTA) or presence of Ca2+ (Ca2+) are shown. Full length protein 4.1G (at 50 nM to 1 µM) was pre-incubated with CaM (5 µM) and either 0.1 mM EGTA (EGTA) or 1.1 mM CaCl2 and 1.0 mM EGTA (Ca2+) for 30 min at 25 °C in buffer D. The complex of 4.1G and CaM was applied to band 3cyt, GPCcyt, p55 or CD44cyt immobilized on aminosilane cuvettes. ka, kd and K(D) were calculated from three independent experiments represents (mean ± S.D.).
No binding: No signal appeared when 2 µM of Analyte applied to the Ligand immobilized cuvette.
Based on the fact that CaM bound to the 30kDa domain on 4.1R80 in the absence of Ca2+ and decreased K(D) for its binding partners in a Ca2+-dependent manner [14, 15], we also examined the effect of CaM binding to G30 on its binding to membrane proteins using RHP-G30. Binding affinities of RHP-G30 for band 3cyt, GPCcyt, p55 and CD44cyt were measured in the presence or absence of Ca2+ and CaM (Table 4). The K(D) obtained for each binding partner in the absence of CaM was similar to that obtained with full length 4.1G (Table 1). In contrast, binding assays performed with RHP-G30 pre-incubated with Ca2+/CaM showed a major decrease in binding affinity (7–10 fold in K(D)) of RHP-G30 for band 3cyt, GPCcyt and p55 and a less extensive decrease (2.5 fold in K(D)) for CD44cyt. These results indicated that although CaM can bind to G30 independent of Ca2+, G30 interactions with membrane proteins were regulated by CaM in a Ca2+-dependent manner. The RHP-G30 indeed co-pelleted with IOVs (supplementary Figure S5).
Table 4.
Ca2+-dependent CaM regulation of RHP-G30 binding to membrane proteins
| Analyte | Ligand | Condition | ka (M−1 s−1) | kd (s−1) | K(D) (nM) |
|---|---|---|---|---|---|
| RHP-G30/CaM | band 3cyt | EGTA | 6.4 ± 0.4 × 104 | 1.2 ± 0.2 × 10−2 | 183 ± 32 |
| Ca2+ | 2.0 ± 0.2 × 104 | 4.3 ± 0.4 × 10−2 | 2188 ± 78 | ||
| GPCcyt | EGTA | 6.5 ± 0.3 × 104 | 1.5 ± 0.3 × 10−2 | 237 ± 23 | |
| Ca2+ | 1.4 ± 0.1 × 104 | 1.9 ± 0.4 × 10−2 | 1478 ± 182 | ||
| p55 | EGTA | 3.0 ± 0.2 × 105 | 3.2 ± 0.4 × 10−2 | 113 ± 12 | |
| Ca2+ | 6.8 ± 0.4 × 103 | 1.2 ± 0.1 × 10−2 | 1763 ± 43 | ||
| CD44cyt | EGTA | 5.1 ± 0.2 × 104 | 1.6 ± 0.4 × 10−2 | 314 ± 41 | |
| Ca2+ | 2.5 ± 0.2 × 104 | 1.9 ± 0.1 × 10−2 | 737 ± 50 |
K(D) for the interactions of the complex of RHP-G30 and CaM complex (RHP-G30/CaM) with cytoplasmic domain of band 3 (band 3cyt), that of GPC (GPCcyt), that of CD44 (CD44cyt) or with p55 in the absence (EGTA) or presence of Ca2+ (Ca2+) are shown. RHP-G30 as analyte (at 50nM to 2.5 µM) was incubated with CaM (5 µM) in either 0.1 mM EGTA (EGTA) or 1.1 mM CaCl2 and 1.0 mM EGTA (Ca2+) for 30 min at 25 °C. The RHP-G30/CaM complex was applied to band 3cyt, GPCcyt, p55 or CD44cyt immobilized on aminosilane cuvettes. From the binding curves obtained by the resonant mirror detection method, K(D) were determined using the software package FAST-Fit®. ka, kd and K(D) were calculated from three independent experiments represents (mean ± S.D.).
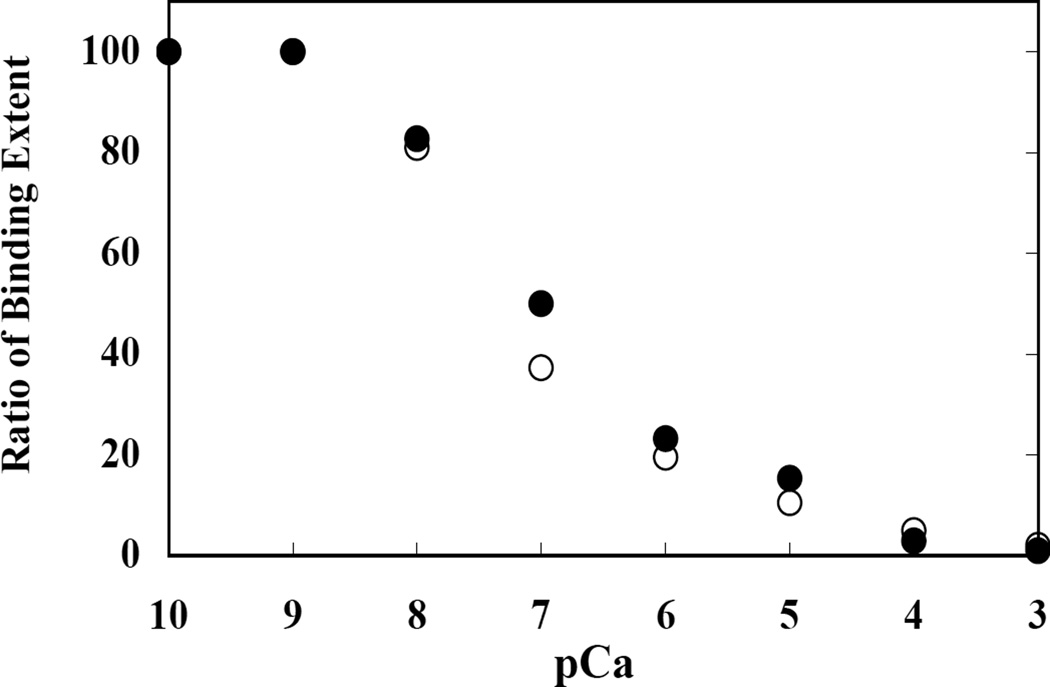
The Ca2+ concentration dependence of the CaM-modulated interaction of GHP-G30 with band 3cyt and GPCcyt was measured (Figure 6). At Ca2+ concentrations greater than 0.01 µM (pCa= 8), the extent of GHP-G30 binding to band 3cyt and GPCcyt started to decline, and maximal inhibition of the binding was noted at Ca2+ concentrations of 100 µM and higher (pCa=4). Half-maximal effect was seen at a Ca2+ concentration of ~0.1 µM (pCa=7). In the absence of CaM, the binding affinities were not altered (data not shown).
Figure 6. Ca2+ concentration dependence of 4.1G (GHP-G30) binding to the cytoplasmic domain of band 3 and GPC.
GHP-G30 binding to cytoplasmic domain of band 3 (●) and GPC (○) was measured at various concentrations of Ca2+ either in the presence of 5 µM CaM. Ca2+ concentrations were maintained by a Ca2+/EGTA buffer system. The maximal extent of binding under different experimental conditions was quantitated as described under “Materials and Methods.” Maximal binding in the presence of EGTA was used to normalize the extent of binding under different experimental conditions. pCa represents ionized Ca2+ concentration. The extent of GHP-G30 binding to cytoplasmic domain of band 3 and GPC is plotted as a function of Ca2+ concentration. In the absence of CaM, there was no change in the binding of GHP-G30 to the cytoplasmic domain of the transmembrane proteins as a function of Ca2+ concentration (data not shown).
Expression of 4.1G and 4.1R135 in human erythroblasts
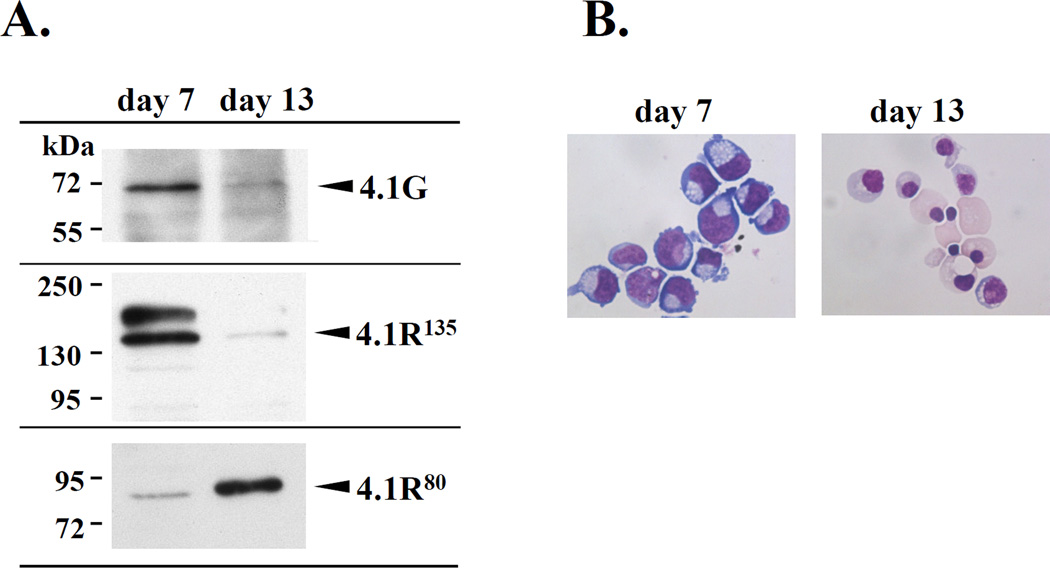
To establish the biologic significance of our biochemical findings, we studied the expression pattern of 4.1G and 4.1R135 during terminal differentiation of human erythroblasts (Figure 7). Western blot analysis using antibodies specific for 4.1R and 4.1G showed that both 4.1G and 4.1R135 were highly expressed in proerythroblasts (day 7 of culture) but their expression level was markedly decreased in orthochromatic erythroblasts (day 13 of culture). In contrast, the expression level of 4.1R80 that was low in proerythroblasts was markedly elevated in orthochromatic erythroblasts.
Figure 7. Expression of 4.1G, 4.1R135 and 4.1R80 during human erythroblast differentiation.
(A) Immuno-blot analysis of protein extracts from human erythroblasts at early (day7) and late stages (day13) of differentiation (see “Materials and Methods”) using antibodies specific for 4.1G and 4.1R. (B) Cellular morphology was assessed by cytospin on a daily basis followed by May-Grünwald Giemsa staining and light microscopy (Magnification is X100).
DISCUSSION
In the present study, we demonstrated that 4.1G binds through its 30kDa domain to various previously defined binding partners for 4.1R, including transmembrane proteins such as band 3, GPC and CD44, and p55. These interactions were not affected by the HP region, but Ca2+-dependent CaM binding to the HP region had a profound effect on the interaction of 4.1G with its binding partners. The documented binding profiles of 4.1G are markedly different from that previously reported for 4.1R135 [12]. Since the primary structure of the 30kDa domain of 4.1G and 4.1R is highly conserved (71% sequence identity), the differences in binding profiles arise primarily from the non-conserved HP region. We provide here extensive support for this hypothesis through the use of several recombinant proteins, GHP, G30, GHP-G30 as well as the chimera protein, RHP-G30.
Computational calculation indicates that the 3D structure of the 30kDa domain of 4.1G is very similar to that of 4.1R, a clover-like structure adopting a globular conformation [30]. The high similarity of 3D structure is supported by in vitro binding assays. Strikingly, the binding sites for each major binding partner of this domain are located on a different lobe of the clover-like structure [26]: band 3 on the N-lobe [31], GPC on the α-lobe [11], and p55 [11] and CD44 on the C-lobe [32]. We previously showed an important role for the HP region in regulating 4.1R135 30kDa domain binding to membrane proteins [12]; the HP region itself improved accessibility of the N-lobe to band 3, impaired accessibility of the α-lobe to GPC and did not have much influence on the C-lobe [12]. In contrast, the HP of 4.1G does not seem to affect the 30kDa domain interactions with membrane proteins, the binding profile of 4.1G and GHP-G30 being similar to that of G30. This indicates that in contrast to HP region of 4.1R135, HP region of 4.1G has little effect on the binding sites in the 30kDa domain of 4.1G. The calculated pI values of GHP and GHP-G30 are 5.16 and 6.65, while that of RHP and RHP-R30 are 4.46 and 5.30, respectively. Thus differences in electrostatic interactions may account for the documented differences in the affinity of interaction of 4.1G and 4.1R135 with band 3cyt and GPCcyt. Considering that the HP region of both 4.1G and 4.1R135 appear to adopt an intrinsically disordered structure, the spatial organization of the HP region and the 30kDa domain must be different in 4.1G and 4.1R135. Our study highlights that GHP-G30 and RHP-G30 represent unique structural and functional modules despite the high amino acid sequence identity of their 30kDa domains and the conservation of CaM binding sites. Delineation of the structure of the HP region alone and in combination with the 30kDa domain will be critical for validating these hypotheses.
The present results indicate that CaM bound to the HP region of 4.1G in a Ca2+-dependent manner but not to the 30kDa domain as has been previously documented for 4.1R80 [15]. The HP region of 4.1G contains the sequence S71RGISRFIPPWLKKQKS which is 76% identical (13/17 residues) to the CaM binding site in the HP region of 4.1R (S76RGLSRLFSSFLKRPKS) [12, 16]. While Ca2+-independent CaM binding sequence previously identified in the 30kDa domain of 4.1R80 is also conserved in 4.1G [15], our findings imply that CaM binds to the HP region but not to the 30kDa domain of 4.1G. It should be emphasized that although the HP by itself does not affect the binding to 30kDa domain of 4.1G to various membrane proteins, Ca2+/CaM binding to the HP markedly inhibits the ability of 4.1G to interact with its various binding partners. These findings have enabled us to document similarities and differences in the structural and functional properties of 4.1G and 4.1R135.
Our finding that the half maximal binding of GHP-G30 to band 3cyt and GPCcyt occurred at Ca2+ concentrations in the submicromolar range suggests potential biologic relevance of our biochemical findings. Ca2+/CaM modulations of 4.1G binding to membrane proteins may be modulated upon signal transduction during erythroid development since at early stages of erythropoiesis it has been documented that following binding of EPO to its receptor intracellular calcium levels increased to 259±49nM from a basal level of 55±5nM [33, 34]. This level of increase in intracellular calcium levels is sufficient to modulate the interaction of 4.1G with its binding partners in erythroid cells.
Our findings further suggest that 4.1G offers a unique opportunity to explore divergence of protein structure and function during evolution and development. In erythroblasts, we showed that 4.1G and 4.1R135 are both expressed during terminal erythroid differentiation consistent with an earlier report [13] and both these proteins can interact with transmembrane proteins, band 3, GPC and CD44. Different binding affinities and Ca2+/CaM modulation to band 3 and GPC suggest that these 4.1 proteins may play specific roles in membrane biogenesis during terminal erythroid differentiation.
Supplementary Material
The secondary structure of 30kDa domain of 4.1G, 4.1B (2HE7A) and 4.1R (1GG3A) derived using the software package DSSP based on defined structures of 4.1B and 4.1R from the Protein Data Base [20].
Probability of order/disorder in the structure of HP region and 30kDa domain of 4.1G (upper panel) and 4.1R135 (lower panel) calculated using the PrDOS software package (http://prdos.hgc.jp/cgi-bin/top.cgi). Values above the 0.5 cut off (line) reflect a disordered structure. For this modeling experiment, the false positive rate was set at 5%. The HP region has a very disordered structure (0.8–0.9). In contrast, the 30kDa domain has a highly ordered structure (0.1–0.4). The location of the predicted CaM binding sequence in 4.1G HP (sequence shown in Figure 1), based on the one identified in 4.1R HP, is highlighted in gray. Note that, unlike the rest of the HP region, this short sequence has an ordered structure (0.3).
Purified GHP-G30 and G30 were analyzed using 8.4% and 12% SDS-PAGE gels, respectively. The protein bands were stained with CBB. M represents marker proteins. Note that GHP-G30 migrated at the position corresponding to a polypeptide with a molecular mass of 75kDa (differing from its calculated molecular weight of 57kDa based on its sequence).
Purified human IgG (150kDa), BSA (68kDa), and ovalbumin (43kDa) in the Buffer C without 2-ME were used as marker proteins for standardization of the column (see the Methods section). The elution position of GHP-G30 is presented as a closed circle (●) and the predicted elution position for a 57kDa polypeptide as a square (■). Apparent molecular weight of GHP-G30 was estimated to be 110kDa.
A chimera protein of RHP-G30 was co-pelleted with IOVs. Bound fractions were recovered by centrifugation as described in the Method section. Left panel: Coomassie Brilliant Blue-stained gel (CBB). Right panel: immuno-blot probed with anti-G30 (α-30). Lanes 1 and 2 indicate the input and IOVs pelleted protein, respectively.
Acknowledgments
FUNDING
This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education Culture, Sport, Science and Technology of Japan 15570123 for WN and by Grants of National Institute of Health DK 26263, HL31579 and DK 32094 for NM.
The abbreviations used are
- 2-ME
2-mercaptoethanol
- 4.1G
protein 4.1G
- 4.1R80
80kDa human erythrocyte protein 4.1
- band 3cyt
cytoplasmic domain of band 3
- CaM
calmodulin
- CD44cyt
cytoplasmic domain of CD44
- CTD
carboxyl terminal domain
- FBS
fetal bovine serum
- FERM
Four-one, Ezrin, Radixin, Moesin
- EPO
erythropoietin
- G30
30kDa membrane binding domain of protein 4.1G
- GHP
headpiece region of protein 4.1G
- GHP-G30
fusion protein of GHP and G30
- GPC
glycophorin C
- GPCcyt
cytoplasmic domain of GPC
- GST
glutathione-S-transferase
- HP
headpiece region
- IOVs
inside-out vesicles of human erythrocytes
- ka
association rate constant
- kd
dissociation rate constant
- K(D)
dissociation constant at equilibrium
- PBS
phosphate-buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- PI
photometric intensity
- PVDF
polyvinylidene fluoride
- R30
30kDa domain of protein 4.1R
- RHP
headpiece region of protein 4.1R
- RHP-R30
fusion protein of RHP and R30
- RHP-G30
chimera protein of RHP and G30
- RMD
resonant mirror detection
- SAB
spectrin and actin binding
- SEC
size exclusion chromatography
- SCF
stem cell factor
Footnotes
AUTHOR CONTRIBUTION
Wataru Nunomura conceived the study, designed the experiments, performed biochemical experiments, analyzed and interpreted the data, and wrote the manuscript. Kengo Kinoshita performed in silico structural analyses of protein 4.1G. Marilyn Parra established the bacterial system for expression of recombinant proteins. Philippe Gascard designed the chimera protein expression system and contributed to editing of the manuscript. Xiuli An performed the experiments using cultured erythroblasts. Narla Mohandas and Yuichi Takakuwa contributed to the interpretation of the data and edited the manuscript.
REFERENCES
- 1.Diakowski W, Grzybek M, Sikorski AF. Protein 4.1, a component of the erythrocyte membrane skeleton and its related homologue proteins forming the protein 4.1/FERM superfamily. Folia Histochemica et Cyrobiologica. 2006;44:231–248. [PubMed] [Google Scholar]
- 2.Parra M, Gascard P, Walensky LD, Snyder SH, Mohandas N, Conboy JG. Cloning and characterization of 4.1G (EPB41L2), a new member of the skeletal protein 4.1 (EPB41) gene family. Genomics. 1998;49:298–306. doi: 10.1006/geno.1998.5265. [DOI] [PubMed] [Google Scholar]
- 3.Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HUG, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH. A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J. Neuroscience. 1999;19:6457–6467. doi: 10.1523/JNEUROSCI.19-15-06457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parra M, Gascard P, Walensky LD, Gimm JA, Blackshaw S, Chan N, Takakuwa Y, Berger T, Lee G, Chasis JA, Snyder SH, Mohandas N, Conboy JG. Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. J. Biol. Chem. 2000;275:3247–3255. doi: 10.1074/jbc.275.5.3247. [DOI] [PubMed] [Google Scholar]
- 5.Conboy JG, Chan J, Mohandas N, Kan YW. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl. Acad. Sci. USA. 1988;85:9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gascard P, Lee G, Coulombel L, Auffray I, Lum M, Parra M, Conboy JG, Mohandas N, Chasis JA. Characterization of multiple isoforms of protein 4.1R expressed during erythroid terminal differentiation. Blood. 1998;92:4404–4414. [PubMed] [Google Scholar]
- 7.Anderson RA, Lovrien RE. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- 8.Pasternack GR, Anderson RA, Leto TL, Marchesi VT. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J. Biol. Chem. 1985;260:3676–3683. [PubMed] [Google Scholar]
- 9.Nunomura W, Takakuwa Y, Tokimitsu R, Kawashima M, Krauss S, Mohandas N. Regulation of CD44-protein 4.1 interaction by Ca2+ and calmodulin. Implications for modulation of CD44-ankyrin interaction. J. Biol. Chem. 1997;272:30322–30328. doi: 10.1074/jbc.272.48.30322. [DOI] [PubMed] [Google Scholar]
- 10.Marfatia SM, Morais-Cabral JH, Kim AC, Byron O, Chishti AH. The PDZ domain of human erythrocyte p55 mediates its binding to the cytoplasmic carboxyl terminus of glycophorin C. Analysis of the binding interface by in vitro mutagenesis. J. Biol. Chem. 1997;272:24191–2417. doi: 10.1074/jbc.272.39.24191. [DOI] [PubMed] [Google Scholar]
- 11.Nunomura W, Takakuwa Y, Parra M, Conboy J, Mohandas N. Regulation of protein 4.1R, p55, and glycophorin C ternary complex in human erythrocyte membrane. J. Biol. Chem. 2000;275:24540–24546. doi: 10.1074/jbc.M002492200. [DOI] [PubMed] [Google Scholar]
- 12.Nunomura W, Parra M, Hebiguchi M, Swada K, Mohandas N, Takakuwa Y. Marked difference in membrane protein binding properties of the two isoforms of protein 4.1R expressed at early and late stages of erythroid differentiation. Biochem. J. 2009;417:141–148. doi: 10.1042/BJ20081372. [DOI] [PubMed] [Google Scholar]
- 13.Delhommeau F, Vasseur-Godbillon C, Leclerc P, Schischmanoff PO, Croisille L, Rince P, Morinière M, Benz EJ, Jr, Tchernia G, Tamagnini T, Ribeiro L, Delaunay J, Baklouti F. A splicing alteration of 4.1R pre-mRNA generates 2�protein isoforms with distinct assembly to spindle poles in mitotic cells. Blood. 2002;100:2629–2636. doi: 10.1182/blood.V100.7.2629. [DOI] [PubMed] [Google Scholar]
- 14.Nunomura W, Takakuwa Y. Regulation of protein 4.1R interactions with membrane proteins by Ca2+ and calmodulin. Front Biosci. 2006;11:1522–1539. doi: 10.2741/1901. [DOI] [PubMed] [Google Scholar]
- 15.Nunomura W, Takakuwa Y, Parra M, Conboy J, Mohandas N. Ca2+-dependent and Ca2+-independent calmodulin binding sites in erythrocyte protein 4.1. Implications for regulation of protein 4.1 interactions with transmembrane proteins. J. Biol. Chem. 2000;275:6360–6367. doi: 10.1074/jbc.275.9.6360. [DOI] [PubMed] [Google Scholar]
- 16.Leclerc E, Vetter S. Characterization of a calcium-dependent calmodulin-binding domain in the 135-kD human protein 4.1 isoform. Eur. J. Biochem. 1998;258:567–571. doi: 10.1046/j.1432-1327.1998.2580567.x. [DOI] [PubMed] [Google Scholar]
- 17.Kang Q, Wang T, Zhang H, Mohandas N, An X. A Golgi-associated protein 4.1B variant is required for assimilation of proteins in the membrane. J. Cell Sci. 2009;122(Pt 8):1091–1099. doi: 10.1242/jcs.039644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishida T, Kinoshita K. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic. Acids. Res. 2007;35:W460–W464. doi: 10.1093/nar/gkm363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marti-Renom MA, Stuart A, Fiser A, Sánchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 20.Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 21.Danilov YN, Fennell R, Ling E, Cohen CM. Selective modulation of Band 4.1 binding to erythrocyte membranes by protein kinase C. J. Biol. Chem. 1990;265:2556–2562. [PubMed] [Google Scholar]
- 22.An X, Takakuwa Y, Nunomura W, Manno S, Mohandas N. Modulation of band 3-ankyrin interaction by protein 4.1. Functional implications in regulation of erythrocyte membrane mechanical properties. J. Biol. Chem. 1996;271:33187–33191. doi: 10.1074/jbc.271.52.33187. [DOI] [PubMed] [Google Scholar]
- 23.Cush R, Cronin JM, Stewart WJ, Maule CH, Molloy J, Goddard NJ. The resonant mirror: a novel optical biosensor for direct sensing of biomolecular interactions Part I: Principle of operation and associated instrumentation. Biosensors & Bioelectronics. 1993;8:347–353. [Google Scholar]
- 24.Nunomura W, Takakuwa Y, Cherr GN, Murata K. Characterization of protein 4.1R in erythrocytes of zebrafish (Danio rerio): Unique binding properties with transmembrane proteins and calmodulin. Comp. Biochem. Physiol. Part B. 2007;148:124–138. doi: 10.1016/j.cbpb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Higgins DG, Thompson JD, Gibson TJ. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]
- 26.Tompa P. Intrinsically unstructured proteins. Trends in Biochem. Sci. 2002;27:527–533. doi: 10.1016/s0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 27.Radivojac PR, Iakoucheva LM, Oldfield CJ, Obradovic Z, Uversky V, Dunker AK. Intrinsic disorder and functional proteomics. Biophysic. J. 2007;92:1439–1465. doi: 10.1529/biophysj.106.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uversky VN, Dunker AK. Understanding protein non-folding. Biochim. Biophys. Acta. 2010;1804:1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porchet R, Probst A, Bouras C, Dráberová E, Dráber R, Riederer BM. Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics. 2003;3:1476–1485. doi: 10.1002/pmic.200300456. [DOI] [PubMed] [Google Scholar]
- 30.Han BG, Nunomura W, Takakuwa Y, Mohandas N, Jap JK. Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat. Struct. Biol. 2000;7:871–875. doi: 10.1038/82819. [DOI] [PubMed] [Google Scholar]
- 31.Jöns T, Drenckhahn D. Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to the erythrocyte anion exchanger. EMBO J. 1992;11:2863–2867. doi: 10.1002/j.1460-2075.1992.tb05354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattori M, Nunomura W, Ito E, Ohta H, Takakuwa Y. Regulation of ankyrin interaction with CD44 by protein 4.1 in HeLa cells. Membrane. 2006;31:107–114. [Google Scholar]
- 33.Yelamarty RV, Miller BA, Scaduto RC, Jr, Yu FTS, Tillotson DL, Cheung JY. Three-dimensional intracellular gradients in single human brust-forming units-erythroid-derived erythroblasts induced by erythropoietin. J. Clin. Invest. 1990;85:17799–1809. doi: 10.1172/JCI114638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller BA, Scaduto RC, Jr, Tillotson DL, Botti J, Cheung JY. Erythropoietin stimulates a rise in intracellular free calcium concentartion in single early human erythroid precursors. J. Clin. Invest. 1988;825:309–315. doi: 10.1172/JCI113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The secondary structure of 30kDa domain of 4.1G, 4.1B (2HE7A) and 4.1R (1GG3A) derived using the software package DSSP based on defined structures of 4.1B and 4.1R from the Protein Data Base [20].
Probability of order/disorder in the structure of HP region and 30kDa domain of 4.1G (upper panel) and 4.1R135 (lower panel) calculated using the PrDOS software package (http://prdos.hgc.jp/cgi-bin/top.cgi). Values above the 0.5 cut off (line) reflect a disordered structure. For this modeling experiment, the false positive rate was set at 5%. The HP region has a very disordered structure (0.8–0.9). In contrast, the 30kDa domain has a highly ordered structure (0.1–0.4). The location of the predicted CaM binding sequence in 4.1G HP (sequence shown in Figure 1), based on the one identified in 4.1R HP, is highlighted in gray. Note that, unlike the rest of the HP region, this short sequence has an ordered structure (0.3).
Purified GHP-G30 and G30 were analyzed using 8.4% and 12% SDS-PAGE gels, respectively. The protein bands were stained with CBB. M represents marker proteins. Note that GHP-G30 migrated at the position corresponding to a polypeptide with a molecular mass of 75kDa (differing from its calculated molecular weight of 57kDa based on its sequence).
Purified human IgG (150kDa), BSA (68kDa), and ovalbumin (43kDa) in the Buffer C without 2-ME were used as marker proteins for standardization of the column (see the Methods section). The elution position of GHP-G30 is presented as a closed circle (●) and the predicted elution position for a 57kDa polypeptide as a square (■). Apparent molecular weight of GHP-G30 was estimated to be 110kDa.
A chimera protein of RHP-G30 was co-pelleted with IOVs. Bound fractions were recovered by centrifugation as described in the Method section. Left panel: Coomassie Brilliant Blue-stained gel (CBB). Right panel: immuno-blot probed with anti-G30 (α-30). Lanes 1 and 2 indicate the input and IOVs pelleted protein, respectively.