Abstract
Terpinolene (TPO) is a monocyclic monoterpene found in the essential oils of various fir and pine species. Recent reports indicated that several monoterpenes could exhibit antioxidant effects in both human and animal experimental models. However, so far, the nature and/or biological roles of TPO have not been elucidated in human models yet. The aim of this study was to investigate the genetic, oxidative and cytotoxic effects of TPO in cultured human blood cells (n = 5) for the first time. Human blood cells were treated with TPO (0–200 mg/L) for 24 and 48 h, and then cytotoxicity was detected by lactate dehydrogenase (LDH) release and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay, while DNA damage was also analyzed by micronucleus assay, sister chromatid exchanges assay and 8-oxo-2-deoxyguanosine (8-OH-dG) level. In addition, biochemical parameters [total antioxidant capacity (TAC) and total oxidative stress (TOS)] were examined to determine oxidative effects. The results of LDH and MTT assays showed that TPO (at concentrations greater than 100 mg/L) decreased cell viability. In our in vitro test systems, it was observed that TPO had no genotoxicity on human lymphocytes. Again, TPO (at 10, 25, 50 and 75 mg/L) treatment caused statistically important (p < 0.05) increases of TAC levels in human lymphocytes without changing TOS levels. In conclusion, TPO can be a new resource of therapeutics as recognized in this study with its non-genotoxic and antioxidant features.
Keywords: Cytotoxicity, Human lymphocytes, Genotoxicity, Oxidative effects, Terpinolene
Introduction
Essential oils are aromatic oily liquids obtained from plant materials (flowers, buds, seeds, leaves, roots, etc.) (Van de Braak and Leijten 1999; Prabuseenivasan et al. 2006). Monoterpenes are found in essential oils of plants of some fruits, vegetables and herbs (Loza-Tavera 1999). Essential oils and their monoterpenes were effective in the treatment of some diseases such as seizures, epilepsy, gastric ulcer, bone resorption and osteoporosis (Mühlbauer 2002; Al-Bayati 2008; Rozza and Pellizzon 2013). Monoterpenes have long been investigated for their biological activities anticancer (Yeruva et al. 2010), antimicrobial (Saddiq and Khayyat 2010), antifungal (Marei et al. 2012), antigenotoxic (Archana et al. 2011), anti-inflammatory (da Rocha et al. 2013), insecticidal (Lee et al. 2010), and more recently antioxidant (Costa et al. 2012) activity.
Antioxidants are important compounds that inhibit or delay the oxidation of other molecules by inhibiting the initiation or propagation of oxidizing chain reactions. A variety of synthetic antioxidants like butylated hydroxyanisole, butylated hydroxytoluene, and tertbutylhydroquinone are commonly used within the food industry although restrictions on the use of synthetic antioxidants are being imposed because of their toxicity (Grice 1986; Wichi 1988; Luo et al. 2010). Much attention has recently been focused on the development of safe and effective antioxidants (Choi et al. 2000), because toxic free radicals play a role in the etiology of many disorders including neurodegenerative and cardiovascular diseases (Lorgis et al. 2010; Dumont and Beal 2011), diabetes (Giacco and Brownlee 2010) and certain cancers (Ziech et al. 2010). Therefore, the development and utilization of more effective and less harmful antioxidants of natural origins are reported to be very desirable (Luo et al. 2010).
Nowadays, extensive efforts are made to investigate therapeutic substances capable of reducing the genotoxicity of various natural and man-made genotoxicants in human life. These include vitamins, fatty acids, lichen, plant extract and antibody (Turkez and Geyikoglu 2010; Aydin and Turkez 2011a, b, 2012; Turkez et al. 2012a, b, c, d, e; Dirican et al. 2012). Terpinolene (TPO) is a monocyclic monoterpene found in the essential oils of various fir and pine species, as well as plants such as Manilla elemi, Nectranda elaiophora and Dacrydium colensoi (Burdock 1995; Brauss et al. 1999). TPO may also be vaporized from some terpene-based cleaners, perfumes and air fresheners (Ma and Marston 2009). Blood and liver tissues are the main targets for exogenous materials for toxicity evaluations. Blood cells are potentially vulnerable as the highest significantly adverse effects from environmental agents reach other tissues through body fluids. Moreover, studies on cultured cells have shown that the human peripheral blood lymphocyte is an extremely sensitive indicator of both in vivo and in vitro induced chromosome structural changes (Lerda et al. 2005). And in contrast to separated erythrocytes whole blood represents an independent physiological compartment with functions of host defence and regulatory functions against cell damaging effects produced by oxidative stress (Hadnagy et al. 2003). The aim of this investigation was to determine its safety profile in vitro, using peripheral blood samples of healthy human donors. So, in our present study, we examined the effects of TPO on the viability of cells on cultured human blood cells (by LDH and MTT assays). We also evaluated the role of TPO on antioxidant capacity (by TAC and TOS analysis) and DNA damage [by micronucleus (MN), sister chromatid exchanges (SCE) rates and 8-oxo-2-deoxyguanosine (8-OH-dG levels)] after TPO-treatment in cultured human blood cells.
Materials and methods
Blood sampling
Whole heparinized human blood from five healthy non-smoking male donors between the ages 22 and 25 was used in our experiments. Questionnaires were obtained for each blood donor to evaluate exposure history, and in addition, informed consent forms were signed by each donor. In all the volunteers involved in this study, hematological and biochemical parameters were analyzed and no pathology was detected. Approximately, 20 mL of blood was collected, by venipuncture, into syringes containing sodium heparin as anticoagulant. Blood was taken the same day of the initiation of the experiment between 9.00 and 9.30 am to minimize possible confounding effects of dietary factors. Human peripheral blood lymphocyte cultures were set up according to a slight modification of the protocol described by Evans and O’Riordan (1975). The heparinized blood (0.5 mL) was cultured in 5 mL of culture medium (Chromosome Medium B, Biochrom®, Berlin, Germany) with 5 μg/mL of phytohemagglutinin (Biochrom®). Various concentrations (0, 10, 25, 50, 75, 100, 150 and 200 mg/L) of TPO (C10H16, CAS No. 586-62-9; Sigma-Aldrich®, St Louis, MO, USA) were tested in blood cultures. The doses were selected according to previous studies (Zhou et al. 2004; Yu et al. 2008; Buyukleyla and Rencuzogullari 2009; Aristatile et al. 2011; Aydin et al. 2013). Experiments conformed to the guidelines of the World Medical Assembly (Declaration of Helsinki). The cultures without extracts were studied as control− group. Mitomycin C (10−7 M) was used as the positive control in MN, SCE and 8-OH-dG assays. Likewise, ascorbic acid (10 μM) and hydrogen peroxide (25 μM) were also used as the positive controls in TAC and TOS analysis, respectively.
Lactate dehydrogenase release assay
Human blood cells were placed in a 24-well culture plate and treated with 10, 25, 50, 75, 100, 150 and 200 mg/L of TPO for 24 and 48 h. Lactate dehydrogenase (LDH) released from damaged cells in culture medium was quantified by using LDH assay kit (Cayman Chemical Company®, Ann Arbor, MI, USA). A total of 120 μL of cell medium was used for LDH analysis. Released LDH catalyzed the oxidation of lactate to pyruvate with simultaneous reduction of NAD+ to NADH. The rate of NAD+ reduction was measured as an increase in absorbance at 490 nm. The rate of NAD+ reduction was directly proportional to LDH activity in the Chromosome medium B.
Cell viability assay
The human blood cells were seeded in 48-well plates. Cells were incubated at 37 °C in a humidified 5 % CO2/95 % air mixture and treated with TPO at different concentrations (0, 10, 25, 50, 75, 100, 150 and 200 mg/L) for 24 h. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) substrate solution was used according to the manufacturer’s instructions (Cayman Chemical Company®). Briefly, MTT was added to the cell cultures for 3 h. Formed formazan crystals were dissolved in dimethyl sulfoxide (Sigma-Aldrich®), and the plates were analyzed using Microquant reader at 570 nm wavelength.
Cytogenetic assays
Human blood cells (0.5 mL) were placed in culture tubes and treated with 10, 25, 50, 75, 100, 150 and 200 mg/L of TPO. A total of 6 mL of cell medium (Chromosome Medium B, Biochrom®) with phytohemagglutinin was used. The MN test was performed by adding cytochalasin B (Sigma®; final concentration of 6 μg/mL) after 44 h of culture as previously described by Fenech and Morley (1985). At the end of the 72 h incubation period, after hypotonic treatment (0.075 M KCl) followed by three repetitive cycles of fixation in methanol/acetic acid solution (3:1, v/v), centrifugation, and resuspension, the cell suspension was dropped onto chilled, grease free microscopic slides, air-dried, aged, and then differentially stained for the inspection of micronuclei rate according to fluorescence plus Giemsa (FPG) (Sigma®, Munich, Germany) procedure (Perry and Wolff 1974). The criteria for scoring micronuclei were as described by Fenech (1993). At least 1,000 binucleated lymphocytes were examined per concentration for the presence of one, two, or more micronuclei.
In order to provide successive visualization of sister chromatid exchanges (SCEs), 5-bromo-20-deoxyuridine (Sigma®; final concentration of 10 μg/mL) was added after culture initiation. At exactly 70 h and 30 min after beginning incubations, colcemid (Sigma®; final concentration of 10 μg/mL) was added to the cultures. After hypotonic treatment (0.075 M KCl) followed by three repetitive cycles of fixation in methanol/acetic acid solution (3:1, v/v), centrifugation, and resuspension, the cell suspension was dropped onto chilled, grease free microscopic slides, air-dried, aged, and then differentially stained for the inspection of SCE rate according to fluorescence plus Giemsa (FPG) procedure (Perry and Wolff 1974). For each treatment condition, 25 well-spread second division metaphases were scored by a single observer (E. Aydin), and the values obtained were calculated as SCEs per cell.
Nucleic acid oxidation
DNA oxidation was determined by measuring the intracellular amount of 8-OH-dG adducts. DNA was digested by incubation with DNAase I, endonuclease, and alkaline phosphatase (Schneider et al. 1993). The amount of 8-OH-dG was measured by high performance liquid chromatography (HPLC) with electrochemical detection as described previously (Floyd et al. 1993).
Total antioxidant capacity and total oxidant status analysis
Since the measurement of different oxidant molecules separately is not practical and their oxidant effects are additive, the total oxidant status (TOS) of a sample was measured named total peroxide (TP), serum oxidation activity (SOA), reactive oxygen metabolites (ROM) or some other synonyms (Erel 2005). The automated Trolox equivalent total antioxidant capacity (TAC) and TOS assays were carried out in plasma samples obtained from blood cultures for 2 h by commercially available kits (Rel Assay Diagnostics®, Gaziantep, Turkey) (Erel 2004).
Statistical analysis
Statistical analysis was performed using SPSS software (version 13.0, SPSS, Chicago, IL, USA). The Duncan’s test was used to determine whether any treatment significantly differed from controls or each other. Statistical decisions were made with a significance level of 0.05.
Results
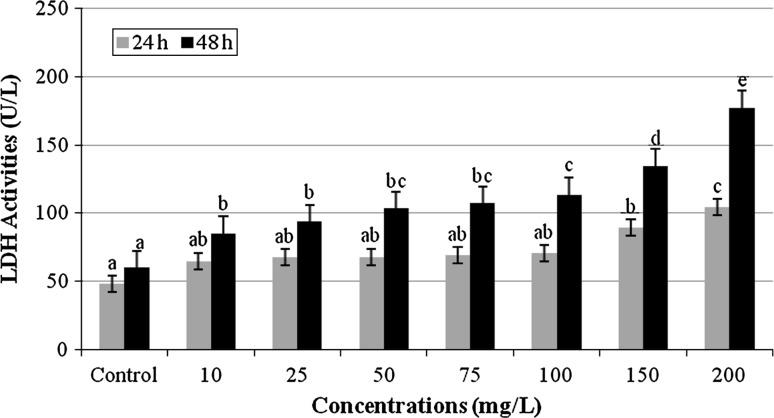
Cell membrane damage was reflected by LDH levels in the cell medium after TPO exposure for 24 and 48 h. Figure 1 indicates that TPO induced significant increase of cytotoxicity in a time- and dose-dependent manner on cultured human blood cells. LDH enzyme activity in the supernatant increased as the number of dead cells (or cells with damaged plasma membranes) increased and thus, the number of viable cells decreased.
Fig. 1.
LDH activities of cultured human blood cells treated with 10, 25, 50, 75, 100, 150 and 200 mg/L of TPO for 24 and 48 h (Control: whole blood without terpinolene. Each value represents mean ± SD of five experiments; means in the figure followed by different letters present significant differences at the p < 0.05 level)
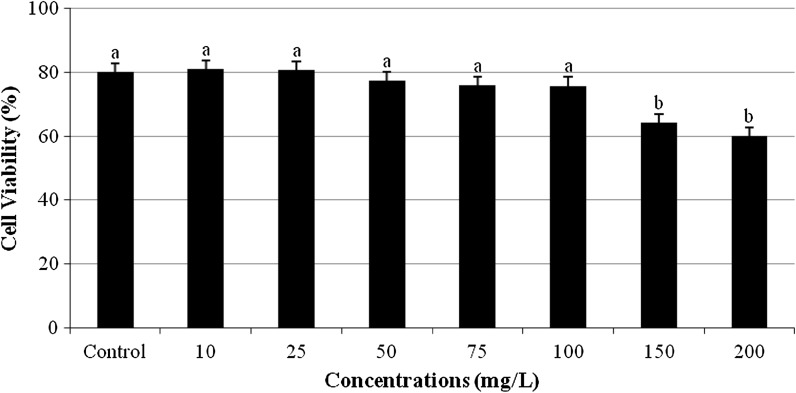
The MTT assay, a colorimetric method of determining cell viability, was used to assess the cytotoxicity activity of TPO. A typical concentration–response curve for TPO of cultured human blood cells obtained with the MTT assay is shown in Fig. 2. No significant change in the cell viability was observed after treatment with concentrations below 150 mg/L TPO for 24 h. However, when cells were treated with 150 and 200 mg/L TPO for 24 h, the number of viable cells significantly decreased compared to control.
Fig. 2.
Cytotoxic effect of TPO on human blood cells (Control: whole blood without terpinolene. Each value represents mean ± SD of five experiments; means in the figure followed by different letters present significant differences at the p < 0.05 level)
The frequency of MN and SCE on human peripheral blood lymphocyte treated with TPO are given in Table 1. TPO did not induce significant (p < 0.05) increases in MN and SCE for any dose. However, Mitomycin C, which was used as a positive control, also caused the expected large increases on human peripheral blood lymphocyte compared to the control− group (Fig. 3).
Table 1.
The frequencies of SCE and MN values in human lymphocyte treated with TPO in cultures for 72 h
| Treatments | MN/1,000 cell | SCEs/cell |
|---|---|---|
| Control− | 3.21 ± 0372a | 6.56 ± 0.92a |
| Control+ | 8.26 ± 1.18b | 12.45 ± 2.21b |
| 10 mg/L | 3.37 ± 0.45a | 6.62 ± 0.98a |
| 25 mg/L | 3.26 ± 0.41a | 6.76 ± 1.08a |
| 50 mg/L | 3.20 ± 0.47a | 6.81 ± 0.89a |
| 75 mg/L | 3.32 ± 0.49a | 6.93 ± 1.06a |
| 100 mg/L | 3.25 ± 0.43a | 6.87 ± 0.99a |
| 150 mg/L | 3.24 ± 0.48a | 6.65 ± 0.98a |
| 200 mg/L | 3.33 ± 0.42a | 6.90 ± 1.02a |
Positive control: mitomycin C (10−7 M). Negative control: whole blood without terpinolene. Each value represents mean ± SD of five experiments; means in the table followed by different letters present significant differences at the p < 0.05 level
Fig. 3.
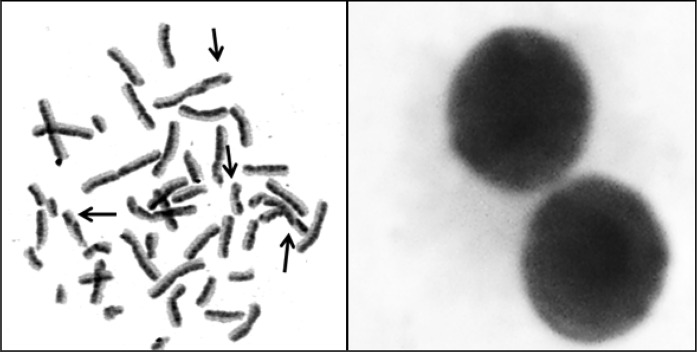
The photomicrographs of sample metaphase (left) and binucleated cells (right) from TPO (150 mg/L) treated cultures (Arrows show sister chromatid exchange points)
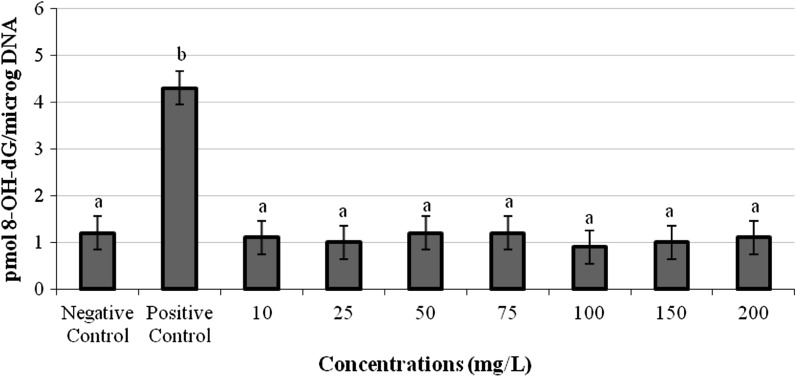
The formation of 8-OH-dG in human lymphocytes exposed to all concentrations of TPO is presented in Fig. 4. All concentrations of TPO did not lead to any increase or decrease of 8-OH-dG levels in human lymphocytes.
Fig. 4.
8-OH-dG adducts in cultured human blood cells maintained 72 h in the presence of TPO (Positive control: mitomycin C (10−7 M). Negative control: whole blood without terpinolene. Each value represents mean ± SD of five experiments; means in the figure followed by different letters present significant differences at the p < 0.05 level)
Table 2 provides an overview on oxidant/antioxidant status of TPO in human whole blood cells. It is apparent from Table 2 that 100 and 150 mg/L concentrations of TPO did not lead to any alterations in the TAC levels while 10, 25, 50 and 75 mg/L of TPO treatments caused significant increases of TAC levels in cultured human blood cells as compared to control value. Also, TPO caused significant decreases of the TAC levels at the highest concentration (at 200 mg/L). On the other hand, the TOS levels increased at 150 and 200 mg/L doses of TPO in cultured human blood cells. However, TPO did not change the TOS levels in cultured human blood cells at doses lower than 150 mg/L.
Table 2.
Total antioxidant capacity (TAC) and total oxidative stress (TOS) levels in cultured human blood cells
| Treatments | TAC (mmol Trolox equiv./L) | TOS (mmol H2O2 equiv./L) |
|---|---|---|
| Control− | 6.28 ± 0.68b | 11.62 ± 2.71a |
| Control+ | 12.67 ± 0.92d | 39.25 ± 4.63c |
| 10 mg/L | 8.13 ± 0.63c | 11.58 ± 2.60a |
| 25 mg/L | 9.58 ± 0.60c | 11.27 ± 2.19a |
| 50 mg/L | 9.22 ± 0.58c | 11.36 ± 2.57a |
| 75 mg/L | 8.79 ± 0.72c | 11.79 ± 3.02a |
| 100 mg/L | 6.73 ± 0.50b | 11.42 ± 2.43a |
| 150 mg/L | 6.20 ± 0.53b | 18.67 ± 3.09b |
| 200 mg/L | 5.20 ± 0.44a | 19.81 ± 2.73b |
Positive control: ascorbic acid (10 μM) and hydrogen peroxide (25 μM) in TAC and TOS analysis, respectively. Negative control: whole blood without terpinolene. Each value represents mean ± SD of five experiments; means in the table followed by different letters present significant differences at the p < 0.05 level
Discussion
Terpinolene and other monoterpenes are lipophilic and are highly soluble in blood (Falk et al. 1990). As a matter of fact, inhaled monoterpenes by humans and animals are absorbed almost entirely in the lungs and then delivered to the liver, where they are completely metabolized by detoxification enzymes (Igimi and Nishimura 1974; Foley et al. 1987; Boyle et al. 1999, 2000). Also, specifically, a single study on the distribution of orally administered limonene, a monoterpene similar to TPO, suggested that monoterpenes are highly absorbed in the intestine (Igimi and Nishimura 1974). Although there is no information about metabolites of TPO in literature, limonene, which is a monoterpene similar to it, is metabolized to oxidized metabolites in rats and in humans (Crowell 1999). In rats, serum metabolites of limonene are perillic acid and dihydroperillic acid (Crowell et al. 1992). Humans produce limonene-1,2-diol in addition to these two serum metabolites (Crowell et al. 1994). On the other hand, glycine and glucuronide conjugates of perillic acid and uroterpenol (p-mentha-8,9-diol) have been detected in the urine of many limonene-fed mammals (Kodama et al. 1976; Regan and Bjeldanes 1976). Yeruva et al. (2007) reported that perillic acid elicited dose-dependent cytotoxicity, induced cell cycle arrest and apoptosis with increasing expression of bax, p21 and caspase-3 activity in non small cell lung cancer (NSCLC, A549, and H520) lines. Again, perillic acid reduced DNA damage in bone marrow of mice exposed to gamma radiation (Pratheeshkumar et al. 2010).
The interactions in human body of natural products are one of the most crucial issues in pharmacotherapeutic safety. TPO is used as a synthetic food flavoring additive or fragrance enhancer, some terpene-based air fresheners, perfumes and cleaners. Thus, it has high potential for human exposure (Ma and Marston 2009; Okumura et al. 2012). In the present study, we evaluated the cytotoxic effects of TPO on cultured human blood cells via LDH and MTT assays for the first time. We demonstrated that TPO significantly increased LDH levels on cultured human blood cells in a time and dose-dependent manner. LDH is a stable cytoplasmic enzyme in serum as a biological indicator for increased cellular damage (Park et al. 2010). Cell death leads to a rise in the concentration of the LDH enzyme in serum and tissues. LDH released into the medium provides an index of cell death and membrane permeability to LDH and an increase in LDH activity in the medium occurs as a result of cell membrane disintegration and enzyme leakage (Yokogawa et al. 2004). In addition, cytotoxicity, the degree to which a chemical can cause cell damage, is assessed in this study by the means of MTT assay. Overall, TPO (at 150 and 200 mg/L) significantly decreases (p < 0.05) the viability of human blood cells. There is no study in literature about the cytotoxic effects of TPO. Therefore, we have discussed the cytotoxic effects of other monoterpenes. Consistent with our finding, MTT assay indicated that the viabilities of human epithelial prostate, Vero monkey kidney and human fetal fibroblast cells were significantly decreased after monoterpenes (such as d-limonene, α-pinene, myrcene, linalool and stylosin) treatment (Silva et al. 2007; Rabi and Bishayee 2009; Rassouli et al. 2011). The exact mechanisms of the cytotoxic action of TPO are not known, but oxidative stress is thought to be the main responsible mechanism in its cellular toxicity. In addition to oxidative stress, previous studies reported that different mechanisms have been linked to cytotoxicity of plant products including (1) proteasome inhibition, (2) topoisomerase inhibition, (3) inhibition of fatty acid synthesis, (4) accumulation of p53, (5) induction of cell cycle arrest, (6) inhibition of phosphatidyl-inositol 3-kinase, (7) inhibition of cyclin D1 gene and (8) enhanced expression of c-fos and c-myc (Constantinou et al. 1995; Lepley et al. 1996; Plaumann et al. 1996; Agullo et al. 1997; Zhou et al. 1997; Chen et al. 1998, 2005; Bienvenu et al. 2001; Kazi et al. 2004; Brusselmans et al. 2005; Shimura et al. 2012).
According to this study, there is considerable evidence that TPO is non-genotoxic in lymphocytes. The results obtained through the present study did not indicate any significant increases in the ratios of the MN and SCE in lymphocytes exposed to TPO as compared to control values. In fact, MN assay provides a measure of both chromosome breakage and chromosome loss or non-disjunction in clastogenic and aneugenic events, respectively (Karaman et al. 2009). And damaged DNA can lead to aneuploidy and/or chromosomal instability, which is believed to be major contributor to tumor progression (Erol 2010). SCE is a sensitive indicator of chromosome (DNA) instability, since the SCE patterns can reveal general genome instability (Konat 2003). An even closer parallel to our findings is provided by the work of Slamenova et al. (2011) who have recently found that chromosomal aberration assay (CA) in rat primary hepatocytes did not testify any genotoxic activity of carvacrol and thymol. Again, Turner et al. (2001) reported that limonene, which is a monoterpene, did not exhibit genotoxic effects in male Big Blue rats. In another study, it was determined that β-myrcene, α-terpinene, and (+) and (−)-α-pinene are not genotoxic in the Salmonella/microsome assay (TA100, TA98, TA97a and TA1535 tester strains) (Gomes-Carneiro et al. 2005). In contrast to our findings, a previous study reported that d-carvone can induce exchanges in sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary cells (National Toxicology Program, 1990). 8-OH-dG is a major product of oxidative DNA damage and as such is regarded as a useful and relevant marker for cellular oxidative stress, particularly with respect to carcinogenesis (Breimer 1990; Halliwell 1999). In our investigation, it was determined that TPO did not change significantly 8-OH-dG levels as compared to control value.
To appraise the antioxidative activity of TPO, TAC and TOS assays were performed in this study. TPO (at concentrations of 10, 25, 50 and 75 mg/L) led to increases of TAC level. As a matter of fact, Bourgou et al. (2012) reported that TPO showed antioxidant activity by using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) assay in normal human skin fibroblast (WS1) cells. Recent studies also demonstrated that TPO was also reported to have antioxidant properties by 2,2-diphenyl-1-picrylhydrazyl (DPPH), hexanal/hexanoic acid assay, thiobarbituric acid reactive species (TBARS), β-carotene agar diffusion methods (Dorman et al. 2000; Ruberto and Baratta 2000; Kim et al. 2004; Grassmann et al. 2005; Mohamed et al. 2010). In an in vivo study, Costa et al. (2012) reported that cyano-carvone (at 25, 50 and 75 mg/kg) showed antioxidant activity by a decrease in the level of lipid peroxidation in mice hippocampus. On the other hand, TPO (at concentrations below 150 mg/L) did not lead to any alterations in TOS levels while a treatment with 150 and 200 mg/L of TPO caused significant increases of TOS levels in cultured human blood cells as compared to control value.
In the light of the findings obtained in the present study, it is suggested that TPO had no genotoxic effects on human lymphocytes. Furthermore, TPO exhibited antioxidant properties due to the applied dose added to the cultures. So, the TPO has the potential of being utilized as novel bio-resource for naturally occurring anti-oxidant therapies when used in low concentrations. However, the dose should be carefully adjusted since it is both an ingredient of functional foods and used for pharmaceutical purposes.
Acknowledgments
The authors are grateful to all volunteers for the blood samples.
References
- Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, Payrastre B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53:1649–1657. doi: 10.1016/S0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- Al-Bayati FA. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J Ethnopharmacol. 2008;116:403–406. doi: 10.1016/j.jep.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Archana PR, Nageshwar Rao B, Satish Rao BS. Modulation of gamma ray-induced genotoxic effect by thymol, a monoterpene phenol derivative of cymene. Integr Cancer Ther. 2011;10:374–383. doi: 10.1177/1534735410387421. [DOI] [PubMed] [Google Scholar]
- Aristatile B, Al-Numair KS, Al-Assaf AH, Pugalendi KV. Pharmacological effect of carvacrol on D: -galactosamine-induced mitochondrial enzymes and DNA damage by single-cell gel electrophoresis. J Nat Med. 2011;65:568–577. doi: 10.1007/s11418-011-0544-8. [DOI] [PubMed] [Google Scholar]
- Aydin E, Turkez H. Antioxidant and genotoxicity screening of aqueous extracts of four lichens collected from North East Anatolia. Fresen Environ Bull. 2011;20:2085–2091. [Google Scholar]
- Aydin E, Turkez H. Effects of lichenic extracts (Bryoria capillaris, Peltigera rufescens and Xanthoria elegans) on human blood cells: a cytogenetic and biochemical study. Fresen Environ Bull. 2011;20:2992–2998. [Google Scholar]
- Aydın E, Türkez H, Keleş MS (2013) The effect of carvacrol on healthy neurons and N2a cancer cells: some biochemical, anticancerogenicity and genotoxicity studies. Cytotechnology 66:149–157 [DOI] [PMC free article] [PubMed]
- Bienvenu F, Gascan H, Coqueret O. Cyclin D1 represses STAT3 activation through a Cdk4-independent mechanism. J Biol Chem. 2001;276:16840–16847. doi: 10.1074/jbc.M100795200. [DOI] [PubMed] [Google Scholar]
- Bourgou S, Pichette A, Lavoie S, Marzouk B, Legault J. Terpenoids isolated from Tunisian Nigella sativa L. essential oil with antioxidant activity and the ability to inhibit nitric oxide production. Flavour Frag J. 2012;27:69–74. doi: 10.1002/ffj.2085. [DOI] [Google Scholar]
- Boyle R, McLean S, Foley WJ, Davies NW. Comparative metabolism of dietary terpene, p-cymene, in generalist and specialist folivorous marsupials. J Chem Ecol. 1999;25:2109–2127. doi: 10.1023/A:1021092908058. [DOI] [Google Scholar]
- Boyle R, McLean S, Davies NW. Biotransformation of 1,8-cineole in the brushtail possum (Trichosurus vulpecula) Xenobiotica. 2000;30:915–932. doi: 10.1080/004982500433336. [DOI] [PubMed] [Google Scholar]
- Brauss MS, Linforth RST, Cayeux I, Harvey B, Taylor AJ. Altering the fat content affects flavor release in a model yogurt system. J Agric Food Chem. 1999;47:2055–2059. doi: 10.1021/jf9810719. [DOI] [PubMed] [Google Scholar]
- Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3:188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- Burdock GA (1995) Fenaroli’s hanbook of flavor ingredients. 3rd edn. CRC Press, Boca Raton, pp 350
- Buyukleyla M, Rencuzogullari E. The effects of thymol on sister chromatid exchange, chromosome aberration and micronucleus in human lymphocytes. Ecotoxicol Environ Saf. 2009;72:943–947. doi: 10.1016/j.ecoenv.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173–179. doi: 10.1016/S0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem Pharmacol. 2005;69:1421–1432. doi: 10.1016/j.bcp.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Choi JS, Chung HY, Jung HA, Park HJ, Yokozawa T. Comparative evaluation of antioxidant potential of alaternin (2-hydroxyemodin) and emodin. J Agric Food Chem Toxicol. 2000;48:6347–6351. doi: 10.1021/jf000936r. [DOI] [PubMed] [Google Scholar]
- Constantinou A, Mehta R, Runyan C, Rao K, Vaughan A, Moon R. Flavonoids as DNA topoisomerase antagonists and poisons: structure-activity relationships. J Nat Prod. 1995;58:217–225. doi: 10.1021/np50116a009. [DOI] [PubMed] [Google Scholar]
- Costa DA, de Oliveira GA, Lima TC, dos Santos PS, de Sousa DP, de Freitas RM. Anticonvulsant and antioxidant effects of cyano-carvone and its action on acetylcholinesterase activity in mice hippocampus. Cell Mol Neurobiol. 2012;32:633–640. doi: 10.1007/s10571-012-9812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775–778. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- Crowell PL, Lin S, Vedejs E, Gould MN. Identification of metabolites of the antitumor agent d-limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother Pharmacol. 1992;31:205–212. doi: 10.1007/BF00685549. [DOI] [PubMed] [Google Scholar]
- Crowell PL, Ren Z, Lin S, Vedejs E, Gould MN. Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem Pharmacol. 1994;47:1405–1415. doi: 10.1016/0006-2952(94)90341-7. [DOI] [PubMed] [Google Scholar]
- da Rocha ML, Oliveira LE, Patrício Santos CC, de Sousa DP, de Almeida RN, Araújo DA. Antinociceptive and anti-inflammatory effects of the monoterpene α, β-epoxy-carvone in mice. J Nat Med. 2013;67:743–749. doi: 10.1007/s11418-012-0738-8. [DOI] [PubMed] [Google Scholar]
- Dirican E, Turkez H, Toğar B. Modulatory effects of Thymbra spicata L. different extracts against the mercury induced genotoxicity in human lymphocytes in vitro. Cytotechnology. 2012;64:181–186. doi: 10.1007/s10616-011-9406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman HJD, Figueiredo AC, Barroso JG, Deans SG. In vitro evaluation of antioxidant activity of essential oils and their components. Flavour Fragr J. 2000;15:12–16. doi: 10.1002/(SICI)1099-1026(200001/02)15:1<12::AID-FFJ858>3.0.CO;2-V. [DOI] [Google Scholar]
- Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 2011;51:1014–1026. doi: 10.1016/j.freeradbiomed.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–2785. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Erol A. Systemic DNA damage response and metabolic syndrome as a premalignant state. Curr Mol Med. 2010;10:321–334. doi: 10.2174/156652410791065282. [DOI] [PubMed] [Google Scholar]
- Evans HJ, O’Riordan ML (1975) Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res 31:135–148 [DOI] [PubMed]
- Falk AA, Hagberg MT, Lof AE, Wigaeus-Hjelm EM, Zhiping W. Uptake, distribution and elimination of alpha-pinene in man after exposure by inhalation. Scand J Work Environ Health. 1990;16:372–378. doi: 10.5271/sjweh.1771. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-L. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1993;1:163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Foley WJ, Lassak EV, Brophy J. Digestion and absorption of Eucalyptus essential oils in greater glider (Petauroides volans) and brushtail possums (Trichosurus vulpecula) J Chem Ecol. 1987;13:2115–2130. doi: 10.1007/BF01012875. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Carneiro MR, Viana ME, Felzenszwalb I, Paumgartten FJ. Evaluation of β-myrcene, α-terpinene and (+)- and (−)-α-pinene in the Salmonella/microsome assay. Food Chem Toxicol. 2005;43:247–252. doi: 10.1016/j.fct.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Grassmann J, Hippeli S, Spitzenberger R, Elstner EF. The monoterpene terpinolene from the oil of Pinus mugo L. in concert with alpha-tocopherol and beta-carotene effectively prevents oxidation of LDL. Phytomedicine. 2005;12:416–423. doi: 10.1016/j.phymed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Grice HC. Safety evaluation of butylated hydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food Chem Toxicol. 1986;24:1127–1130. doi: 10.1016/0278-6915(86)90298-X. [DOI] [PubMed] [Google Scholar]
- Hadnagy W, Marsetz B, Idel H. Hemolytic activity of crystalline silica—separated erythrocytes versus whole blood. Int J Hyg Environ Health. 2003;206:103–107. doi: 10.1078/1438-4639-00200. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Establishing the significance and optimal intake of dietary antioxidants: the biomarker concept. Nutr Rev. 1999;57:104–113. doi: 10.1111/j.1753-4887.1999.tb06933.x. [DOI] [PubMed] [Google Scholar]
- Igimi H, Nishimura M. Studies on the metabolism of d-limonene (p-Mentha-1,8-diene). I. The absorption, distribution, and excretion of d-limonene in rats. Xenobiotica. 1974;4:77–84. doi: 10.3109/00498257409049347. [DOI] [PubMed] [Google Scholar]
- Karaman A, Kadı M, Kara F. Sister chromatid exchange and micronucleus studies in patients with Behçet’s disease. J Cutan Pathol. 2009;36:831–837. doi: 10.1111/j.1600-0560.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure activity relationships of synthetic analogs of (−)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004;24:943–954. [PubMed] [Google Scholar]
- Kim HJ, Chen F, Wu C, Wang X, Chung HY, Jin Z. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J Agric Food Chem. 2004;52:2849–2854. doi: 10.1021/jf035377d. [DOI] [PubMed] [Google Scholar]
- Kodama R, Yano T, Furukawa K, Noda K, Ide H. Studies on the metabolism of d-limonene (p-mentha-1,8-diene). IV: isolation and characterization of new metabolites and species differences in metabolism. Xenobiotica. 1976;6:377–389. doi: 10.3109/00498257609151649. [DOI] [PubMed] [Google Scholar]
- Konat GW. H2O2-induced higher order chromatin degradation: a novel mechanism of oxidative genotoxicity. J Biosci. 2003;28:57–60. doi: 10.1007/BF02970132. [DOI] [PubMed] [Google Scholar]
- Lee CH, Lee SG, Lee HS. Acaricidal effects of Thymus vulgaris leaf-derived materials and monoterpene alcohols against Dermatophagoides spp. J Korean Soc Appl Biol Chem. 2010;53:170–174. doi: 10.3839/jksabc.2010.028. [DOI] [Google Scholar]
- Lepley DM, Li B, Birt DF, Pelling JC. The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis. 1996;17:2367–2375. doi: 10.1093/carcin/17.11.2367. [DOI] [PubMed] [Google Scholar]
- Lerda D, Biaggi Bistoni M, Peralta N, Ychari S, Vazquez M, Bosio G. Fumonisins in foods from Cordoba (Argentina), presence and genotoxicity. Food Chem Toxicol. 2005;43:691–698. doi: 10.1016/j.fct.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Lorgis L, Zeller M, Dentan G, Sicard P, Richard C, Buffet P, L’Huillier I, Beer JC, Cottin Y, Rochette L, Vergely C. The free oxygen radicals test (FORT) to assess circulating oxidative stress in patients with acute myocardial infarction. Atherosclerosis. 2010;213:616–621. doi: 10.1016/j.atherosclerosis.2010.09.018. [DOI] [PubMed] [Google Scholar]
- Loza-Tavera H. Monoterpenes in essential oils: biosynthesis and properties. Adv Exp Med Biol. 1999;464:49–62. doi: 10.1007/978-1-4615-4729-7_5. [DOI] [PubMed] [Google Scholar]
- Luo H, Yamamoto Y, Liu Y, Jung JS, Kahng HY, Koh YJ, Hur JS. The in vitro antioxidant properties of Chinese highland lichens. J Microbiol Biotechnol. 2010;20:1524–1528. doi: 10.4014/jmb.1003.03029. [DOI] [PubMed] [Google Scholar]
- Ma Y, Marston G. Formation of organic acids from the gas-phase ozonolysis of terpinolene. Phys Chem Chem Phys. 2009;11:4198–4209. doi: 10.1039/b818789d. [DOI] [PubMed] [Google Scholar]
- Marei GI, Rasoul MAA, Abdelgaleil SAM. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic Biochem Physiol. 2012;103:56–61. doi: 10.1016/j.pestbp.2012.03.004. [DOI] [Google Scholar]
- Mohamed AA, El-Emary GA, Ali HF. Influence of some citrus essential oils on cell viability, glutathione-S-transferase and lipid peroxidation in Ehrlich ascites carcinoma cells. J Am Sci. 2010;6:820–826. [Google Scholar]
- Mühlbauer R (2002) Essential oils and chemically related species for the treatment of increased bone resorption. WO Patent WO 60:226-355
- National Toxicology Program NTP toxicology and carcinogenesis studies of d-carvone (CAS No. 2244-16-8) in B6C3F1mice (gavage studies) Natl Toxicol Program Tech Rep. 1990;381:1–113. [PubMed] [Google Scholar]
- Okumura N, Yoshida H, Nishimura Y, Kitagishi Y, Matsuda S. Terpinolene, a component of herbal sage, downregulates AKT1 expressionin K562 cells. Oncol Lett. 2012;3:321–324. doi: 10.3892/ol.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CM, Cha YS, Youn HJ, Cho CW, Song YS. Amelioration of oxidative stress by dandelion extract through CYP2E1 suppression against acute liver injury induced by carbon tetrachloride in Sprague–Dawley rats. Phytother Res. 2010;24:1347–1353. doi: 10.1002/ptr.3121. [DOI] [PubMed] [Google Scholar]
- Perry P, Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Plaumann B, Fritsche M, Rimpler H, Brandner G, Hess RD. Flavonoids activate wild-type p53. Oncogene. 1996;13:1605–1614. [PubMed] [Google Scholar]
- Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;30:6–39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheeshkumar P, Raphael TJ, Kuttan G. Protective role of perillic acid against radiation-induced oxidative stress, cytokine profile, DNA damage, and intestinal toxicity in mice. J Environ Pathol Toxicol Oncol. 2010;29:199–212. doi: 10.1615/JEnvironPatholToxicolOncol.v29.i3.40. [DOI] [PubMed] [Google Scholar]
- Rabi T, Bishayee A. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: generation of reactive oxygen species and induction of apoptosis. J Carcinog. 2009;8:1–9. doi: 10.4103/1477-3163.45315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassouli FB, Matin MM, Iranshahi M, Bahrami AR. Investigating the cytotoxic and apoptosis inducing effects of monoterpenoid stylosin in vitro. Fitoterapia. 2011;82:742–749. doi: 10.1016/j.fitote.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Regan JW, Bjeldanes LF. Metabolism of (+)-limonene in rats. J Agric Food Chem. 1976;24:377–380. doi: 10.1021/jf60204a031. [DOI] [PubMed] [Google Scholar]
- Rozza AL, Pellizzon CH. Essential oils from medicinal and aromatic plants: a review of the gastroprotective and ulcer-healing activities. Fundam Clin Pharmacol. 2013;27:51–63. doi: 10.1111/j.1472-8206.2012.01067.x. [DOI] [PubMed] [Google Scholar]
- Ruberto G, Baratta MT. Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chem. 2000;69:167–174. doi: 10.1016/S0308-8146(99)00247-2. [DOI] [PubMed] [Google Scholar]
- Saddiq AA, Khayyat SA. Chemical and antimicrobial studies of monoterpene: citral. Pestic Biochem Physiol. 2010;98:89–93. doi: 10.1016/j.pestbp.2010.05.004. [DOI] [Google Scholar]
- Schneider JE, Phillips JR, Pye Q, Maidt ML, Price S, Floyd RA. Methylene blue and rose bengala photoinactivation of RNA bacteriophages: comparative studies of 8-oxoguanine formation in isolated RNA. Arch Biochem Biophys. 1993;301:91–97. doi: 10.1006/abbi.1993.1119. [DOI] [PubMed] [Google Scholar]
- Shimura T, Noma N, Oikawa T, Ochiai Y, Kakuda S, Kuwahara Y, Takai Y, Takahashi A, Fukumoto M. Activation of the AKT/cyclin D1/Cdk4 survival signaling pathway in radioresistant cancer stem cells. Oncogenesis. 2012;1:e12. doi: 10.1038/oncsis.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SL, Figueiredo PM, Yano T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz. 2007;37:281–286. [Google Scholar]
- Slamenova D, Horvathova E, Chalupa I, Wsolova L, Navarova J. Ex vivo assessment of protective effects of carvacrol against DNA lesions induced in primary rat cells by visible light excited methylene blue (VL + MB) Neoplasma. 2011;58:14–19. doi: 10.4149/neo_2011_01_14. [DOI] [PubMed] [Google Scholar]
- Turkez H, Aydin E (2012) Anti-genotoxic role of eicosapentaenoic acid against imazalil-induced DNA damage in vitro. Toxicol Ind Health 29:584–590 [DOI] [PubMed]
- Turkez H, Geyikoglu F. Boric acid: a potential chemoprotective agent against aflatoxin b1 toxicity in human blood. Cytotechnology. 2010;62:157–165. doi: 10.1007/s10616-010-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoğlu F, Dirican E, Tatar A. In vitro studies on chemoprotective effect of borax against aflatoxin B1-induced genetic damage in human lymphocytes. Cytotechnology. 2012;64:607–612. doi: 10.1007/s10616-012-9454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Aydin E, Aslan A. Xanthoria elegans (Link) (lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology. 2012;64:679–686. doi: 10.1007/s10616-012-9447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Yousef MI, Celik K, Bakir TO. Ameliorative effect of supplementation with L-glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat hepatocyte cultures. Cytotechnology. 2012;64:687–699. doi: 10.1007/s10616-012-9449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Togar B, Polat E. Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology. 2012;64:459–464. doi: 10.1007/s10616-011-9424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Mokhtar YI, Togar B. Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology. 2012;64:15–25. doi: 10.1007/s10616-011-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SD, Tinwell H, Piegorsch W, Schmezer P, Ashby J. The male rat carcinogens limonene and sodium saccharin are not mutagenic to male Big Blue rats. Mutagenesis. 2001;16:329–332. doi: 10.1093/mutage/16.4.329. [DOI] [PubMed] [Google Scholar]
- Van de Braak SAAJ, Leijten GCJJ (1999) Essential oils and oleoresins: a survey in the Netherlands and other major markets in the European Union. CBI, Centre for the Promotion of Imports from Developing Countries, Rotterdam, p 116
- Wichi HP. Enhanced tumor development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium. Food Chem Toxicol. 1988;26:717–723. doi: 10.1016/0278-6915(88)90072-5. [DOI] [PubMed] [Google Scholar]
- Yeruva L, Pierre KJ, Elegbede A, Wang RC, Carper SW. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007;257:216–226. doi: 10.1016/j.canlet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Yeruva L, Hall C, Elegbede JA, Carper SW. Perillyl alcohol and methyl jasmonate sensitize cancer cells to cisplatin. Anticancer Drugs. 2010;21:1–9. doi: 10.1097/CAD.0b013e32832a68ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa K, Watanabe M, Takeshita H, Nomura M, Mano Y, Miyamoto K. Serum aminotransferase activity as a predictor of clearance of drugs metabolized by CYP isoforms in rats with acute hepatic failure induced by carbon tetrachloride. Int J Pharm. 2004;269:479–489. doi: 10.1016/j.ijpharm.2003.09.045. [DOI] [PubMed] [Google Scholar]
- Yu Z, Wang W, Xu L, Dong J, Jing Y. D-Limonene and d-carvone induce apoptosis in HL-60 cells through activation of caspase-8. Asian J Trad Med. 2008;3:134–143. [Google Scholar]
- Zhou Q, Stetler-Stevenson M, Steeg P. Inhibition of cyclin D expression in human breast carcinoma cells by retinoids in vitro. Oncogene. 1997;15:107–115. doi: 10.1038/sj.onc.1201142. [DOI] [PubMed] [Google Scholar]
- Zhou JY, Tang FD, Mao GG, Bian RL. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol Sin. 2004;25:480–484. [PubMed] [Google Scholar]
- Ziech D, Franco R, Georgakilas AG, Georgakila S, Malamou-Mitsi V, Schoneveld O, Pappa A, Panayiotidis MI. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem Biol Interact. 2010;188:334–339. doi: 10.1016/j.cbi.2010.07.010. [DOI] [PubMed] [Google Scholar]