Abstract
Myeloablative allogeneic transplantation in follicular lymphoma has been found to be particularly effective in patients with relapsed disease and an inadequate bone marrow reserve or massive bone marrow involvement. Allogeneic transplantation carries the promise of long-term disease control by graft-versus-lymphoma immunity but is associated with a 30% to 40% risk of transplant-related mortality. Nonmyeloablative stem cell transplantation exploits the graft-versus-lymphoma effect without the attendant toxicity of myeloablative conditioning. The results of several recent reports suggest that it has a high likelihood of resulting in long-term disease-free survival in patients up to 70 years of age with a good performance status, chemotherapy-sensitive disease, and HLA-matched sibling donors. At The University of Texas MD Anderson Cancer Center, the standard NST conditioning regimen for patients with follicular lymphoma is fludarabine, cyclophosphamide, and rituximab. This regimen results in a transplantation-related mortality rate of 10%, and 85% of patients are alive without disease at 8 years. In this article, we discuss the current issues in NST for follicular lymphoma, including chemosensitivity, conditioning intensity, graft-versus-host disease, donor lymphocyte infusion's role, and ongoing strategies to treat refractory disease..
Keywords: Follicular lymphoma, allogeneic transplantation
Introduction
Conventional chemoimmunotherapy and radioimmunotherapy for advanced, relapsed follicular lymphoma (FL) has much improved, but it is not curative [1,2]. Autologous hematopoietic stem cell transplantation (SCT) has long been available for patients with relapsed chemosensitive disease [3]. In the pre-rituximab era, however, there was little evidence that autologous SCT conferred any survival advantage compared with conventional treatments for FL. Furthermore, the observed 5- to 15-fold increased incidence of secondary myelodysplasia [4,5] is of major concern, particularly for patients with indolent lymphoma, whose major benefit from autologous SCT is prolonged remission, not cure. The mortality rate in patients with these secondary malignancies partially negates the lower short-term treatment-related mortality (TRM) rate associated with this approach.
Allogeneic SCT offers the advantages of lymphoma-free grafts and the immunologic graft-versus-lymphoma (GVL) effect, which have been found to confer long-term remission [6-8]. Allogeneic SCT also permits the use of high-dose cytotoxic therapy and cell products devoid of tumor cells and prior chemotherapy-induced DNA damage. The widespread application of myeloablative allogeneic SCT, however, is limited by rates of upfront mortality of up to 40%, occurring in part because allogeneic SCT has traditionally been used in patients with more advanced, chemorefractory disease.
Autologous versus allogeneic SCT in FL
Unlike allogeneic SCT, autologous SCT has been associated with a TRM rate of < 5% but has also been associated with a higher risk of relapse, related to graft contamination and the persistence of minimal residual disease, which results from a lack of a GVL effect.
The only prospective comparison being conducted of autologous and allogeneic hematopoietic SCT for relapsed FL closed early as a result of poor accrual [9]. The other comparisons were based on retrospective analyses of registry data, and which have shown a lower relapse rate and a longer disease-free survival after allogeneic SCT than after autologous SCT. The high TRM rate associated with myeloablative allogeneic SCT, however, offsets any potential survival benefits [6,7].
Is there GVL in FL?
A retrospective analysis of non-Hodgkin's lymphoma patients in the International Bone Marrow Transplant Registry and European Bone Marrow Transplant Registry called the GVL phenomenon into question by demonstrating equally low rates of relapse in syngeneic and full-intensity allogeneic transplantation recipients [10]. Importantly, chemosensitivity was inversely related to relapse on multivariate analysis, and more allograft recipients demonstrated chemorefractoriness prior to transplantation than did syngeneic graft recipients. This discrepancy, along with the retrospective nature of the study, suggests that unmeasured differences between patient groups existed. The inclusion of diverse non-Hodgkin's lymphoma histologic types also precludes specific conclusions regarding FL.
Clinical evidence of a GVL effect is suggested by a plateau in relapse risk that is reached after 2 to 5 years of allogeneic SCT in most studies, indicating that a substantial proportion of patients derive long-term disease control from transplantation [6-8]. One of the most compelling lines of evidence of a GVL effect is the success of allogeneic SCT after autologous SCT has failed to result in a durable response or the use of allogeneic SCT as consolidative therapy after autologous SCT [11,12]. In addition, observations of marked tumor response after withdrawal of immunosuppression and donor lymphocyte infusion in patients who experience relapse after allograft underscore the robustness of the GVL effect in FL and its capacity to maintain long-term remission [12,13].
Nonmyeloablative SCT in FL
Approximately 10% to 20% of allogeneic SCT recipients experience disease relapse after transplantation, but a plateau in relapse risk is reached after 2 to 5 years in most studies, indicating that a substantial proportion of patients derive long-term disease control from transplantation. To exploit this GVL effect without the toxicity associated with myeloablative SCT, our research group has explored the use of nonmyeloablative SCT (NST) in patients with advanced lymphoma [15]. Reduced-intensity conditioning (RIC) regimens were first reported in patients with relapsed lymphoma almost 13 years ago and were intended to make allogeneic transplantation feasible for older, heavily pretreated patients.
Lessons from prospective trials: Regime intensity, chemosensitivity and t-cell-replete graft
In 1998, we reported the feasibility of HLA-identical sibling NST in 15 patients with advanced relapsed/refractory indolent B-lymphoid malignancies, including four patients with transformed disease [15]. The conditioning regimen was nonmyeloablative (ie, not requiring transplanted cells to achieve recovery of blood counts within 28 days), consisting of a combination of fludarabine and cyclophosphamide (FC) at conventional doses. Our observation from this trial was that NST was effective in patients with chemosensitive disease but was less active in patients with chemorefractory relapses: at follow-up, five of six patients with chemosensitive disease at the time of transplantation remained alive, compared with two of nine patients with chemorefractory disease. Since then, four prospective prospective studies of NST in FL have reported favorable outcomes for TRM rate, graft-versus-host disease (GVHD) incidence, and survival rates [16-19] (Table 1). All four trials included patients with relapsed but mostly chemosensitive disease.
Table 1. Prospective Trials of Nonmyeloablative Allogeneic Transplantation in FL.
| Report | N | Conditioning Regimen | Median Age (range) | %Sib,% MUD | Median follow-up, mos | aGVHD, cGVHD | Prog% | %Survival |
|---|---|---|---|---|---|---|---|---|
| Khouri 2008 | 47 | FCR | 53 (33 – 68) | 96, 4 | 60 | 11%, 36% | 4 | 85 (8y) |
| CALGB 2007 | 23 | FC | 53 | 100, 0 | 31.2 | 27%,18% | NA | 76 (2y) |
| Thomson 2010 | 82 | Flu/MEL/Alemtuzumab | 45 (26 – 65) | 46,52 | 43 | 13%,32% (pre & post DLI) | 26 | 76 (4y) |
| Pinana 2010 | 37 | Flu/MEL | 50 (34 – 62) | 100,0 | 52 | 51%, 53% | 8 | 57 (4y) |
FCR, fludarabine, cyclophosphamide & rituximab; Flu/MEL, fludarabine and melphalan; Sib, sibling; MUD, matched unrelated donor; aGVHD, acute II-IV graft-versus-host disease; cGVHD, chronic extensive GVHD; DLI, donor lymphocyte infusion; Prog, progression.
We have recently reported our 8-year experience with the fludarabine, cyclophosphamide, and rituximab (FCR) regimen in 47 FL patients who underwent sibling donor (n = 45) or matched unrelated donor (n = 2) NST in 2009 [16]. A high-dose rituximab schedule of 375 mg/m2 on day -13, followed by 1000 mg/m2 on days -6, +1, and +8, was used, Tacrolimus and methotrexate were used for GVHD prophylaxis. The median patient age was 53 years (range, 33-68 years), and all patients had chemosensitive disease. At the time of NST, 62% of patients were in partial remission (PR); after transplantation, 100% experienced a complete response (CR). Acute GVHD and chronic extensive GVHD occurred in 11% and 36%, respectively. In addition, a unique finding in that report is that 20 of 28 (71%) patients who experienced chronic GVHD had de novo onset, which had no major negative impact on survival. The 1-year TRM rate was 10%. Furthermore, only six of the 47 patients died of infections, even though the regimen targets both cellular and humoral immunity. At a median 60 months of follow-up, only two cases of disease progression after CR had occurred (4%). The progression-free survival (PFS) and overall survival (OS) were 83% and 85%, respectively
Favorable outcomes were also reported by the Cancer and Leukemia Group B 109901 trial [17] in 23 patients with relapsed chemosensitive FL. The nonmyeloablative conditioning regimen consisted of 20 mg/m2 of fludarabine for 5 days and 1000 mg/m2 of cyclophosphamide daily for 3 days. With a median follow-up of 31.2 months, the 2-year OS and PFS rates were 71% and 76%, respectively.
Compared with studies employing nonmyeloablative conditioning (such as FC or FCR), there is no evidence that the more substantial “reduced-intensity” (RIC) regimens (fludarabine and melphalan) provide any advantage in disease control in patients with chemosensitive disease. Importantly, reduced-intensity regimens appear to be asscociated with more severe toxicities and GVHD.
The GELTAMO group, for example, have recently reported the outcome in 37 FL patients who underwent a sibling donor transplant. Melphalan and fludarabine conditioning was used. Cyclosporin and methotrexate were used for GVHD prophylaxis [18]. The median patient age was 50 years (range, 34-62 years). Thirty patients (82%) were transplanted with sensitive disease and 15 (18%) with refractory disease. At the time of transplantation, 39% of patients were in CR, 43% in PR, and 18% had unresponsive or progressive disease (PD). Mucositis grades II-IV was observed in 43% of the patients, and acute renal failure in 18%. Acute II-IV GVHD and chronic extensive GVHD occurred in 51%, and 53%, respectively. Although the incidence of relapse for the whole group was only 8%, progressive or refractory disease at the time of transplantation was associated with a significantly higher NRM. At a median 52 months of follow-up, the 4-year disease-free survival rates for patients with PD, PR or CR at transplantation were 29%, 48% and 64%, respectively, whereas the 4-year cumulative incidences of non-relapse mortality (NRM) were 71%, 33%, and 26%, respectively.
In order to decrease the risk of GVHD associated with reduced-intensity regimens, one study has evaluated the impact of in vivo T-cell depletion using alemtuzumab. Thomson et al. reported the outcome of conditioning with fludarabine, melphalan, and alemtuzumab (total alemtuzumab dose, 100 mg) in 82 FL patients undergoing sibling (46%) or unrelated (52%) transplantation [19]. Nearly all patients (90%) had chemosensitive disease. The risk of relapse was high (26%), and donor lymphocyte infusion (DLI) was frequently needed (41 patients, 50%) than the reports involving t-cell-replete grafts. The risk of extensive chronic GVHD occurring before and/or post-DLI GVHD was 32% at 4 years.
Myeloablative versus NST allogeneic transplantation in FL: A retrospective analysis
NST outcomes in FL vary widely in the medical literature. The inherent difficulties of interpreting these disparate results are study heterogeneity (selection bias, single-center vs multicenter cooperative group vs registry studies), patient heterogeneity (in particular, whether only patients with FL were included), and NST procedure limitations (conditioning regimen, GVHD prophylaxis, and stem cell source).
The most recent retrospective analysis of RIC versus myeloablative conditioning regimens for FL patients was conducted by the European Group for Blood and Marrow Transplantation [20]. The study included 44 patients who received matched unrelated allografts after myeloablative conditioning and 87 who underwent RIC between 2000 and 2005. RIC patients were significantly older, with a longer interval from diagnosis to transplant and had failed a previous autograft more frequently than myeloablative recipients. On multivariate analysis, RIC regimens were associated with a lower NRM (P = 0.01), and a significantly longer PFS (P = 0.01) and OS (P = 0.01).
These results were in contrast to the ones reported in another registry study that included 88 patients with FL who received various nonmyeloablative and RIC regimens; the outcomes were compared with those in 120 patients who received matched sibling grafts after myeloablative conditioning [21]. NST and RIC patients were older (50% of NST patients were older than age 50 years, compared with 15% in the myeloablative group), more likely to be in or past second remission, more likely to have received rituximab, and more likely to receive peripheral blood stem cells rather than marrow-derived stem cells. The higher rate of peripheral blood stem cell use and absence of methotrexate in the GVHD prophylaxis regimen resulted in a higher incidence of chronic GVHD in the NST group than in the myeloablative group (P = 0.03). Despite this difference and the larger population studied, no statistically significant differences in PFS, OS, or TRM rates emerged. The reported 20% TRM rate in the myeloablative group, however, was lower than the 30%-40% reported by the same authors and in other single-institution trials, which suggest that there were likely unmeasured variables, such as organ dysfunction and comorbidities that led to differences in patient selection for myeloablative regimens over NST.
Comorbidities were evaluated by the Seattle Consortium in a study to determine outcome in 41 FL patients who underwent in RIC and myeloablative treatment [22]. Patients had a trend toward a lower relapse risk with myeloablative conditioning but a higher TRM risk (P = 0.02). When the findings were analyzed according to the previously validated hematopoietic SCT -specific comorbidity index, patients without comorbidities were found to have similar TRM and OS rates, regardless of conditioning intensity, whereas patients with high comorbidity scores had lower rates with RIC (HR for TRM, 0.47: P = 0.009; HR for OS, 0.63; P = 0.04)
Optimizing NST strategies for FL
Together, the reported results of allogeneic transplantation suggest that further improvement is required before NST can be widely accepted as the treatment of choice for recurrent FL. Areas to be addressed include the judicious use of DLI, optimizing the conditioning regimen intensity for treatment of refractory disease, and appropriate patient selection for transplantation.
Role of DLI
The precise criteria for DLI administration in NST for FL are not always clear. DLI is often used to augment disease control in patients with progressive or resistant lymphoma but may also be given to patients with mixed donor chimerism to achieve full donor chimerism, even in the absence of measurable disease. This represents a high risk for GVHD and is a major cause of mortality and morbidity after NST.
We evaluated the relationship between disease response, risk of relapse, the incidence of chronic GVHD and donor T-cell chimerism by day 90 in FL patients who received a t-cell-replete grafts [16]. T-cell chimerism was evaluated in 33 patients, and 17 (52%) had mixed chimerism in this compartment. Twelve (71%) of these 17 patients were in PR at transplantation. All achieved CR without DLI. There was no difference in the rate of chronic GVHD and risk of relapse in patients with mixed chimerism compared with the patients who had 100% donor cells by day 90. This observation suggests that experiencing an early full donor chimerism is not a requirement for disease control in follicular lymphoma after T cell–replete transplantation and that the use of DLI for treatment of mixed chimerism should be avoided
RIC regimens that incorporate the lymphocytotoxic CD52 antibody alemtuzumab demonstrate efficient engraftment and reduced GVHD. However, these protocols substantially impair posttransplantation antitumor immunity, partly because the antibody is detectable for up to 56 days after transplantation [19]. Mixed chimerism in this setting has been associated with an increased risk of relapse. Researchers have used prophylactic DLI to decrease the risk of disease progression after alemtuzumab treatment. Meyer et al [23] used prophylactic transfer of CD8-depleted donor lymphocytes after T-cell-depleted reduced-intensity transplantations. However, of 23 patients for whom the strategy was intended, only 11 were able to receive the DLI. The use of natural killer cells after T-cell-deplete grafts is under investigation.
Treatment of refractory disease: potential role for immunotherapy-based conditioning
Various strategies are being investigated to improve the outcome in patients with refractory disease after nonmyeloablative transplantation, including incorporating novel agents into the conditioning regimen to increase effectiveness without increasing toxicity and enhancing GVL effects through tumor-specific immunization or posttransplantation immunomodulation.
The monoclonal anti-CD20 antibody rituximab has significant single-agent activity in B-cell lymphoid malignancies. It may also enhance GVL through antibody-dependent cytotoxicity [24] and by increasing dendritic cell uptake and presentation of tumor cell–derived peptides [25,26]. We have recently demonstrated the importance and effectiveness of this strategy in chronic lymphocytic leukemia. We hypothesized that radioummunotherapy with an anti-CD20 antibody with yttrium-90-ibritumomab tiuxetan (90YIT)[27] would enhance the GVL effect, as it delivers radiation not only to the tumor cells that bind the antibody but also, because of a crossfire effect, to neighboring tumor cells that are inaccessible to the antibody or have insufficient antigen expression.
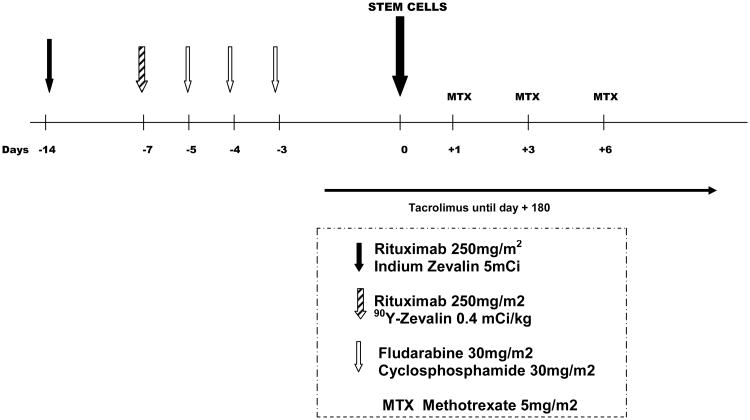
The 90YIT was studied in a phase II NST trial [28]. Patients received single doses of 90YIT (0.4 mCi/kg on day -14) with FCR (Figure 1). The cohort included 34 patients (FL=11, chronic lymphocytic leukemia =20, diffuse large B-cell lymphoma=2, and mantle cell lymphoma=1) with a median age of 59 years (range, 29-70 years). Eight of the FL patients had high [n=6 (55%)] or intermediate [n=2 (18%)] FLIPI (FL international Prognostic Index) score at study entry; 8 (73%) had FDG-avid positron emission tomography scans, and 6 (55%) had refractory disease (that had non-responded or progressed after at least two lines of therapy such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone or rituximab, ifosfamide, carboplatin, and etoposide). Peripheral blood from HLA-compatible sibling donors was the graft source in all patients. Tacrolimus and methotrexate were used for GVHD prophylaxis. Toxicity was similar to that for the FCR regimen alone, with a median time to neutrophil engraftment of 11 days (range, 8-17 days) and to > 20,000 mm3 platelets of 10 days (range, 0-55 days). Median donor T cell counts at days 30 and 90 were 90% and 100%, respectively. The cumulative incidence of acute II-IV GVHD was 32% (no patients had grade III, and one had grade IV), and the cumulative incidence of extensive chronic GVHD was 44%. With a median follow-up of 22 months (range, 3-73 months), the PFS rates at 3 years for FL were both 100%. Verification of our initial results in a larger cohort of patients continues.
Figure 1. 90Yttrium ibritumomab tiuxetan (Zevalin)- containing preparative regimen for nonmyeloablataive stem cell transplantation in patients with FL.
Patient selection for NST
The timing of NST in patients with relapsed FL is still a matter of debate. Some advocate the use of allogeneic transplantation only for patients with disease progression after an autologous transplantation. At our center, we found that about 20% of such patients would not qualify for an allogeneic NST because of rapidly progressive or transformed disease, organ dysfunction, or secondary myelodysplasia (Khouri et al, unpublished data). As a result, and because of the impressive results with the FCR regimen, we have generally preferred allogeneic to autologous transplants. Our approach for relapsed FL is to treat patients with NST if they have a matched donor, especially if the patients are experiencing their second or later relapse, have undergone more than two lines of therapy, or have undergone a prior autologous transplantation. We usually limit this strategy to patients with good organ function and a good performance status.
Conclusions
Substantial progress has been made in the field of allogeneic transplantation for FL. The use of nonmyeloablative conditioning has extended the use of allogeneic transplantation to older patients, with treatment-related mortality rates of 10%-15% and an 8-year disease-free survival rate of 83%. Disease chemosensitivity remains the major determinant of transplantation success. These promising results deserve further investigation in prospective, multicenter trials. Novel strategies, including the addition of radioimmunotherapy to conditioning, are required for patients with chemorefractory disease. Patients with relapsed disease should be referred to transplantation centers for enrollment in forthcoming clinical trials.
Footnotes
Conflict of interest statement: No conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher R, I, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 2.Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23:5019–5026. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 3.Rohatiner AZ, Nadler L, Davies AJ, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: Long-term follow-up. J Clin Oncol. 2007;25:2554–2559. doi: 10.1200/JCO.2006.09.8327. [DOI] [PubMed] [Google Scholar]
- 4.Lenz G, Dreyling M, Schiegnitz E, et al. Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem-cell transplantation in patients with indolent lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. J Clin Oncol. 2004;22:4926–4933. doi: 10.1200/JCO.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Sacchi S, Marcheselli L, Bari A, et al. Secondary malignancies after treatment for indolent non-Hodgkin's lymphoma: A 16-year follow-up study. Haematologica. 2008;93:398–404. doi: 10.3324/haematol.12120. [DOI] [PubMed] [Google Scholar]
- 6*.Van Besien K, Loberiza FRJ, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 7.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 8.Hosing C, Saliba RM, McLaughlin P, et al. Long-term results favor allogeneic over autologous hematopoietic stem cell transplantation in patients with refractory or recurrent indolent non-Hodgkin's lymphoma. Ann Oncol. 2003;14:737–744. doi: 10.1093/annonc/mdg200. [DOI] [PubMed] [Google Scholar]
- 9.Laport G, Bredeson CN, Tomblyn M, et al. Autologous versus reduced intensity allogeneic hematopoietic cell transplantation for patients with follicular non-Hodgkin's lymphoma (FL) beyond first complete response or first partial response [abstract 7041] J Clin Oncol. 2008;26 doi: 10.1016/j.bbmt.2010.11.004. Abstract 7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bierman PJ, Sweetenham JW, Loberiza FRJ, et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin's lymphoma: a comparison with allogeneic and autologous transplantation—The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003;21:3744–3753. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 11.Escalon MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin's lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 12.Branson K, Chopra R, Kottaridis PD, et al. Role of nonmyeloablative allogeneic stem-cell transplantation after failure of autologous transplantation in patients with lymphoproliferative malignancies. J Clin Oncol. 2002;20:4022–4031. doi: 10.1200/JCO.2002.11.088. [DOI] [PubMed] [Google Scholar]
- 13.Mandigers CM, Verdonck LF, Meijerink JP, et al. Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003;32:1159–1163. doi: 10.1038/sj.bmt.1704290. [DOI] [PubMed] [Google Scholar]
- 14.van Besien KW, de Lima M, Giralt SA, et al. Management of lymphoma recurrence after allogeneic transplantation: The relevance of graft-versus-lymphoma effect. Bone Marrow Transplant. 1997;19:977–982. doi: 10.1038/sj.bmt.1700781. [DOI] [PubMed] [Google Scholar]
- 15.Khouri IF, Keating M, Korbling M, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 16*.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shea TC, Johnston J, Walsh W, et al. Reduced intensity allogeneic transplantation provides high disease-free and overall survival in patients (Pts) with advanced indolent NHL and CLL: CALGB 109901. Blood. 2007;110:150a. doi: 10.1016/j.bbmt.2011.01.016. abstract 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piñana JL, Martino R, Gayoso J, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Thomson KJ, Morris EM, Milligon D, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28:3695–3700. doi: 10.1200/JCO.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 20.Avivi I, Montoto S, Canals C, et al. Matched unrelated donor stem cell transplant in 131 patients with follicular lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Br J Haematol. 2009;147:719–728. doi: 10.1111/j.1365-2141.2009.07905.x. [DOI] [PubMed] [Google Scholar]
- 21.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: Higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Storer BE, Maloney DG, et al. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Meyer RG, Britten CM, Wehler D, et al. Prophylactic transfer of CD8-depleted donor lymphocytes after T-cell-depleted reduced-intensity transplantation. Blood. 2007;109:374–382. doi: 10.1182/blood-2006-03-005769. [DOI] [PubMed] [Google Scholar]
- 24.Khouri IF, Lee MS, Saliba RM, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: impact of rituximab on immunomodulation and survival. Exp Hematol. 2004;32:28–35. doi: 10.1016/j.exphem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 26.Hsu FJ, Komarovskaya M. CTLA4 blockade maximizes antitumor T-cell activation by dendritic cells presenting idiotype protein or opsonized anti-CD20 antibody-coated lymphoma cells. J Immunother. 2002;25:455–468. doi: 10.1097/00002371-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Witzig TE, Molina A, Gordon LI, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109:1804–1810. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 28.Khouri IF, Harrell R, Valverde R, et al. Stem cell transplantation with 90yttrium ibritumumab tiuxetan (90YIT) in non-Hodgkin's lymphoma (NHL): observations from PET pre-transplant imaging and responses in allografted refractory follicular histologies. Blood (ASH Annual Meeting Abstracts) 2008;114 Abstract 868. [Google Scholar]